Pulses are a key component of crop production systems in numerous countries to their rotational benefits and potential profit margins. However, cultivation in temperate cropping systems is limited by low soil water availability and subsoil constraints. This limitation of soil water is compounded by the irregular rainfall, resulting in the absence of plant-available water at depth. Given the propensity for many pulse cropping regions to experience moderate in-season droughts, a new root ideotype that combines a range of root architectural traits is proposed. This ideotype has a root length density that is concentrated in the upper soil layers to capture in-season rainfall before it is lost due to evaporation. This high root length density would be achieved through more hypocotyl roots, a wide root angle allowing for lateral root growth in a shallow soil profile, thin lateral roots, and thin xylem diameter with a high number of root hairs to increase surface area to volume ratio.
- dicotyledon
- indeterminacy
- tolerance
- avoidance
- dryland cropping
- constraints
- morphology
1. Introduction
Pulses are a key seed crop both in Australia and globally due to their production value, their ability to increase soil nitrogen through fixation, forming a disease break in crop rotations, and for leaving residual moisture deeper in the soil profile for use by subsequent, deep-rooted crops [1,2]. In Australia, the annual pulse crop was valued at AUD $1.8 million in 2018 [3]. Furthermore, approximately 3 million tonnes of nitrogen are fixed annually via root nodules in pulse crops, which translates into approximately AUD $4 billion [4]. If constraints are addressed, predicted pulse production can reach 4.2 million tonnes, with a combined commodity value and farm system benefit of AUD 2 billion [3].
Considerable research effort over several decades has been expended on physiological improvements of crop adaptation to water stress [5,6]. A relatively recent line of enquiry has targeted features of the root system architecture (RSA) that specifically improve access to soil water and nutrients. The root system architecture of monocots has been thoroughly characterised in terminal drought conditions such as in that of the Northern Australian cropping system. However, there is comparatively little information available on dicotyledon roots, especially pulses. In contrast to cereals, pulses are an indeterminate crop; hence, they differ significantly in their interactions between phenology, root development and response to seasonal variation [3]. Understanding the RSA of pulses and exploring the genetic variability that favours constraint tolerance or avoidance when combined with appropriate management strategies could provide a direction to breeders to improve the productivity of pulses under challenging climate and soil conditions.
Recently, a “Steep, Deep and Cheap” ideotype [7] has been proposed to increase soil water and nitrogen acquisition in an effective manner in monocotyledons. Steep root angles and thick long laterals allow deep roots to reach soil water and nitrate at depth, whilst a structure with few laterals, large cortical cells and aerenchyma reduces the photosynthate demand on the plant (Figure 1a). The focus on deeper roots is supported by research that has demonstrated the value of water stored deep in the soil profile to seed yield in environments characterised by terminal drought [8], particularly in sub-tropical regions, where crops rely on water stored during summer fallows and “opportunity cropping” [9]. However, deep roots may not be as important in major cropping regions in temperate Southern Australia. Here, rain predominantly falls during the cropping season, heavy clay soils limit the ability of water to permeate deeper down the profile, and fallows are limited to summer when limited recharge occurs. This can result in a lack of stored subsoil water and deeper roots may not provide a benefit during water-deficit conditions. Furthermore, many cropping soils in the southern region contain soil physicochemical constraints such as high boron, sodicity, salinity, high soil strength/poor soil structure and nutrient deficiencies that potentially restrict root growth and access to soil water and nutrients [10] (Supplementary Figures S1 and S2). Thus, there is a need to investigate root traits specific to water stress adaption in the dryland cropping systems of Southern Australia.
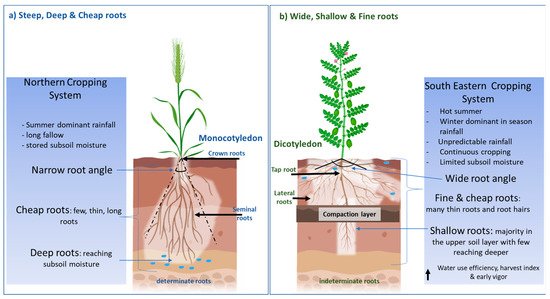
Figure 1. (a) The “Steep, Deep and Cheap” root architecture that has been largely investigated and applied to monocotyledons such as maize and wheat cultivated in environments that experience terminal drought with presence of subsoil water. (b) The proposed alternative, “Wide, Shallow and Fine” roots, for the southeastern cropping system of Australia, which experiences unpredictable rainfall and absence/unavailability of stored subsoil water.
Studies to date on pulse root system architecture (RSA) have identified key traits such as rooting depth, lateral root intensity, root length distribution, root angle and root diameter that have the potential to increase plant adaptation to water stress [11,12,13]. Ramamoorthy et al. [14] investigated some of these traits in in chickpeas and observed that genotypes with higher root length density closer to the soil surface, with greater root dry weight at depth, showed the best tolerance to drought. Similarly, Kashiwagi et al. [15] observed that genotypes with deeper roots and more soil exploration capability perform better under drought stress. Other studies, such as those by Gorim and Vandenberg [16] and Idrissi et al. [17], have examined root trait diversity in selected germplasms and found significant genetic variability in root traits and their response to drought. However, it is difficult to compare the value of various traits across existing studies due to the range of different methods that have been used. Consolidating root traits would provide breeders and agronomists with a new perspective in approaching the development of climate-ready pulse crops, especially when attempting to improve crop adaptation to specific environments.
2. Ideotype Context and Traits
2.1. Soil, Climate and Constraints
Pulse cultivation in the temperate regions of Australia with low- to mid-rainfall zones is mainly undertaken in dryland systems. Rainfall in the low-rainfall zone is <350 mm, medium 350–550, and in the high-rainfall zone, it is >550 mm. Pulses in temperate Australia are typically sown in late autumn and experience cool wet winters, with terminal drought often ending the season in early summer [19]. Existing cultivars of these crops are best adapted to alkaline soils and mostly grown on Vertosols, Sodosols and Calcarosols with a high-clay subsoil. Faba bean appears to be able to tolerate more acidic soil and waterlogging in high-rainfall areas. However, most other pulses cannot and are more limited to higher soil pH in medium- and low-rainfall areas (Supplementary Figure S1). Topsoils can be both clay and loam, although sands become increasingly dominant in some of the lower rainfall areas. Environmental conditions in Southern Australia drylands result in a range of physicochemical and nutrient constraints, including water stress, subsoil compaction, salinity, sodicity, boron, extremes in pH and nitrogen and phosphorous deficiencies.
Despite the numerous abiotic and biotic constraints to pulse productivity in dryland cropping systems, the principal abiotic constraint is drought [10,20]. Dryland cropping systems in the medium- and low-rainfall zones of Australia typically experience unpredictable rainfall, often in insufficient amounts to replenish soil water throughout the potential rooting zone of annual crops, whilst in higher-rainfall locations, high precipitation: evaporation rates often lead to waterlogging during winter. Furthermore, the large episodic events that can occur in summer are also inefficient in recharging subsoil water storage due to high runoff and evaporation rates [19,21]. Verburg, McBeath, Armstrong, Tavakkoli, Wilhelm, Mclaughlin, Haling, Richardson, Mason, Kirkegaard and Sandral [21] found that, in certain areas, insufficient and irregular rainfall resulted in low soil water, starting at depths of only 10 cm in the soil profile.
In the case of dryland cropping systems in Southern Australia, where subsoil water can be limited due to unpredictable rainfall and continuous cropping, traits associated with developing deep roots may provide little benefit in most temperate cropping systems and may even reduce productivity as excess energy is used in developing roots that provide little value. However, critical knowledge can be extrapolated from these traits and applied to root system architecture suited to alternative seasonal rainfall patterns and soil types.
2.2. A New Root Ideotype—Wide, Shallow and Fine
Given the propensity for many pulse cropping regions to experience moderate in-season droughts, a new root ideotype that combines a range of root architectural traits is proposed. This ideotype has a root length density that is concentrated in the upper soil layers to capture in-season rainfall before it is lost due to evaporation. This high root length density would be achieved through more hypocotyl roots, a wide root angle allowing for lateral root growth in a shallow soil profile, thin lateral roots and thin xylem diameter with a high number of root hairs to increase surface area to volume ratio. Lynch [7] proposed cheap roots in terms of construction costs, by going deep quickly, and this was important to ensure a balance on the photosynthate demand on the plant. Shallow roots are inherently cheap to produce as there is no wastage on extensive root production whilst searching for water; shallow roots are already at the optimal position for water and nutrient uptake in ephemeral rainfall systems. A high water use efficiency (WUE)/transpiration efficiency and harvest index are also preferred for the optimal extraction of soil moisture and to convert it effectively to biomass and yield. Furthermore, the development of early root vigour, aimed at establishing extensive colonisation of upper soil layers when soil water is available and root penetration is easier, would likely benefit productivity. Early vigour above ground can also help to trap soil moisture by reducing evaporative losses. Early root and shoot vigour would accelerate the accumulation of shoot biomass early in the season, before water deficiency reaches limiting levels, and could act in concert with efficient translocation of assimilation to seed later in the season. The proposed ideotype would also help in competitiveness against weeds. Ramamoorthy, Lakshmanan, Upadhyaya, Vadez and Varshney [14] have also proposed a more profuse root length density in the topsoil and few thick roots at depth as adaption traits for water stress. The possession of high hereditability and plasti[1][2][3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70][71]city in this ideotype is also important, so that in years with above-average rainfall, yield potential is still able to be fully achieved.
This entry is adapted from the peer-reviewed paper 10.3390/plants10040692
References
- Campbell, C.A.; Zentner, R.P.; Basnyat, P.; Wang, H.; Selles, F.; McConkey, B.G.; Gan, Y.T.; Cutforth, H.W. Water use efficiency and water and nitrate distribution in soil in the semiarid prairie: Effect of crop type over 21 years. Canadian Journal of Plant Science 2007, 87, 815-827, doi:10.4141/CJPS06034.
- Gan, Y.; Campbell, C.A.; Liu, L.; Basnyat, P.; McDonald, C.L. Water use and distribution profile under pulse and oilseed crops in semiarid northern high latitude areas. Agricultural Water Management 2009, 96, 337-348, doi:https://doi.org/10.1016/j.agwat.2008.08.012.
- Pulse Australia. Pulse Australia. Available online: (accessed on
- Drew, E.; Herridge, D.; Ballard, R.; O’Hara, G.; Deaker, R.; Denton, M.; Yates, R.; Gemell, G.; Hartley, E.; Phillips, L.; et al. Inoculating legumes: Apracticle guide; Grains Research and Development Organisation: https://grdc.com.au/resources-and-publications/all-publications/bookshop/2015/07/inoculating-legumes, 2012.
- Sreeman, S.M.; Vijayaraghavareddy, P.; Sreevathsa, R.; Rajendrareddy, S.; Arakesh, S.; Bharti, P.; Dharmappa, P.; Soola-nayakanahally, R. Introgression of Physiological Traits for a Comprehensive Improvement of Drought Adaptation in Crop Plants. Frontiers in Chemistry 2018, 6, doi:10.3389/fchem.2018.00092.
- Kebede, A.; Manjit, S.K.; Endashaw, B. Advances in mechanisms of drought tolerance in crops, with emphasis on barley. In Advances in Agronomy, Donald, L.S., Ed.; Zoe Kruze: 2019; Volume 156, pp. 266-300.
- Lynch, J.P. Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Ann Bot 2013, 112, 347-357, doi:10.1093/aob/mcs293.
- Lilley, J.M.; Kirkegaard, J.A. Seasonal variation in the value of subsoil water to wheat: simulation studies in southern New South Wales. Australian Journal of Agricultural Research 2007, 58, 1115-1128, doi:https://doi.org/10.1071/AR07046.
- Carroll, C.; Halpin, M.; Burger, P.; Bell, K.; Sallaway, M.M.; Yule, D.F. The effect of crop type, crop rotation, and tillage practice on runoff and soil loss on a Vertisol in central Queensland. Soil Research 1997, 35, 925-940, doi:https://doi.org/10.1071/S96017.
- Adcock, D.; McNeill, A.M.; McDonald, G.K.; Armstrong, R.D. Subsoil constraints to crop production on neutral and alkaline soils in south-eastern Australia: a review of current knowledge and management strategies. Australian Journal of Experimental Agriculture 2007, 47, 1245-1261, doi:https://doi.org/10.1071/EA06250.
- Lynch, J.P.; Chimungu, J.G.; Brown, K.M. Root anatomical phenes associated with water acquisition from drying soil: targets for crop improvement. Journal of Experimental Botany 2014, 65, 6155-6166, doi:10.1093/jxb/eru162.
- Liu, L.; Gan, Y.; Bueckert, R.; Van Rees, K. Rooting systems of oilseed and pulse crops. II: Vertical distribution patterns across the soil profile. Field Crops Research 2011, 122, 248-255, doi:https://doi.org/10.1016/j.fcr.2011.04.003.
- Lynch, J. Root Architecture and Plant Productivity. Plant Physiol 1995, 109, 7-13, doi:10.1104/pp.109.1.7.
- Ramamoorthy, P.; Lakshmanan, K.; Upadhyaya, H.D.; Vadez, V.; Varshney, R.K. Root traits confer grain yield advantages under terminal drought in chickpea (Cicer arietinum L.). Field Crops Research 2017, 201, 146-161, doi:https://doi.org/10.1016/j.fcr.2016.11.004.
- Kashiwagi, J.; Krishnamurthy, L.; Purushothaman, R.; Upadhyaya, H.D.; Gaur, P.M.; Gowda, C.L.L.; Ito, O.; Varshney, R.K. Scope for improvement of yield under drought through the root traits in chickpea (Cicer arietinum L.). Field Crops Research 2015, 170, 47-54, doi:https://doi.org/10.1016/j.fcr.2014.10.003.
- Gorim, L.Y.; Vandenberg, A. Evaluation of Wild Lentil Species as Genetic Resources to Improve Drought Tolerance in Cul-tivated Lentil. Front Plant Sci 2017, 8, 1129-1129, doi:10.3389/fpls.2017.01129.
- Idrissi, O.; Houasli, C.; Udupa, S.M.; De Keyser, E.; Van Damme, P.; De Riek, J. Genetic variability for root and shoot traits in a lentil (Lens culinaris Medik.) recombinant inbred line population and their association with drought tolerance. Euphytica 2015, 204, 693-709, doi:10.1007/s10681-015-1373-8.
- Redden, R.J.; Berger, J.D. History and orgin of chickpea. In Chickpea Breeding and management, Yadav, S.S., Ed.; CAB Interna-tional: UK, 2007; pp. 1-13.
- Nuttall, J.G.; Armstrong, R.D.; Connor, D.J.; Matassa, V.J. Interrelationships between edaphic factors potentially limiting cereal growth on alkaline soils in north-western Victoria. Soil Research 2003, 41, 277-292, doi:https://doi.org/10.1071/SR02022.
- Sadras, V.O.; Hayman, P.T.; Rodriguez, D.; Monjardino, M.; Bielich, M.; Unkovich, M.; Mudge, B.; Wang, E. Interactions be-tween water and nitrogen in Australian cropping systems: physiological, agronomic, economic, breeding and modelling perspectives. Crop and Pasture Science 2016, 67, 1019-1053, doi:https://doi.org/10.1071/CP16027.
- Verburg, K.; McBeath, T.M.; Armstrong, R.D.; Tavakkoli, E.; Wilhelm, N.; Mclaughlin, M.J.; Haling, R.E.; Richardson, A.E.; Mason, S.D.; Kirkegaard, J.A.; et al. Soil water dynamics as a function of soil and climate to inform phosphorus placement strategies. In Proceedings of the Soil Science Australia and New Zealand Scoiety of Soil Science Joint Conference, Cains, Australia, 2020.
- Reid, C.D.; Strain, B.R. Effects of CO2 enrichment on whole-plant carbon budget of seedlings of Fagus grandifolia and Acer saccharum in low irradiance. Oecologia 1994, 98, 31-39, doi:10.1007/BF00326087.
- Gutschick, V.P. 2 - Photosynthesis, Growth Rate, and Biomass Allocation. In Ecology in Agriculture, Jackson, L.E., Ed.; Academic Press: 1997; pp. 39-78.
- Urry, L.A.; Meyers, N.; Cain, M.L.; Wasserma, S.A.; Minorsk, P.V.; Reece, J.B. Campbell Biology: Australian and New Zealand edition (11th ed.); Pearson, Australia, 2017.
- Adu, O.M. Identifying key contributing root system traits to genetic diversity in field-grown cowpea (Vigna unguiculata L. Walp.) genotypes. Field Crops Research 2019, v. 232, pp. 106-118-2019 v.2232, doi:10.1016/j.fcr.2018.12.015.
- Gregory, P.J. Root growth of chickpea, faba bean, lentil, and pea and effects of water and salt stresses. In Current Plant Science and Biotechnology in Agriculture R.J., S., Ed.; World crops: Cool season food legumes; Springer: Dordrecht, 1988; Volume 5.
- Manschadi, A.M.; Christopher, J.; deVoil, P.; Hammer, G.L. The role of root architectural traits in adaptation of wheat to wa-ter-limited environments. Functional Plant Biology 2006, 33, 823-837, doi:https://doi.org/10.1071/FP06055.
- Lopes, M.S.; Reynolds, M.P. Partitioning of assimilates to deeper roots is associated with cooler canopies and increased yield under drought in wheat. Functional Plant Biology 2010, 37, 147-156, doi:https://doi.org/10.1071/FP09121.
- Bengough, A.G.; McKenzie, B.M.; Hallett, P.D.; Valentine, T.A. Root elongation, water stress, and mechanical impedance: a review of limiting stresses and beneficial root tip traits. Journal of Experimental Botany 2011, 62, 59-68, doi:10.1093/jxb/erq350.
- Lucas, M.; Schlüter, S.; Vogel, H.-J.; Vetterlein, D. Roots compact the surrounding soil depending on the structures they en-counter. Scientific Reports 2019, 9, 16236, doi:10.1038/s41598-019-52665-w.
- Han, E.; Kautz, T.; Perkons, U.; Uteau, D.; Peth, S.; Huang, N.; Horn, R.; Köpke, U. Root growth dynamics inside and outside of soil biopores as affected by crop sequence determined with the profile wall method. Biology and Fertility of Soils 2015, 51, 847-856, doi:10.1007/s00374-015-1032-1.
- Reynolds, M.; Ortiz-Monasterio;; A;, M. Application of physiological in wheat breeding; CIMMYT: Mexico,D.F., 2001; Volume 31, pp. 162-171.
- Le Marié, C.A.; York, L.M.; Strigens, A.; Malosetti, M.; Camp, K.-H.; Giuliani, S.; Lynch, J.P.; Hund, A. Shovelomics root traits assessed on the EURoot maize panel are highly heritable across environments but show low genotype-by-nitrogen interaction. Euphytica 2019, 215, 173, doi:10.1007/s10681-019-2472-8.
- Sarker, A.; Erskine, W.; Singh, M. Variation in shoot and root characteristics and their association with drought tolerance in lentil landraces. Genetic Resources and Crop Evolution 2005, 52, 89-97, doi:10.1007/s10722-005-0289-x.
- Futsaether, C.M.; Oxaal, U. A growth chamber for idealized studies of seedling root growth dynamics and structure. Plant and Soil 2002, 246, 221-230, doi:10.1023/a:1020609224525.
- Chen, Y.; Ghanem, M.E.; Siddique, K.H. Characterising root trait variability in chickpea (Cicer arietinum L.) germplasm. Journal of Experiental Botany 2017, 68, 1987-1999, doi:10.1093/jxb/erw368.
- Belachew, K.Y.; Nagel, K.A.; Fiorani, F.; Stoddard, F.L. Diversity in root growth responses to moisture deficit in young faba bean (Vicia faba L.) plants. PeerJ 2018, 6, e4401-e4401, doi:10.7717/peerj.4401.
- Zarebanadkouki, M.; Kim, Y.X.; Carminati, A. Where do roots take up water? Neutron radiography of water flow into the roots of transpiring plants growing in soil. New Phytologist 2013, 199, 1034-1044, doi:10.1111/nph.12330.
- Popova, L.; van Dusschoten, D.; Nagel, K.A.; Fiorani, F.; Mazzolai, B. Plant root tortuosity: an indicator of root path formation in soil with different composition and density. Annals of Botany, 2016, 118, 685-698, doi:10.1093/aob/mcw057.
- Clark, L.J.; Whalley, W.R.; Barraclough, P.B. How do roots penetrate strong soil? Plant and Soil 2003, 255, 93-104.
- Singh, V.; van Oosterom, E.J.; Jordan, D.R.; Messina, C.D.; Cooper, M.; Hammer, G.L. Morphological and architectural de-velopment of root systems in sorghum and maize. Plant and Soil 2010, 333, 287-299, doi:10.1007/s11104-010-0343-0.
- Van der Bom, F.J.T.; Williams, A.; Bell, M.J. Root architecture for improved resource capture: trade-offs in complex envi-ronments. Journal of Experimental Botany 2020, 71, 5752-5763, doi:10.1093/jxb/eraa324.
- Prince, S.J.; Murphy, M.; Mutava, R.N.; Durnell, L.A.; Valliyodan, B.; Shannon, J.G.; Nguyen, H.T. Root xylem plasticity to improve water use and yield in water-stressed soybean. Journal of Experimental Botany 2017, 68, 2027-2036, doi:10.1093/jxb/erw472.
- Richards, R.; Passioura, J. A breeding program to reduce the diameter of the major xylem vessel in the seminal roots of wheat and its effect on grain yield in rain-fed environments. Australian Journal of Agricultural Research 1989, 40, 943-950, doi:https://doi.org/10.1071/AR9890943.
- Richards, R.A.; Passioura, J.B. Seminal Root Morphology and Water Use of Wheat II. Genetic Variation1. Crop Science 1981, 21, cropsci1981.0011183X002100020012x, doi:10.2135/cropsci1981.0011183X002100020012x.
- Benjamin, J.G.; Nielsen, D.C. Water deficit effects on root distribution of soybean, field pea and chickpea. Field Crops Research 2006, 97, 248-253, doi:https://doi.org/10.1016/j.fcr.2005.10.005.
- Kashiwagi, J.; Krishnamurthy, L.; Crouch, J.H.; Serraj, R. Variability of root length density and its contributions to seed yield in chickpea (Cicer arietinum L.) under terminal drought stress. Field Crops Research 2006, 95, 171-181, doi:https://doi.org/10.1016/j.fcr.2005.02.012.
- Manschadi, A.M.; Sauerborn, J.; Stützel, H.; Göbel, W.; Saxena, M.C. Simulation of faba bean (Vicia faba L.) root system de-velopment under Mediterranean conditions. European Journal of Agronomy 1998, 9, 259-272, doi:https://doi.org/10.1016/S1161-0301(98)00044-6.
- Serraj, R.; Krishnamurthy, L.; Kashiwagi, J.; Kumar, J.; Chandra, S.; Crouch, J.H. Variation in root traits of chickpea (Cicer arietinum L.) grown under terminal drought. Field Crops Research 2004, 88, 115-127, doi:https://doi.org/10.1016/j.fcr.2003.12.001.
- Zaman-Allah, M.; Jenkinson, D.M.; Vadez, V. A conservative pattern of water use, rather than deep or profuse rooting, is critical for the terminal drought tolerance of chickpea. Journal of experimental botany 2011, 62, 4239-4252, doi:10.1093/jxb/err139.
- Gorim, L.Y.; Vandenberg, A. Root Traits, Nodulation and Root Distribution in Soil for Five Wild Lentil Species and Lens culinaris (Medik.) Grown under Well-Watered Conditions. Front Plant Sci 2017, 8, 1632-1632, doi:10.3389/fpls.2017.01632.
- Zaman-Allah, M.; Jenkinson, D.M.; Vadez, V. Chickpea genotypes contrasting for seed yield under terminal drought stress in the field differ for traits related to the control of water use. Functional Plant Biology 2011, 38, 270-281, doi:https://doi.org/10.1071/FP10244.
- Gahoonia, T.S.; Ali, O.; Sarker, A.; Nielsen, N.E.; Rahman, M.M. Genetic Variation in Root Traits and Nutrient Acquisition of Lentil Genotypes. Journal of Plant Nutrition 2006, 29, 643-655, doi:10.1080/01904160600564378.
- Gorim, L.Y.; Rabani, E.M.; Barlow, B.A.; de Silva, D.; Vandenberg, A. Are Artificial Media Valid For Root Analysis? A Case Study Comparing Root Traits of Five Lentil Genotypes in Artificial Media versus Soil. In Proceedings of the Journal of Soil Science & Plant Health, 2017.
- Manschadi, A.M.; Christopher, J.T.; Hammer, G.L.; Devoil, P. Experimental and modelling studies of drought‐adaptive root architectural traits in wheat (Triticum aestivum L.). Plant Biosystems - An International Journal Dealing with all Aspects of Plant Biology 2010, 144, 458-462, doi:10.1080/11263501003731805.
- Wang, L.; Guo, M.; Li, Y.; Ruan, W.; Mo, X.; Wu, Z.; Sturrock, C.J.; Yu, H.; Lu, C.; Peng, J.; et al. LARGE ROOT ANGLE1, en-coding OsPIN2, is involved in root system architecture in rice. Journal of Experimental Botany 2017, 69, 385-397, doi:10.1093/jxb/erx427.
- Waite, J.M.; Collum, T.D.; Dardick, C. AtDRO1 is nuclear localized in root tips under native conditions and impacts auxin localization. Plant Molecular Biology 2020, 103, 197-210, doi:10.1007/s11103-020-00984-2.
- Zhan, A.; Lynch, J.P. Reduced frequency of lateral root branching improves N capture from low-N soils in maize. Journal of Experimental Botany 2015, 66, 2055-2065, doi:10.1093/jxb/erv007.
- Liao, H.; Rubio, G.; Yan, X.; Cao, A.; Brown, K.M.; Lynch, J.P. Effect of phosphorus availability on basal root shallowness in common bean. Plant and Soil 2001, 232, 69-79, doi:10.1023/a:1010381919003.
- Miguel, M.A.; Widrig, A.; Vieira, R.F.; Brown, K.M.; Lynch, J.P. Basal root whorl number: a modulator of phosphorus acqui-sition in common bean (Phaseolus vulgaris). Ann Bot 2013, 112, 973-982, doi:10.1093/aob/mct164.
- Burridge, J.; Jochua, C.N.; Bucksch, A.; Lynch, J.P. Legume shovelomics: High—Throughput phenotyping of common bean (Phaseolus vulgaris L.) and cowpea (Vigna unguiculata subsp, unguiculata) root architecture in the field. Field Crops Research 2016, 192, 21-32, doi:https://doi.org/10.1016/j.fcr.2016.04.008.
- Van Duivenbooden, N.; Pala, M.; Studer, C.; Bielders, C.L.; Beukes, D.J. Cropping systems and crop complementarity in dry-land agriculture to increase soil water use efficiency: a review. NJAS - Wageningen Journal of Life Sciences 2000, 48, 213-236, doi:https://doi.org/10.1016/S1573-5214(00)80015-9.
- Varney, G.T.; Canny, M.J. Rates of water uptake into the mature root system of maize plants. New Phytologist 1993, 123, 775-786, doi:10.1111/j.1469-8137.1993.tb03789.x.
- Ahmed, M.A.; Zarebanadkouki, M.; Meunier, F.; Javaux, M.; Kaestner, A.; Carminati, A. Root type matters: measurement of water uptake by seminal, crown, and lateral roots in maize. Journal of Experimental Botany 2018, 69, 1199-1206, doi:10.1093/jxb/erx439.
- Wiecheteck, L.H.; Giarola, N.F.B.; de Lima, R.P.; Tormena, C.A.; Torres, L.C.; de Paula, A.L. Comparing the classical perma-nent wilting point concept of soil (−15,000 hPa) to biological wilting of wheat and barley plants under contrasting soil textures. Agricultural Water Management 2020, 230, 105965, doi:https://doi.org/10.1016/j.agwat.2019.105965.
- Wang, X.; Gan, Y.; Hamel, C.; Lemke, R.; McDonald, C. Water use profiles across the rooting zones of various pulse crops. Field Crops Research 2012, 134, 130-137, doi:https://doi.org/10.1016/j.fcr.2012.06.002.
- Liu, L.; Gan, Y.; Bueckert, R.; Van Rees, K. Rooting systems of oilseed and pulse crops I: Temporal growth patterns across the plant developmental periods. Field Crops Research 2011, 122, 256-263, doi:https://doi.org/10.1016/j.fcr.2011.04.002.
- Rawson, H.M.; Macpherson, H.G. Irrigated Wheat; FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NA-TIONS: http://www.fao.org/3/x8234e/x8234e00.htm#Contents, 2000.
- Palta, J.A.; Watt, M. Vigorous crop root systems: form and function for improving the capture of water and nutrients. . In In: Crop Physiology. Applications for Genetic Improvement and Agronomy, D.Calderini, V.S.a., Ed.; Elsevier: 2009; pp. 309-325.
- Li, P.; Ma, B.; Palta, J.A.; Ding, T.; Cheng, Z.; Lv, G.; Xiong, Y. Wheat breeding highlights drought tolerance while ignores the advantages of drought avoidance: A meta-analysis. European Journal of Agronomy 2021, 122, 126196, doi:https://doi.org/10.1016/j.eja.2020.126196.
- Siddiqui, M.N.; Léon, J.; Naz, A.A.; Ballvora, A. Genetics and genomics of root system variation in adaptation to drought stress in cereal crops. Journal of Experimental Botany 2020, 72, 1007-1019, doi:10.1093/jxb/eraa487.
