Nephronectin (NPNT) was originally identified as an ECM protein by two independent research groups in 2001 [10,11]. Brandenberger et al. reported NPNT to be involved in the embryonic development of the kidney, hence the name nephronectin (nephron: unit within the kidney; nectin: cellular adhesion proteins) [11]. Morimura and colleagues discovered the same protein to be associated with osteoblast differentiation, and named it preosteoblast epidermal growth factor-like repeat protein with meprin, A5 protein and receptor protein-tyrosine phosphatase µ domain (POEM) [10]. For this review, we will use the name nephronectin (NPNT).
- nephronectin
- cancer
- breast cancer
1. NPNT Structure and Domain Related Functions
NPNT was first reported to be a secreted and glycosylated ECM protein of 70–90 kDa. It was later discovered that NPNT is also located intracellularly [12]. NPNT comprises three functional domains: the five N-terminal epidermal growth factor (EGF)-like repeats, a central linker region with a proline-rich, mucin-like region containing two integrin-binding sequences, and a C-terminal MAM domain (Figure 1) [11,13]. An NPNT homolog also exists, termed epidermal growth factor-like protein 6 (EGFL6, also known as MAEG). However, EGFL6 lacks one of the integrin-binding motifs (LFEIFEIER) [14,15]. The combination of these domains gives NPNT diverse functions which are summarised below.
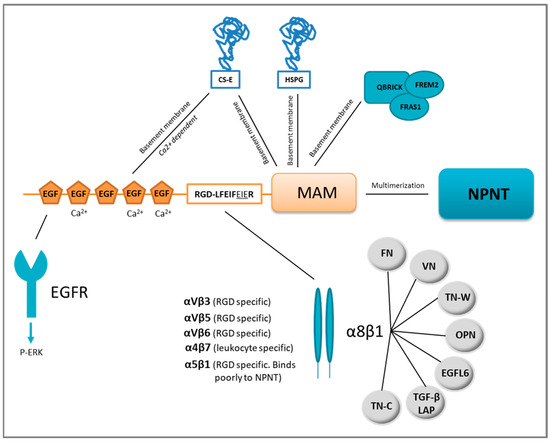
Figure 1. Nephronectin (NPNT) structure, domain-related binding partners and NPNT–integrin redundancy. NPNT consists of three main domains, the N-terminal EGF-like repeats, the linker-region containing two integrin-binding motifs, and the C-terminal MAM domain. The EGF-like repeats may bind the epidermal growth factor receptor (EGFR) and trigger intracellular signalling via ERK phosphorylation (P). Chondroitin sulphate-E (CS-E) can also bind to NPNT via the EGF-like repeats and the MAM domain. The MAM domain is responsible for binding to heparan sulphate proteoglycans (HSPG) in the basement membrane. NPNT may also bind QBRICK, FRAS1 and FREM2 in the basement membrane via its MAM domain. NPNT may multimerise via the MAM domain, and bind several different integrins, most prominently α8β1, which may furthermore associate with several other binding partners. FN = fibronectin, VN = vitronectin, OPN = osteopontin, TN-C = tenascin-C, TN-W = tenascin-W, TGF-β LAP= transforming growth factor-β latency-associated protein.
1.1. EGF-Like Repeats
NPNT contains five EGF-like repeats [11], which are evolutionarily conserved protein domains found in many extracellular proteins [16] including tenascin-C [17], fibrillin-1 [18], jagged 1 [19] and factor IX [20]. Three of the EGF-like repeats of NPNT are believed to be Ca2+-binding [21], a property that is usually associated with interactions of similar domain types, but may also involve other structural motifs [16]. Some EGF-like repeats can bind and activate the EGF receptor (EGFR), triggering proliferation, migration and differentiation [17]. The EGF-like repeats of NPNT were assumed to bind the EGFR, as they were reported to initiate PI3K-AKT signalling downstream of EGFR in dental epithelial stem cells [22]. The EGF-like repeats of NPNT also induce differentiation of pre-osteoblastic cells into mature osteoblasts through ERK phosphorylation [23,24]. More recently, the EGF-like repeats of NPNT were shown to induce angiogenesis in vitro through ERK and p38 MAPK phosphorylation [25]. The NPNT homolog EGFL6 is also reported to induce angiogenesis [26]. Another reported binding partner of the NPNT EGF-like repeats is the ECM protein chondroitin sulphate-E (CS-E) (Figure 1), a binding partially dependent on Ca2+, as it can be reduced by the addition of EDTA. It has been hypothesised that the binding of NPNT to CS-E is needed for deposition of NPNT into basement membranes [27].
1.2. Linker Region (RGD- and LFEIFEIER Peptide Sequences)
Mucins are a family of large and highly O-glycosylated proteins, prominent in the glycocalyx of mucosal epithelia where they are located at the epithelial cell surface. Mucins are important for protective barrier functions of the mucosa and expressed in tissues like the intestine, respiratory tract, eye and middle ear epithelium [28]. The linker region of NPNT resembles a mucin, rich in proline, serine and threonine and highly O-glycosylated with a sialic acid cap [11,13,29]. One N-glycosylation site is also predicted to be present in this mucin-like region [11]. The N-terminal two-thirds of the linker region harbours most of the glycosylations [13], while the remaining portion contains two integrin-binding motifs: RGD (Arg-Gly-Asp) and LFEIFEIER (Leu-Phe-Glu-Ile-Phe-Glu-Ile-Glu-Arg). Integrins reported to bind NPNT are integrin α5β1, αVβ3, αVβ5, αVβ6, α4β7 and α8β1 [11,30] as shown in Figure 1. Of these, the NPNT–α8β1 is best studied and believed to be most prominent, partly due to the overlapping phenotypes of the α8β1 and NPNT knock-out mice [31,32].
The RGD motif of NPNT is critical, however not efficient to secure the binding of NPNT to integrin α8β1 [13]. The LFEIFEIER-motif functions as an integrin-binding enhancer motif, which has thus far only been reported involved in enhancing the specific NPNT–α8β1 binding [13,30]. Sato et al. found that the minimal sequence needed to secure enhancer site binding was EIE (LFEIFEIER) [13], while Sanchez-Cortes et al., using a different method (“self-assembled peptides”), found that the two FEI (LFEIFEIER) motifs were the essential sites. Furthermore, Sanchez-Cortez and colleagues also found that both RGD and LFEIFEIER sites interred equally strong binding to the α8β1 integrin [30].
Using RGD blocking peptides and NPNT with mutated integrin-binding motifs, the RGD motif of NPNT was shown to facilitate cell attachment of cardiomyocytes, endothelial and breast cancer cells [12,33,34]. Furthermore, by mutating the integrin-binding motifs, metastasis of breast cancer cells was significantly reduced [12,34]. Through antibody blocking of the RGD and LFEIFEIER sequence, collagen-induced inflammatory arthritis was reduced [35]. The RGD and partially the LFEIFEIER sequence have also been suggested to be involved in recruitment of CD4+ T cells in both acute and chronic liver hepatitis [36].
1.3. MAM Domain
MAM domains are found in a variety of proteins, including ECM and several transmembrane proteins. The domain consists of approximately 170 amino acids and contains four cysteine residues that potentially form disulphide bridges [27,37]. MAM domains are assumed to contribute to the proteins’ adhesive properties [38], including cell–cell adhesion. When NPNT was expressed in fibroblast-like and pre-osteoblast cells, the protein was not released into the culture medium, but was assumed to be attached to the cell surface. By removing the MAM domain, the protein was released into the medium, indicating that the MAM domain was indeed involved in the cell surface localization of NPNT. When a mutated recombinant NPNT (RGD -> RGE), lacking the EGF domains, was used for coating in cell adhesion studies, leukemic-derived cells stably expressing integrin α8β1 showed reduced survival and cell spreading. Though the cells did not spread out, they adhered to the coated surface through binding to the remaining MAM domain. This shows that the MAM domain plays a role in the initial phases of cell adhesion [10]. Some MAM-containing proteins may form dimers through their MAM domains [39,40], where the NPNT MAM domain is likely involved in the formation of NPNT multimers [10,13]. The importance of multimeric NPNT is not known, but could be related to its localisation and functions in different basement membranes. Through the MAM domain, NPNT binds to basement membrane located proteins. These include heparan sulphate (HS) proteoglycans (HSPG), like agrin and perlecan, chondroitin sulphate (CS)-E, QBRICK, FRAS1 and FREM2 [27,41]. NPNT also binds the highly sulphated glycosaminoglycan, heparin [27]. The MAM domain was, however, not involved in binding to CS-A, CS-C, CS-D, dermatan sulphate or hyaluronan, see Figure 1 [27]. The importance of the localisation of NPNT in the basement membrane is exemplified in the study of Fraser syndrome. Fraser syndrome is a rare autosomal recessive multiorgan congenital disorder that leads to fused eyelids, fingers and toes as well as renal agenesis, pulmonary complications and diverse morphogenetic defects. When the basement membrane protein QBRICK is dysfunctional, it provokes Fraser syndrome. QBRICK facilitates the assembly of NPNT into the basement membrane (together with FRAS1 and FREM2), where the MAM domain ensures the binding. Without QBRICK or NPNT, integrin α8β1 binding to the basement membrane could not occur. Although the NPNT homolog EGFL6/MAEG was present, this could not secure the α8β1 binding. The authors showed that NPNT was a higher-affinity ligand for α8β1. Integrin α8β1 binding is important for cell differentiation and organ development and its loss leads to the widespread defects observed [32,41,42].
2. Expression and Functional Roles of NPNT
NPNT expression is restricted to specific sites during tissue development and tissue homeostasis, where it plays a pivotal role in cell differentiation [32,42,43]. NPNT is expressed in the developing mouse embryo in many different types of tissues. These include the developing renal tubules, parathyroid and thyroid glands, endocrine organs and choroid plexus of the brain, tooth germs, lens of the eyes, the basal lamina of the apical surface of the ear epithelia, Rathke’s pouch, basal lamina of the lip and skin epithelium, basal lamina of the lungs, stomach, developing bone, oesophagus and taste buds of the tongue. Expression is somewhat weaker in the muscles of the tongue, developing pancreas and the lobe of the liver [10,11]. NPNT knock-out mice (NPNT−/−) display renal agenesis and hypoplasia [11,32]. This phenotype resembled that of mice with non-functional integrin α8β1 [31], which triggered Reichardt and colleagues to test whether NPNT was the binding partner of integrin α8β1 [11,32]. Integrin α8β1 is a member of the RGD-binding group of integrins, where the α8 subunit associates exclusively with the β1 subunit [44,45]. The α8 subunit is expressed in vascular and visceral smooth muscle, liver stellate cells, and smooth muscle-like contractile cells. In addition, the kidney mesenchyme (mesangial cells) expresses α8, as well as one cell type in the alveolar wall of the lungs, most likely the alveolar myofibroblasts [44,45,46]. Müller et al. reported expression of the α8 subunit in mesenchymal but not epithelial cells of developing organs such as the gut, lung, gonads and the nephrogenic cord [31]. A crucial role for α8β1 has been uncovered in early steps of kidney morphogenesis in both mice [31] and humans [47]. Integrin α8−/− knock-out mice display frequent renal agenesis or hypoplasia, but also inner ear hair cell (stereocilia) defects [7,31]. Linton et al. could show that the NPNT–α8β1 interaction plays an important role in embryonic kidney development, and is required for the invasion of the metanephric mesenchyme by the epithelial cells of the uretic bud during kidney development. The downstream signalling was shown to be elicited through the glial cell line-derived neurotrophic factor (GDNF), a member of the TGF-β superfamily [32]. Although NPNT is expressed in many specific tissues during development, only the kidneys show macroscopic abnormalities in NPNT−/− mice. This indicates that there is a functional redundancy of the NPNT–integrin α8β1 interaction (Figure 1) [32]. In addition to NPNT, α8β1 can bind [10,11,13] fibronectin (FN) [48], vitronectin (VN) [46], tenascin-C (TN-C) [49], osteopontin (OPN) [50], tenascin-W (TN-W) [51] and the latency-associated peptide (LAP) of transforming growth factor-β1 (TGF-β1) [52] and EGFL6 [42]. Functional redundancy of the NPNT–integrin α8β1 interaction has been confirmed studying hair follicles of the epidermis [42]. Fujiwara et al. found that the stem cells in the hair follicle bulge deposited NPNT into the underlying basement membrane. Mesenchymal cells expressing integrin α8β1 adhered to NPNT and up-regulated smooth muscle markers, triggering arrector pili muscle cell transformation. However, in NPNT−/− mice, the arrector pili muscle cells adhered, not to the bulge, but rather to the follicle above the bulge where the NPNT homolog, EGFL6, was expressed [42]. A similar functional redundancy was observed in the developing heart of zebrafish. NPNT is an upstream regulator of Bmp2-Has2 signalling, and inhibition of the signalling cascade caused failure of heart valve formation. However, integrin α8β1 was not found expressed in the tissue and was therefore most probably not involved in the process [53], indicating the involvement of an alternative receptor.
Whereas the function of NPNT in renal development is well established, much less is known about the function of NPNT expression in the choroid plexus of the brain, lens of the eye, parathyroid and thyroid glands, and the basal lamina of the apical surface of the ear epithelia. Interestingly, α8−/− knock-out mice also display inner ear hair cell (stereocilia) defects [31]. This warrants further investigation. NPNT is also heavily expressed in lung tissue. Increased serum levels of NPNT are linked to silicosis, a type of lung fibrosis [54]. Furthermore, through genome-wide association meta-analysis, NPNT was one of several genes associated with lung function and the susceptibility of developing chronic obstructive pulmonary disease (COPD) [55,56]. A recent preprint indicates that a splice variant of NPNT includes an additional serine residue in the splice site, and is associated with COPD. The serine residue is predicted to be located between the signal peptide and the first EGF-like repeat [57]. The structure and function of this newly discovered variant of NPNT is still to be resolved. NPNT is also elevated in diabetic glomerulosclerosis and nephropathy [58,59] and a maker for glomerular regeneration [60].
As previously reviewed by Sun et al., expression of the NPNT gene is regulated by several different signalling pathways [61]. As summarised in Table 1, NPNT is induced and repressed by a diverse spectrum of factors.
Table 1. Expression and repression of the NPNT gene. Regulation is indicated by colour-coding: up = green, down = red. The downstream signalling pathways/effector(s) are shown and their references. ERK = extracellular signal-regulated kinase, MAPK = mitogen-activated protein kinase, ALK = anaplastic lymphoma kinase, JNK = c-Jun N-terminal kinase, PI3K = phosphoinositide 3-kinase, Klf2 = Kruppel-like factor 2, FGFR = fibroblast growth factor, JAK = Janus kinase, STAT = signal transducer and activator of transcription proteins, TCF = T-cell specific transcription factor, LEF = lymphoid enhancer binding factor, Wnt = wingless-related integration site, IGFR = insulin growth factor receptor, Sp1 = specificity protein 1.
| Regulatory Effect on NPNT | Initiator(s) | Downstream Effector(s) | References |
|---|---|---|---|
| Down-regulation | Tumour necrosis factor α (TNFα) | Nuclear factor-κB (NF-κB) | [62] |
| Transforming growth factor β (TGF-β) | ALK5, Smad2 ERK1/2, JNK, MAPK |
[23,63,64] | |
| Fibroblast growth factor-2 (FGF-2) | JNK PI3K |
[65] | |
| Interleukin-1β (IL-1β) | JNK ERK1/2 |
[66] | |
| Oncostatin M (OSM) | JAK/STAT MAPK |
[67] | |
| Inorganic Phosphate | FGFRs (via Pit) | [68] | |
| Mmu-miRNAs 23a, 101a, 296-5p, 328, 425 | GSK3β, Cyclin D, ERK | [69] | |
| Up-regulation | Wnt3a Wnt |
β-catenin, TCF/LEF | [42,70,71] |
| Bone morphogenetic protein-2 (BMP-2) | [72] | ||
| Vitamin D3 | Vitamin D receptor (VDR) | [73] | |
| Fibroblast growth factor 10 (FGF10) | transcription factor T box 5 (Tbx5) | [43] | |
| Insulin growth factor (IGF) | IGFR—ERK1/2 | [74] | |
| ? | ERK5—Sp1—Klf2/4 | [75] |
Most studies on NPNT regulation (Table 1) are done on osteoblasts, where the protein has a central role in differentiation. However, different effects might be expected in cancer cells. This remains to be tested. Exogenously supplied NPNT induces osteoblast differentiation in pre-osteoblast cells, and the process is mediated through the EGF-like repeats [24]. Fang and colleagues showed that TGF-β1 inhibits NPNT-induced osteoblast differentiation [23]. TGF-β1 stimulates the early steps of osteoblast differentiation, but inhibits the final steps [76]. Cultured pre-osteoblasts stimulated with TGF-β1 down-regulated NPNT expression and differentiation was inhibited [64,77]. The suppression was transferred through TGF-β1-induced phosphorylation of ERK1/2 and JNK in the osteoblasts [64]. It has also been shown that microRNA (miR) plays a part in the regulation of NPNT and osteoblast differentiation [69,78]. Yang and colleagues found five potential miRNAs that can bind to the 3′UTR of NPNT and by that regulate osteoblast differentiation: these included mmu-miR23a, 101a, 296-5p, 328 and 425. The authors suggested that the regulation was through GSK3β, Cyclin D and ERK [69]. Wnt3a is one of the signalling molecules reported to up-regulate NPNT expression in the human pre-osteoblastic cell line MC3T3-E1. The up-regulation was mediated through β-catenin signalling [70], a finding supported by the coinciding observation that NPNT expression is up-regulated in the K14∆β-cateninER mice where a stabilised β-catenin is expressed [42].
This entry is adapted from the peer-reviewed paper 10.3390/cancers13050959
