Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Ophthalmology
Dry eye syndrome (DES) results from insufficient tear production or excessive evaporation of tears, and is associated with symptoms such as dry eye surface, discomfort, visual impairment, and aching. It also leads to an increase in the osmolality of the tear film and inflammation of the ocular surface. The prevalence of DES is estimated as 5–30% in people older than 50 years.
- Dry Eye Syndrome
- hyaluronic acid
1. Introduction
Dry eye syndrome (DES) results from insufficient tear production or excessive evaporation of tears, and is associated with symptoms such as dry eye surface, discomfort, visual impairment, and aching [1]. It also leads to an increase in the osmolality of the tear film and inflammation of the ocular surface. The prevalence of DES is estimated as 5–30% in people older than 50 years [2].
Although DES is a very common disease in adults [3,4], its diagnostic and treatment assessment methods have not yet been standardized. To diagnose patient symptoms, a self-reported questionnaire such as the ocular surface disease index (OSDI) is used. In addition, various clinical tests, including the Schirmer (SH) test, tear breakup time (TBUT), corneal and conjunctival staining, tear meniscus height, tear osmolality, and tear lysozyme analysis, are conducted by clinicians [5].
Several treatment options are available to patients with DES depending on the severity of their symptoms. For treating dry eye, tear replacement products or punctal plugs are used to restore the original homeostasis of the ocular surface and tear film. Recently, several pharmacologic agents have been used to stimulate tear production [6]. Tear replacement with numerous kinds of lubricants is used to improve ocular surface discomfort. Products called artificial tears (ATs), including hyaluronic acid (HA), polyacrylic acid, carboxymethyl cellulose (CMC), dextran, HP-guar, hydroxypropyl methyl cellulose, polyvinyl alcohol, polyvinylpyrrolidone, and polyethylene glycol, are available [6]. Because these products lack the biologically active ingredients found in natural tears [7,8], they may be used in combination with other supplements to enhance lubrication and lengthen the time they last on the ocular surface before evaporation.
HA or sodium hyaluronate is a glycosaminoglycan disaccharide linear biopolymer consisting of repeated alternating sequences of N-acetyl-glucosamine and glucuronate [9]. The topical application of HA has been used to increase the secretion of water and mucin on the ocular surface since the early 1990s [10]. The beneficial effects of various concentrations of HA eye drops on the ocular surface, tear film stability, and dry eye symptoms have been reported in humans [11,12,13,14] and in animal models [15]. However, some studies have been reported that tear supplement with ATs other than HA eye drops significantly improved dry eye signs and symptoms and relieved inflammation [16,17,18,19,20]. It seems that topical preparation of HA has been provided a considerable improvement of subjective and objective outcomes in patients with DES, while there is controversy regarding the efficacy of HA-only eye drops treatment.
Some systematic reviews and meta-analyses performed a pooling analysis of data to compare the efficacies of HA- and non-HA-based eye drops for treating DES [21,22,23]. The results of objective indicators, including TBUT and remission rate, did not show significance [22,23]. In another study, the significant difference of pre- and posttreatment on SH test and TBUT showed 0.238 mm and 0.566 s, respectively [21]. The authors insisted that these differences might be not enough to reflect the clinical significance but are truly comparable. Therefore, there is a need for more reliable results on the effectiveness of HA eye drops for relief of DES.
2. Overview of Recent Studies
2.1. Literature Search
PubMed, the Cochrane Central Register of Controlled Trials, EMBASE, DBpia, KoreaMed, KMBASE, RISS, and KISS databases were searched for studies published up to September 2020. The search terms were (“dry eye” or “keratoconjunctivitis sicca” or “Sjogren’s syndrome” or “xerophthalmia”) and (“hyaluronic acid” or “hyaluronan”). There were no restrictions on sources or languages.
2.2. Inclusion and Exclusion Criteria
The inclusion criteria were as follows: (1) Type of studies: Randomized controlled trials (RCT); (2) Type of participants: Patients with DES, not restrictions for age, gender, or race; (3) Type of interventions: Topical HA-only eye drops with different concentrations; (4) Type of comparisons: Non-HA-based eye drops, including ATs and normal saline; (5) Type of outcomes: At least 1 outcome of SH test, TBUT, corneal fluorescein staining scores (Oxford score scale, 0–5), and OSDI; and (6) Follow-up duration: At least 7 days after the initiation of treatment with eye drops.
The exclusion criteria were as follows: (1) Not RCT, i.e., observational studies, self-controlled studies, clinical trials without contrast, reviews, and letters; (2) Abstracts and conference proceedings; (3) Previously conducted cataracts or ocular surgery; (4) Previously used eye drops for therapeutic purposes (e.g., glaucoma) or contact lens wear; (5) First follow-up after 5 or more weeks; and (6) Not published in English or Korean.
3. Quantitative Analysis
3.1. Schirmer’s (SH) Test
The quantitative data of the SH test (n = 10) were available from nine studies [11,12,16,19,26,29,31,32,35]. A pooled total of 362 cases was randomly allocated to the HA group, and 348 cases were assigned to the non-HA group. A pooled analysis showed that the HA eye drops significantly increased tear production compared with the non-HA group (SMD 0.18; 95% CI 0.03, 0.33), with low heterogeneity (I2 = 0.0%, p = 0.632) (Figure 2).
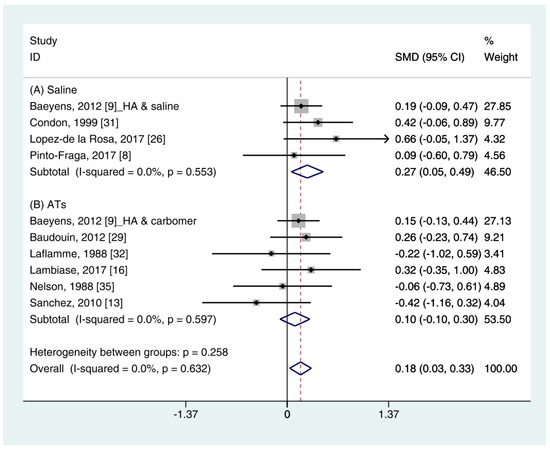
Figure 2. Comparison of the changes on SH test scores (mm/5 min) in HA and non-HA groups using the fixed effect model. The non-HA group was classified into (A) saline and (B) ATs depending on whether lubricant was included. Subgroup analysis was performed between HA and (A) saline and between HA and (B) ATs. SH test: Schirmer’s test; HA: Hyaluronic acid; ATs: Artificial tears; SMD: Standardized Mean Difference; CI: Confidence interval.
In the subgroup analysis, the HA group significantly improved the SH test scores compared with the saline group (SMD 0.27; 95% CI 0.05, 0.49), with low heterogeneity (I2 = 0.0%, p = 0.553). The SH test scores between the HA and the ATs group were similar (SMD 0.10; 95% CI −0.10, 0.30), with low heterogeneity (I2 = 0.0%, p = 0.597). Publication bias was not observed for saline (Egger’s test, t = 0.24, p = 0.833) or ATs (Eggers test, t = 1.64, p = 0.176).
3.2. Tear Break-Up Time (TBUT)
Data (n = 21) from 15 studies were included in the meta-analysis of the TBUT outcomes [11,12,16,18,19,25,26,27,28,29,30,32,33,34,35]. A pooled total of 707 cases was randomly allocated to the HA group, and 693 cases were randomly allocated to the non-HA group. The mean changes in TBUT were similar between the HA and non-HA group (SMD −0.00; 95% CI −0.10, 0.11), and overall heterogeneity was high (I2 = 43.2%, p = 0.021) (Figure 3).
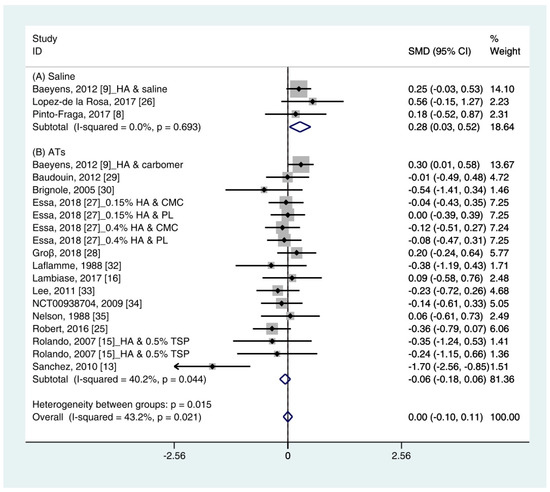
Figure 3. Comparison of the changes on TBUT values (seconds) in HA and non-HA groups using the fixed effect model. The non-HA group was classified into (A) saline and (B) ATs depending on whether lubricant was included. Subgroup analysis was performed between HA and (A) saline and between HA and (B) ATs. TBUT: Tear break-up time; HA: Hyaluronic acid; ATs: Artificial tears; SMD: Standardized Mean Difference; CI: Confidence interval.
In the subgroup analysis, a significant improvement of tear film stability was observed in the HA group (SMD 0.28; 95% CI 0.03, 0.52), with low heterogeneity (I2 = 0.0%, p = 0.693) compared with the saline group. The TBUTs between the HA and the ATs group were similar (SMD −0.06; 95% CI −0.18, 0.06), with high heterogeneity (I2 = 40.2%, p = 0.044). Publication bias was detected in the ATs group (Egger’s test, t = 2.60, p = 0.020). After excluding the source of heterogeneity [16], heterogeneity was decreased (I2 = 0.0%, p = 0.649). However, the HA group showed similar improvement of TBUT compared with the ATs group (SMD −0.03; 95% CI −0.15, 0.09) (Figure S1).
3.3. Corneal Fluorescein Staining Score
Data on corneal fluorescein staining score (n = 7) were obtained from four studies [19,27,29,34]. Pooled data from 286 cases were randomly allocated to the HA group, and data from 272 cases were allocated to the ATs group. The corneal fluorescein staining score was only extracted for ATs. Thus, subgroup analysis was not performed. A similar improvement was observed for HA and ATs (SMD −0.01; 95% CI −0.17, 0.16), with low heterogeneity (I2 = 0.0%, p = 0.613) (Figure 4). The results did not show a publication bias (Eggers test, t = 0.72, p = 0.501).
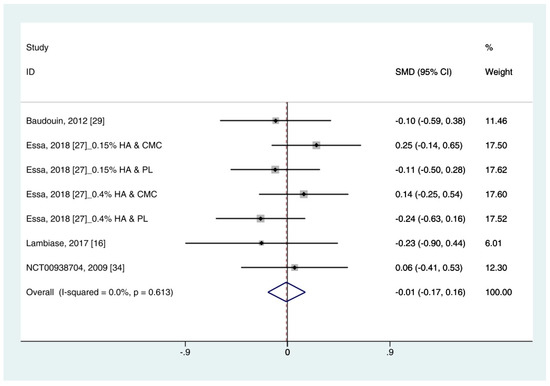
Figure 4. Comparison of the changes on corneal fluorescein staining score (Oxford scale, 0–4) in (HA) and ATs using the fixed effect model. The corneal fluorescein staining score was only extracted from ATs. HA: Hyaluronic acid; ATs: Artificial tears: SMD: Standardized Mean Difference; CI: Confidence interval.
3.4. Ocular Surface Disease Index (OSDI)
Data (n = 8) from five studies were included in the meta-analysis of OSDI outcomes [11,26,27,29,34]. A pooled total of 298 cases was randomly allocated to the HA group, and 284 cases were randomly allocated to the non-HA group. Based on the pooled data, the HA group tended to show decreased symptoms of DES compared with the non-HA group. However, the difference was not significant (SMD −0.14; 95% CI −0.30, 0.02) (Figure 5).
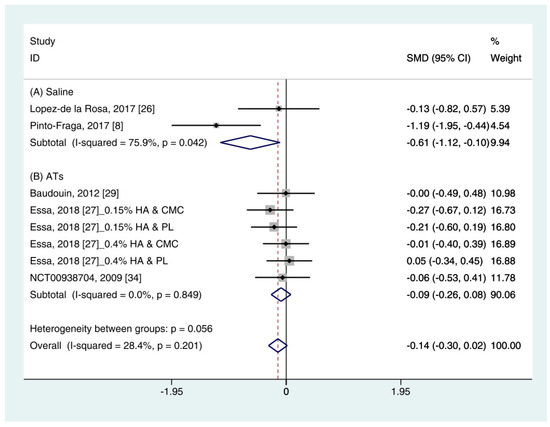
Figure 5. Comparison of the changes on OSDI value in HA and non-HA groups using the fixed effect model. The non-HA group was classified into (A) saline and (B) ATs depending on whether lubricant was included. Subgroup analysis was performed between HA and (A) saline and between HA and (B) ATs. OSDI: Ocular surface disease index; HA: Hyaluronic acid; ATs: Artificial tears; SMD: Standardized Mean Difference; CI: Confidence interval.
In subgroup analysis, the HA group was significantly decreased the symptoms compared with saline (SMD −0.61; 95% CI −1.12, −0.10), with high heterogeneity (I2 = 75.9%, p = 0.042). The OSDI score showed similar between the HA and the Ats groups (SMD −0.09; 95% CI −0.26, 0.08), with low heterogeneity (I2 = 0.0%, p = 0.849).
The publication bias was detected between the HA and the saline groups. However, there were only two studies in the saline group [11,26]. Thus, the OSDI score between the HA group and the saline group were re-examined using the random effect model. The HA group tended to show more improvement of symptoms compared to the saline group. However, statistical significance was not observed (SMD −0.65; 95% CI −1.69, 0.40) (Figure S2).
4. Discussion
The use of HA eye drops has increased in patients with various ocular surface disorders because of its water retention and lubricant properties. In previous studies, the superiority of HA for treating DES was not clearly reported [21,22,23]. Thus, this study aimed to assess the effects of HA eye drops on DES compared with non-HA eye drops, including saline and ATs. For the quantitative analysis, objective tests (e.g., SH test, TBUT, and corneal staining score (Oxford scale, 0–4)) and subjective tests (e.g., OSDI) were used.
A pooled analysis showed that the HA group significantly improved the tear production (based on the SH test) compared with the non-HA group (SMD 0.18; 95% CI 0.03, 0.33) with low heterogeneity (I2 = 0.0%, p = 0.632). The corneal fluorescein staining scores and TBUT values were similar in the HA- and non-HA group were similar. The HA eye drops tended to decrease the OSDI compared with the non-HA eye drops. However, statistical significance was not observed.
In a subgroup analysis, the HA group significantly increased the tear production (based on the SH test) and tear film stability (based on the TBUT) compared with normal saline (SMD 0.27; 95% CI 0.05, 0.49, and SMD 0.28; 95% CI 0.03, 0.52, respectively). Symptom scores (based on the OSDI) in the HA group significantly decreased compared with the saline group (SMD −0.61; 95% CI −1.12, −0.10) with high heterogeneity (I2 = 75.9%, p = 0.042). By random effect modeling, the HA group showed similar OSDI score compared with the saline group (SMD −0.65; 95% CI −1.69, 0.40). The mean changes of SH test score, TBUT, corneal fluorescein score, and OSDI score showed similar between the HA and ATs groups.
The SH test, which measures tear production, is a commonly used diagnostic method in ophthalmology [36]. However, the large measurement error and poor reproducibility are considered limitations [37]. In this study, HA eye drops significantly improved the SH test score compared with non-HA eye drops (Figure 2). Because the changes between the HA- and the non-HA groups was small (0.18 mm), it might not have a significant impact on patient symptoms. Individual studies in this study reported that HA- and non-HA eye drops were effective in the treatment of DES. When considering the various conditions of individual studies, it is reasonable that this degree of difference appears. A previous meta-analysis also showed a significant improvement in the SH test score (0.238 mm) after using an HA eye drops compared with a non-HA eye drops [21]. Two studies were included in the previous meta-analysis but not in our study [38,39]. Those studies reported the beneficial effects of HA on the treatment of DES. However, they did not meet the inclusion criteria in this study because one study used HA-based polyethylene glycol [38] and the other study did not provide a standard deviation [39]. Nonetheless, the overall data showed the significant improvement of tear production after HA treatment compared with the non-HA treatment (Figure 2).
Similar to the SH test score, the TBUT after using HA significantly improved than that of saline and similar to that of ATs (Figure 3). In addition, the ATs outcomes showed high heterogeneity. The TBUT is used to assess tear film stability [40]. It measures the elapses time between the end of a complete blink and the appearance of the first break in the tear film [36]. It is widely used in clinical practice because it can relatively easily measure tear production. A previous meta-analysis showed less improvement in TBUT after using an HA compared with a non-HA preparation, but significance was not observed [21,23].
Among previous studies and our study, only one study reported that non-HA preparation was more effective than HA preparation based on TBUT [16]. The study by Sanchez et al. (2010) [16], which appeared to be the cause of heterogeneity in the previous studies, was also detected as an outlier. This study only reported a significant improvement in TBUT after using ATs compared with HA eye drops. They explained that the difference between the ages of the two groups may have resulted in a difference in the efficacy of treatment. After excluding the study, the heterogeneity was reduced, but there were no significant changes in the SMD of HA and ATs (Figure S1).
The corneal fluorescein staining score after using HA eye drops was similar to that of ATs (Figure 4). Sodium fluorescein, rose Bengal, and lissamine green are widely used staining agents for diagnosing ocular disease. There are various grading systems for recording the severities of ocular surface disorders. This study only used the corneal fluorescein staining score based on the Oxford Scheme (scale 0–4). According to the criteria, low heterogeneity was observed. Although the corneal staining scores is considered informative marker for severe DES, patients with mild/moderate DES showed a poor correlation [41]. Thus, it may be poorly associated with subjective symptoms.
In addition to these objective methods, the HA group showed similar improvement of the OSDI scores compared with the non-HA group (Figure 5). However, significance was not observed. OSDI is widely used to assess DES in clinical trials. It measures the frequency of symptoms, environmental conditions, and vision-related quality of life [36]. Although symptoms are scored by the individual, it is known to produce more reliable and reproducible results than other objective tests [42]. Thus, subjective indicators such as OSDI may be important indicators for evaluating response to DES treatment.
This study has several limitations. First, there was a restriction on the data extraction point. Numerous RCTs used blinding or masking to reduce bias. However, it is difficult to maintain blinding or masking throughout studies because of the instillation frequency, chemical properties, and ocular sensation after instilling HA and non-HA ophthalmic solutions. Thus, we selected the data obtained within 5 weeks and after 1 week of administration to extract homogenous data as much as possible. This period was determined based on a previous study [21]. Second, there were various types of ingredients in the non-HA eye drops, including saline, CMC, phospholipid liposome, emulsions, rebamipid, carmellose, lubricin, and tamarind seed polysaccharide. To reduce heterogeneity and increase accuracy, the comparison group was divided into saline-and AT groups. Third, it is not known whether statistical significance implies clinical improvement. Although the statistically significant beneficial effects of HA, compared with saline, were reflected in the SH test scores and TBUTs, it is difficult to conclude that these differences are clinically meaningful for the treatment of DES. Therefore, further trials are needed to determine the clinical relevance of the symptoms of DES and test outcomes.
Nonetheless, this study included a relatively larger sample size than previous studies that have evaluated the effect of HA. Both objective and subjective indicators were used. In addition, subgroup analysis for saline and ATs was performed.
This entry is adapted from the peer-reviewed paper 10.3390/ijerph18052383
This entry is offline, you can click here to edit this entry!
