Metformin is the first-line pharmacotherapy for treating type 2 diabetes mellitus (T2DM); however, its mechanism of modulating glucose metabolism is elusive. Recent advances have identified the gut as a potential target of metformin. As patients with metabolic disorders exhibit dysbiosis, the gut microbiome has garnered interest as a potential target for metabolic disease. Henceforth, studies have focused on unraveling the relationship of metabolic disorders with the human gut microbiome.
- gut microbiome
- type 2 diabetes mellitus
- metformin
- dysbiosis
Note: The following contents are extract from your paper. The entry will be online only after author check and submit it.
1. Introduction
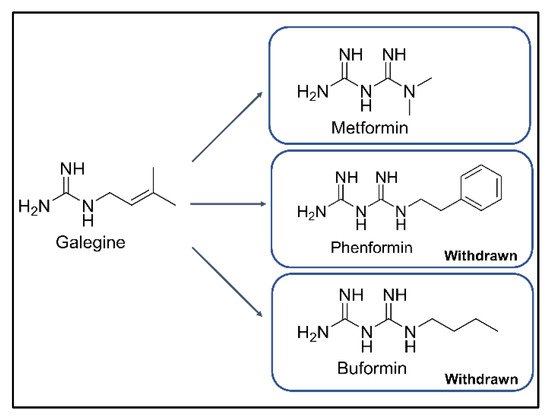
2. Gut Microbiome and T2DM
| Ref * | Population | Study Design | Gut Microbiota | Biochemical Alterations |
|---|---|---|---|---|
| [27] | Chinese | T2DM patients (n = 170) |
versus Healthy subjects (n = 174) Family: Lachnospiraceae ↑, Erysipelotrichaceae ↓ Genus: Alistipes ↑, Clostridium ↑, Eubacterium ↓, Faecalibacterium ↓, Subdoligranulum ↑, Parabacteroides ↑ Species: Akkermansia muciniphila ↑, Bacteroides intestinalis ↑, Clostridium bolteae ↑, Clostridium hatheway ↑, Clostridium ramosum ↑, Clostridium symbiosum ↑, Eggerthella lenta ↑, Escherichia coli ↑, Eubacterium rectale ↓, Faecalibacterium prausnitzii ↓, Haemophilus parainfluenzae ↓, Roseburia intestinalis ↓, Roseburia inulinivorans ↓ |
NA |
| [30] | Danish | T2DM patients (n = 18) |
versus Healthy subjects (n = 18) α-diversity (Chao 1): – Phylum: Bacteroidetes ↑, Firmicutes ↓, Proteobacteria ↑ Class: Bacilli ↑, Bacteroidetes ↑, Betaproteobacteria ↑, Clostridia ↓ Genus: Akkermansia ↑, Alistipes ↑, Bacteroides ↓, Bifidobacterium ↓, Bilophila ↑, Catenibacterium ↓, Dialister ↑, Dorea ↑, Erysipelotrichaceae IS ↑, Faecalibacterium ↓, Lachnospiraceae IS ↓, Lactobacillus ↑, Parabacteroides ↑, Prevotella ↑, Roseburia ↓, Ruminococcus ↓, Sporobacter ↑, Subdoligranulum ↓, Succinivibrio ↑, Sutterella ↑ Species: Dorea longicatena ↓ |
NA |
| [32] | Chinese | T2DM patients (n = 13) |
versus Healthy subjects (n = 44) α-diversity (Chao 1, Shannon index): ↓ Class: Clostridia ↑, Clostridiales ↑ Family: Lachnospiraceae ↑ Genus: Abiotrophia ↑, Bacteroides ↓, Collinsella ↑, Dorea ↑, Eubacterium ↑, Haemophilus ↓, Megamonas ↓, Peptostreptococcus ↑, Prevotella ↑, Roseburia ↓, Ruminococcus ↑, Sporobacter ↑, Subdoligranulum ↑ |
NA |
| [34] | Pakistani | Obese-T2DM patients (n = 40) |
versus Healthy subjects (n = 20) α-diversity (Shannon index): ↓ Phylum: Bacteroidetes ↓, Elusimicrobia ↓, Firmicutes ↓, Proteobacteria ↓, Verrucomicrobioa ↓ Class: Bacilli ↓, Bacteroidia ↓, Clostridia ↑, Coriobacteriia ↑, Deltaproteobacteria ↓, Elusimicrobia ↓, Gammaproteobacteria ↓, Negativicutes ↑, Genus: Allisonella ↑, Bacillus ↓, Christensenellaceae_R_7 ↑, Dialister ↑, Escherichia_Shigella ↓, Eubacterium coprostanoligenes groups ↑, Lactobacillus ↑, Prevotella_9 ↓, Ruminococcus_2 ↓, Subdoligranulum ↑ |
NA |
| Metformin Treatment Effects in T2DM Patients | ||||
| [28] | Japanese | T2DM patients (n = 50) |
versus normal subjects (n = 50) Genus: Atopobium cluster ↓, Lactobacillus ↑, Prevotella ↓ Species: Clostridium coccoides ↓, Lactobacillus plantarum ↑, Lactobacillus reuteri ↑ |
Fecal organic acids ↓ Acetic acid ↓ Propionic acid ↓ Fecal isovaleric acid ↑ CRP ↑, IL-6 ↑ |
| Metformin treated T2DM (n = 17) | versus non treated T2DM (n = 33) Family: Enterobacteriaceae ↑ Genus: Staphylococcus ↑ Species: Clostridium coccoides ↓, Lactobacillus plantarum ↑, Lactobacillus reuteri ↑ |
NA | ||
| [29] | European old woman |
T2DM patients (n = 53) |
versus normal glucose tolerance (n = 43) Class: Clostridiales ↓ Family: Coriobacteriaceae ↓ Genus: Alistipes ↓, Clostridium ↓, Roseburia ↓, Species: Bacteroides intestinalis ↓, Eubacterium eligens ↓, Lactobacillus gasseri ↑, Streptococcus mutans ↑ |
C-peptide ↑ |
| Metformin treated T2DM (n = 20) |
versus non treated T2DM (n = 33) Genus: Clostridium ↓, Escherichia ↑, Eubacterium ↓, Klebsiella ↑, Salmonella ↑, Shigella ↑ Species: Escherichia coli ↑ |
NA | ||
| [31] | Danish | T2DM patients (n = 75) |
versus normal subjects (n = 277) Family: bp Clostridiales ↓, Peptostreptococcaceae ↓ Genus: Akkermansia ↓, Acidaminococcus ↑, Bilophila ↑, Collinsella ↑, Coprococcus ↓, Escherichia ↑, Holdemania ↑, Lactobacillus ↑, Parabacteroides ↑, Roseburia ↓, Veillonella ↓ |
NA |
| Metformin treated T2DM (n = 58) |
versus non treated T2DM (n = 17) Family: Peptostreptococcaceae ↓ Genus: Akkermansia ↑, Bilophila ↓, Escherichia ↑, Holdemania ↑, Roseburia ↑, Veillonella ↓ |
NA | ||
| Swedish female |
T2DM patients (n = 53) |
versus normal subjects (n = 92) Family: Peptostreptococcaceae ↓ Genus: Lactobacillus ↑ |
NA | |
| Metformin treated T2DM (n = 20) |
versus non treated T2DM (n = 33) Family: bp Clostridiales ↓, Peptostreptococcaceae ↓ Genus: Bilophila ↑, Escherichia ↑, Holdemania ↓, Lactobacillus ↑, Roseburia ↓, Veillonella ↓ |
NA | ||
| Chinese | T2DM patients (n = 71) |
versus normal subjects (n = 185) Family: bp Clostridiales ↓, Peptostreptococcaceae ↓, Genus: Acidaminococcus ↑, Bilophila ↑, Collinsella ↑, Coprococcus ↓, Escherichia ↑, Haemophilus ↓, Holdemania ↑, Lactobacillus ↑, Oscillibacter ↑, Roseburia ↓, Veillonella ↓ |
NA | |
| Metformin treated T2DM (n = 15) | versus non treated T2DM (n = 56) Family: bp Clostridiales ↑, Peptostreptococcaceae ↓ Genus: Bilophila ↑, Collinsella ↑, Escherichia ↓, Holdemania ↑, Parabacteroides ↑, Roseburia ↑, Subdoligranulum ↑, Veillonella ↓ |
NA | ||
| [33] | Chinese | T2DM patients (n = 26) |
versus normal subjects (n = 50) α-diversity (Shannon index): ↓ Phylum: Firmicutes ↓ Class: Fusobacteriia ↑ Family: Enterobacteriaceae ↓, Erysipelotrichaceae ↑, Erysipelotrichaceae ↑, Porphyromonadaceae ↑ Genus: Faecalibacterium ↓, Fusobacterium ↑, Lactobacillus ↑, Ruminococcus ↓ |
NA |
| Metformin treated T2DM (n = 51) |
versus non treated T2DM (n = 26) α-diversity (Shannon index): – Phylum: Actinobacteria ↓ Family: Enterobacteriaceae ↓, Spirochaetaceae ↑, Turicibacteraceae ↑ Genus: Fusobacterium ↑, Turicibacter ↑ |
NA | ||
| [55] | British | On metformin T2DM (visit 1 and 4, n = 12) |
versus off metformin T2DM (visit 2 and 3, n = 12) Genus: SMB53 ↓, Adlercreutzia ↓, Eubacterium ↑ |
Serum bile acids ↓ Fecal bile acids ↑ GLP-1 ↑ |
| [56] | Spanish | Metformin treated T2DM for 4 months (n = 22) | versus before metformin treatment in T2DM (n = 22) Phylum: Proteobacteria ↑, Firmicutes ↑ Genus: Actinetobacter ↑, Alkaliphilus ↓, Citrobacter ↑, Cronobacter ↑, Dermcoccus ↑, Desulfurispirillum ↑, Dickeya ↑, Edwardsiella ↑, Enterobacter ↑, Erwinia ↑, Escherichia ↑, Holdemania ↓, Intestinibacter ↓, Klebsiella ↓, Methylobaciilus ↑, Pantoea ↑, Pectobacterium ↑, Photorhabdus ↑, Providencia ↑, Pseudomonas ↑, Rahnella ↑, Rheinheimera ↑, Salmonella ↑, Subdoligranulum ↓, Xanthomonas ↑, Xenohabdus ↑, Yersinia ↑ Species: Akkermansia muciniphila ↑, Bifidobacterium adolescentis ↑ |
Fecal propionate, butyrate, lactate and succinate ↑ Plasma bile acids ↑ |
| [57] | Colombian | T2DM patients (n = 28) |
versus normal subjects (n = 84) Genus: Enterococcus casseliflavus ↓, Clostridiaceae 02d06 ↑, Prevotella ↑ |
NA |
| Metformin treated T2DM (n = 14) |
versus non treated T2DM (n = 14) Genus: Bacnesiellaceae ↓, Butyrivibrio ↑, Clostridiaceae 02d06 ↓, Megasphaera ↑, Oscillospira ↓, Prevotella ↑ |
NA | ||
| [58] | Scandinavian | Metformin treated T2DM (n = 23) |
versus non treated T2DM (n = 7) Family: Enterobacteriaceae ↑, Genus: Bacnesiellaceae ↓, Butyrivibrio ↑, Clostridiaceae 02d06 ↓, Megasphaera ↑, Oscillospira ↓, Prevotella ↑ |
SCFA concentration – |
| [59] | Chinese | Metformin treated for 3 days in T2DM (n = 22) | versus before metformin treatment in T2DM (n = 22) Genus: Bacteroides ↓ Species: Bacteroides fragilis ↓, Bacteroides finegoldii ↓, Bacteroides thetaiotaomicron ↓, Bacteroides uniformis ↓, Bacteroides ovatus ↓, Bacteroides intestinalis ↓, Bacteroides stercoris ↓, Bacteroides eggerthii ↓, Bacteroides fluxus ↓, Bacteroides caccae ↓, Bacteroides dorei ↓ |
GUDCA, Tauroursodeoxycholic acid, Conjugated Secondary bile acids ↑ Total bile acids – |
| Metformin Treatment Effects in Healthy Subjects | ||||
| [60] | Caucasian | Metformin treated for 7 days in healthy subjects (n = 18) |
versus before metformin treatment in healthy subjects (n = 18) α-diversity (Shannon index): ↓ Class: Bacilli ↑, Enterobacteriales ↑, Episilonproteobacteria ↑, Gammaproteobacteria ↑, Negativicutes ↓ Order: Clostridiaceae_1 ↓, Lactobacillales ↑, Peptostreptococcaceae ↓, Selenomonadales ↓ Family: Asaccharospora ↓, Enterobacteriaceae ↑, Romboutsia ↓ Genus: Blautia ↑, Ruminiclostridium_6 ↓, Streptococcus ↑ |
NA |
| [61] | Danish | Metformin treated for 6 weeks in healthy subjects (n = 22) |
versus before metformin treatment in healthy subjects (n = 18) Genus: Bilophila ↑, Caproiciproducens ↑, Clostridium_sensu_stricto_1 ↓, Escherichia-Shigella ↑, Intestinibacter ↓, Prevotella ↑, Terrisporobacter ↓ Species: Alistipes finegoldii ↑, Bilophila wadsworthia ↑, Intestinibacter bartlettii ↓ |
NA |
3. Potential Mechanisms of Metformin on the Gut Microbiome
3.1. Regulation of Glucose Homeostasis
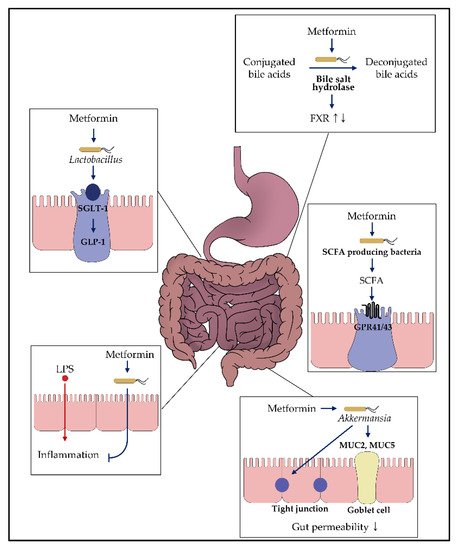
3.2. Effects on Bacteria Producing Short-Chain Fatty Acid
3.3. Enhancement of the Gut Permeability
| Ref * | Animal | Study Design | Gut Microbiota | Biochemical Alterations |
|---|---|---|---|---|
| [76] | Rats | Metformin treatment in high-fat diet | versus without metformin treatment in high fat diet Family: Lactobacillaceae ↑ Genus: Achromobacter –, Acinetobacter –, Azorhiziphilus –, Enterococcus –, Escherichia –, Klebsiella –, tobacillus ↑, Sarcina –, Stenotrophomnas – |
NA |
| [88] | Mice | Metformin treatment in high-fat diet | versus without metformin treatment in high fat diet α-diversity (Shannon): ↓ Phylum: Bacteroidetes ↑, Verrucomicrobia ↑ Family: Bacteroidaceae ↑, Clostridiales familyXIII ↑, Incertae sedis ↑, Rikenellaceae ↑, Ruminococcaceae ↑, Verrucomicrobioaceae ↑ Species: Akkermansia muciniphila ↑, Clostridium cocleatum ↑ |
Inflammation scores – |
| Metformin treatment in normal diet | versus without metformin treatment in normal diet α-diversity (Shannon): – Phylum: Bacteroidetes – Family: Rikenellaceae ↑, Ruminococcaceae ↑, Verrucomicrobioaceae ↑ Genus: Alistipes spp. ↑, Akkermansia spp. ↑, Clostridium spp. ↑ |
NA | ||
| [89] | Mice | Metformin treatment for 16 weeks in high-fat diet | versus without metformin treatment in high fat diet α-diversity (observed OTU): – Phylum: Bacteroidetes ↑, Firmicutes ↓, Verrucomicrobia ↑ Genus: Akkermansia ↑, Bacteroides ↑, Butyricimonas ↑, Parabacteroides ↑ |
IL-6 mRNA ↓ IL-1β mRNA ↓ |
| [90] | Mice | Metformin treatment for 24 weeks in high-fat diet | versus without metformin treatment in high fat diet α-diversity (Shannon): ↓ Phylum: Bacteroidetes ↑, Firmicutes ↓, Verrucomicrobia ↑ Family: Desulfovibrionaceae Genus: Akkermansia ↑, Bacteroides ↑, Christensenella ↑, Coprococcus↓, Dorea ↓, Lachnoclostridium ↓, Parabacteroides ↑, Papillibacter ↓, Oscillospira ↓, Ruminococcus ↓, Desulfovibrio ↓, Muribaculum ↓ |
Plasma threonine ↓, methionine sulfoxide ↓, Tetradecanoylcarnitine ↓, Hexadecenoylcarnitine ↓ |
| [91] | Mice | Metformin treatment in obese mice (db/db mice) | versus without metformin treatment in obese mice (db/db mice) α-diversity (Shannon): ↑ Genus: Akkermansia ↑, Butyricimonas ↑, Clostridium ↓, Coprococcus ↑, Dehalobacterium ↑, Dorea ↑, Lactobacillus ↑, Oscillospira ↑, Parabacteroides ↓, Paraprevotella ↑, Prevotella ↓, Proteus ↓, Ruminococcus ↑ |
Total SCFA concentration in feces ↑ Acetic acid ↑, Butyric acid ↑ LPS levels ↓ |
| [92] | Mice | Metformin treatment in high-fat diet | versus without metformin treatment in high fat diet α-diversity (Shannon, evenness): – Phylum: Bacteroidetes ↑ Family: Coriobacteriaceae ↓, Ruminococcaceae ↑, S24_7 ↑, Veilonellaceae ↓ Genus: Dorea ↓, Dehalobacterium ↓, Lactobacillus ↓, Lactococcus ↑, Roseburia ↓, SMB53 ↓ |
IL-6 ↓, IL-1β ↓, TNF α ↓ Taurine ↑, Butyrate ↑, Total Bile acids ↑, Propionate ↑, Leucine ↑, Creatinine ↓, Sarcosine ↓, Glutamate ↓, Pyruvate ↓, Formate ↓ |
| [93] | Rats | Metformin treatment in high-fat diet combined with a low dose streptozocin | versus without metformin treatment in high fat diet α-diversity (Simpson, Shannon): ↑ Class: Coriobacteriia ↑ Family: S24_7 ↑ |
Total SCFAs ↑, Butyric acid ↑, Isovaleric acid ↑ |
| [94] | Rats | Metformin treatment in high-fat diet combined with a low dose streptozocin | versus without metformin treatment in high fat diet α-diversity (Chao1): ↑ Family: S24_7 ↓ Genus: Anaerotruncus ↑, Escherichia-Shiegella ↓, Eubacterium xylanophilum ↑, Lachnospiraceae NK4A136 ↑, Lachnospiraceae-UCG_006 ↑, Roseburia ↑ |
Serum LPS ↓, Serum CRP↓, Serum TNF α ↓, Serum IL-6 ↓ Propionate in cecum ↑, Butyrate in cecum ↑ |
| [99] | Mice (female) |
Metformin treatment in fat, fructose and cholesterol rich diet |
versus without metformin treatment in fructose and cholesterol rich diet Family: Alloprevotella ↓ Genus: Bacteroides –, Romboutsia ↓ Species: Akkermansia muciniphila –, Lactobacillus animalis ↓ |
TNF α ↓ Endotoxin ↓ |
| [109] | Mice | Metformin treatment for 5 weeks in high-fat diet combined with a low dose streptozocin |
versus without metformin treatment in high fat diet α-diversity (observed OTU): – Genus: Akkermansia ↑, Bacteroides spp. ↓ |
NA |
| [110] | Mice | Metformin treatment in high-fat diet | versus without metformin treatment in high fat diet Phylum: Verrucomicrobia ↑, Genus: Akkermansia ↑, Alistipes ↑, Anaerotruncus ↓, Blautia ↓, Lactococcus ↓, Lactonifactor ↓, Lawsonia ↓, Odoribacter ↓, Parabacteroides ↓ |
IL-6 mRNA ↓ IL-1β mRNA ↓ |
| [121] | Mice | Metformin treatment for 30 days |
versus without metformin treatment in healthy mice α-diversity (Shannon): – Class: Lachnopiraceae ↓, Porphyromonadaceae ↑, Prevoltellaceae ↑, Rhodobacteraceae ↓, Rikenellaceae ↑, Verrucomicrobiaceae ↑ |
NA |
| [123] | Rats | Metformin treatment in high-fat diet |
versus without metformin treatment in high fat diet α-diversity (Shannon): ↓ Phylum: Bacteroidetes –, Firmicutes –, Proteobacteria ↑ Species: Akkermansia ↑, Allobaculum ↑, Bacteroides ↑, Blautia ↑, Butyricoccus ↑, Clostridium ↓, Klebsiella ↑, Lactobacillus ↑, Parasutterella ↑, Phascolarctobacterium ↑, Prevotella ↑, Roseburia ↓ |
NA |
| [124] | Rats | Metformin treatment in high-fat diet combined with a low dose streptozocin |
versus without metformin treatment in high fat diet α-diversity (Chao1, Shannon): ↑ Phylum: Bacteroidetes ↑, Firmicutes ↓, Proteobacteria ↓ Order: Clostridiales ↑, Enterobacteriales ↓, Lactobacillales ↑ Genus: Akkermansia ↑, Desulfovibrio ↓, Lachnospiraceae NK4A136 ↓, Lactobacillus ↑, Roseburia ↑ |
NA |
| [125] | Mice | Metformin treatment for 3 weeks in high-fat diet | versus without metformin treatment in high fat diet α-diversity (Shannon, evenness): – Genus: Akkermansia ↑, Allobaculum ↓, Clostridium ↓, Enterococcus ↓, Lactococcus ↓, Leuconostoc ↓, Oscillospira ↑, Parabacteroides ↑, Prevotella ↑, Ruminococcus ↓, Streptococcus ↓ |
NA |
| [126] | Mice with | Metformin treatment for 5 weeks in high-fat diet combined with a low dose streptozocin |
versus without metformin treatment in high fat diet α-diversity (Chao1): ↓ Phylum: Bacteroidetes ↓, Firmicutes ↑, Proteobacteria ↓ Genus: Lactobacillus ↑ |
NA |
| [127] | Mice | Metformin treatment in high-fat diet |
versus without metformin treatment in high fat diet α-diversity (Shannon, evenness): – Species: Bacteriodetes fragilis ↓, Escherichia coli ↓ |
Serum endotoxin ↓ IL-6 ↓, TLR4 ↓ |
| [128] | Rats | Metformin treatment in high-fat diet combined with a low dose streptozocin |
versus without metformin treatment in high fat diet α-diversity (Chao1): ↑ Phylum: Bacteroidetes ↑, Proteobacteria ↓, Verrucomicrobia ↓ Family: Alcaligenaceae ↑, Peptococcaceae ↑, Prevotellaceae ↑, S24_7 ↑ Genus: Prevotella ↑, Sutterella ↑, 02d06 ↑, rc4 ↑ |
IL-6 mRNA in pancrease ↓, TNF α mRNA in pancrease ↓, LPS ↓ |
| [129] | Rats | Metformin treatment in high-fat diet combined with a low dose streptozocin |
versus without metformin treatment in high fat diet Genus: Bifidobacterium ↑, Lactobacillus ↑ Species: Clostridium perfringens ↓, Escherichia coli ↓ |
Plasma endotoxin ↓, Total SCFAs in cecum ↑, Lactic acid in cecum ↑, Acetic acid in cecum ↑ |
| [130] | Rats | Metformin treatment in Otsuka Long-Evans Tokushima Fatty (OLETF) rats |
versus without metformin treatment Genus: Akkermansia ↑, Prevotella ↓, Roseburia ↑ Species: Escherichia coli ↓ |
Serum endotoxin ↓, Fecal endotoxin ↓, serum TNF α ↓, serum IL-6 ↓ |
| [131] | Rats | Metformin treatment in Zucker diabetic fatty rats | versus without metformin treatment α-diversity (Shannon): – Phylum: Bacteroidetes –, Firmicutes –↑, Proteobacteria ↓, Tenericutes –, Verrucomicrobia ↑ Genus: Lactobacillus ↑ Species: Lactobacillus intestinalis ↑, Lactobacillus johnsonii ↑ |
NA |
3.4. Modulation of the Immune Response
3.5. Actions on the Circulation of the Bile Acids
4. Relationships between Metformin and Gut Microbiome in Human Studies
| Clinical Trials.gov Identifier | Study Title | Country | Study Population | Interventions |
|---|---|---|---|---|
| NCT04194515 | Gut Microbiota and Bile Acids in Type 2 Diabetes Mellitus | Taiwan | Outpatients and treatment-naïve male patients with type 2 diabetes |
Drug: YH1 Drug: metformin |
| NCT04287387 | Response of Gut Microbiota in Type 2 Diabetes to Hypoglycemic Agents | China | Type 2 diabetes patients (18–65 years) |
Drug: Glucophage 500 mg Tablet Drug: Acarbose Tablets Drug: Sitagliptin tablet Drug: Dapagliflozin Tablet Drug: Pioglitazone Tablets Drug: Glimepiride Tablets |
| NCT04639492 | Postbiotic MBS and Metformin Combination in Patients With T2DM |
Taiwan | Type 2 diabetes patients (20–70 years) |
Dietary Supplement: MBS oral solution Oral BIDAC, twice a day before breakfast and dinner times |
| NCT02960659 | Title: Therapeutic Targets in African-American Youth With Type 2 Diabetes |
United States | African-American (12–25 years) |
Drug: Metformin and Liraglutide Drug: Metformin |
| NCT03558867 | Personalized Medicine in Pre-diabetes and Early Type 2 Diabetes |
Australia | Pre-diabetes or newly-diagnosed with type 2 diabetes (in the last 6 months) |
Drug: Metformin + Healthy diet Drug: Metformin + Personalized diet |
| NCT03732690 | The Interaction Between Protein Intake, Gut Microbiota and Type 2 Diabetes in Subjects With Different Ethnic Backgrounds |
France | T2DM patients: Caucasian (n = 80), Caribbean (n = 40) stable dose of metformin and do not use insulin or proton-pump inhibitors. |
Other: Diet HP Other: Diet LP |
| NCT04089280 | Probiotics in Metformin Intolerant Patients With Type 2 Diabetes | Poland | T2DM patients (18–75 years) with metformin treatment in the last 3 months (<1500 mg/d) | Dietary Supplement: Sanprobi Barrier-multispecies probiotic Other: Placebo Comparator |
| NCT03718715 | The Interaction Between Metformin and Microbiota—The MEMO Study. (MEMO) |
Sweden | Newly diagnosed patients with type 2 diabetes without previous treatment with metformin (40–80 years). |
Drug: Metformin |
| NCT03489317 | Gut Microbiomes in Patients With Metabolic Syndrome |
Hongkong | Residents in Hongkong (no metabolic syndrome, metabolic syndrome-partial, metabolic syndrome-full) | Drug: Metformin Behavioral: lifestyle modification Drug: Simvastatin 10 mg Drug: Amlodipine 5 mg |
| NCT02609815 | Initial Combination of Gemigliptin and Metformin on Microbiota Change |
Republic of Korea | Type 2 patients with drug naive for 6 weeks | Drug: gemigliptin/metformin Drug: glimepiride/metformin |
| NCT04341571 | Effect of Probiotics Versus Metformin on Glycemic Control, Insulin Sensitivity and Insulin Secretion in Prediabetes. |
Mexico | Pre-diabetes | Dietary Supplement: Probiotics Drug: Metformin |
| NCT04209075 | Prebiotics and Metformin Improve Gut and Hormones in Type 2 Diabetes in Youth (MIGHTY-fiber) | United States | Type 2 patients (10–25 years) | Dietary Supplement: Biomebliss Drug: Metformin Dietary Supplement: Placebo |
This entry is adapted from the peer-reviewed paper 10.3390/ijms22073566
