Digital twins in the field of tissue culture include the mechanistic of the biological system in the form of diverse mathematical models, which describe the interaction between tissue culture techniques and cell growth, metabolism, and the quality of the tissue.
- digital twin
- tissue engineering
- 3D culture
- organ-on-a-chip
- mathematical model
1. Digital Twins: Concept and Advantages for Tissue Cultures
Please notice that this definition focus on the digital twin concept in the field of tissue culture techniques/tissue engineering solely.
Historically, a DT was defined as a computational entity of a machine (e.g., airplane, spacecraft, car manufacturing) used as an engineering handle to deal with the increased complexity of these machines (reviewed in [1][2]). Universally applied definitions are still scarce and not sufficient, since DTs are differently defined in industry sectors. Barricelli et al. (2019) investigated the evolvement of the DT concept in literature and defined them rather broad:
“DTs can be defined as (physical and/or virtual) machines or computer-based models that are simulating, emulating, mirroring, or “twinning” the life of a physical entity, which may be an object, a process, a human, or a human-related feature.” [1].
Most of the recent efforts were made in the field of mechanical engineering and aircraft design, since the object of investigation is well known and based on physical laws (e.g., material specifications, mechanical properties). Moreover, modern design processes of machines and manufacturing sides are made using computer-aided design software, which can be the basis for a DT in the respective field. A major overcame obstacle to implement DTs was the continuous increase in computational power, enabling Big Data methods, such as Artificial Intelligence (AI) or data mining techniques.
In the past years, the DT concept is more and more discussed in medical-related fields, such as the design and optimization of manufacturing processes for biopharmaceuticals, the development of personalized medicine, and for the characterization, design, and optimizing of 3D tissue culture systems[3][4][5][6][7]. Tissue culture refers to a micro-structured cell culture under in vitro conditions that take into account the three-dimensionality (3D) and the physiology of the tissue. Areas of application cover basic research (e.g. stem cell research, tumor models), pharmacology and toxicity tests, regenerative medicine (tissue engineering for implantation), as well as in vitro meat, among others. With respect to pharmacology and toxicity test, tissue culture are regarded as more suitable than using laboratory animals, even if the interactions between the organs in an organism are not considered. There are indications that organ-like behavior of the cell culture leads to more meaningful results when the mode of action of a new drug is to be tested and conclusions about its pharmacokinetics and pharmacodynamics have to be drawn[8][9]. Additionally, there are various 3D organ models for the respective entry point and place of action of the substance, for example for the skin, liver, pancreas, or the lungs[10]. Meanwhile, it is common sense that proper development, utilization, and data interpretation in the above-mentioned applications requires in silico mathematical modelling to address the tremendous biological and technical complexity. A transient from a technology-driven science-focused field toward a patient-driven, manufacturing-focused one can be seen, driven by innovations at the interface between biology and technology, including robust biological building blocks, precise biomanufacturing technologies, advanced analytical tools, and in silico models [3].
The main challenge in tissue culture is the translation of biological knowledge on complex cell and tissue behavior into a predictive and robust engineering process including proper analytical tools and predictive control methods. This involves the quantification and optimizing of tissue engineering production processes and the investigation of the influence of the cultivation environment/environmental conditions in vitro on the quality of the cultivated tissue, especially an increasing understanding of the pathophysiology, design treatment strategies, and an appropriate quality control. [11] In the author’s opinion, a DT-based framework is seen as a knowledge-based process development and engineering strategy, for which the main advantages are:
- A comprehensive summary of knowledge of the investigated experimental system into a computational system, with the linkage of physical laws and metabolic understanding to design and evaluate culture systems, also at different scales. This increases the level of knowledge about the process steadily through reiteration processes.
- Increasing understanding of the process and its influence on cell growth, phenotyping, epigenetic criteria, prognostic markers, etc., in many process steps.
- Usage of next-generation computational tools to improve the understanding of the cultivation concepts, e.g., optimizers, learning algorithms.
- Decrease of the development cost for experimental design to define fast and efficient cell expansion, resulting in accelerated time to clinic.
- Support of regulatory documentation within serial/routine manufacturing to treat individual patients with customized medications.
- Evolving of the DT during the lifecycle of the medical product, including the prediction of defects and capturing platform knowledge and transferability along the product life cycle with donor-specific manufacturing processes.
- Evaluation, screening, and virtual testing of new configurations/settings prior to experiments.
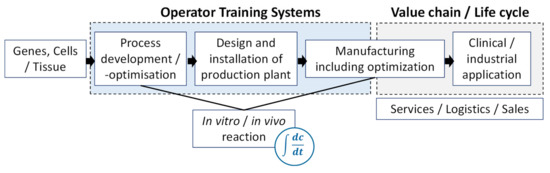
It should be noticed that the benefits of the application of DTs heavily rely on their intended use and that the above-described points are only valid for the design, characterization, and operation of the culture system and the respective cultivation process. As can be seen in Figure 2, potential areas of the application of DTs can be found over the whole lifecycle of tissue engineered products.
Figure 2. Potential areas for Digital Twins (DTs) in tissue culture.
Starting with the genes, cells, or the tissue, the expansion process is developed and optimized, and the manufacturing plant is designed, qualified, and installed accordingly. Then, the process in the respective scale needs to be optimized and scale-up needs to be validated[12][13]. The DTs act as an engineering tool for process design and optimization and to design special culture systems considering e.g., mass transfer and shear effects. Furthermore, tailored materials for cell immobilization considering mass transfer problems could be focused on (e.g., biodegradability, oxygen supply). To address such tasks, the DT should be able to describe all or parts of the mechanical integrity including the microstructure and the biostability and biochemical activity of the cultured tissue. Furthermore, different cellular properties, like cell differentiation, proliferation, metabolism, and cell and surface interactions might be considered by the DT.
Within these steps, an operator training system (OTS) including a DT and capturing the main in vivo and in vitro reactions is beneficial (see list of advantages above)[14][15]. OTSs offer the possibility to train future reactor operators and bioprocess engineers in a very practical way without carrying out the real process. Furthermore, they offer enormous potential for the development of control strategies. After manufacturing of the tissue culture, a DT can assist in the value chain to support the delivery and sales up to the clinical or industrial application[16][17].
2. Efforts toward “Digital Twin” Concepts for Tissue Culture
As summarized in Table 1, numerous studies have been performed using mathematical models for tissue models, especially CFD simulations. It would be beyond the scope of this review to discuss all this work in detail. The intention of the following section is to show that the complexity of the transition from modelling to DT is fluent and includes an increasing complexity. Therefore, examples were selected, which show on the one hand the potential, and on the other hand, the still-existing limitations of model assisted/DT-concepts for 3D tissue culture.
5.1. Expansion of Stem and Immune Cells
A decisive step for the therapeutic use of stem and immune cells is the increase in the number of cells. In some cases, expansion factors in the range of 500–1000 are required here without affecting the differentiation behavior of the cells [18]. As already mentioned in Section 2.2, a widely applied method for the expansion of these mostly adherent cells is cultivation on microcarriers in suspension reactors, e.g., stirred reactors. However, pilot and industrial-scale reactors (e.g., starting from 2 L up to a few m3) are characterized by very heterogeneous flow conditions and concentration environmental gradients, such as inhomogeneous distributions of dissolved oxygen or pH [19][20][21][22]. There is strong evidence that the growth and differentiation of tissue cells, especially stem cells, are affected by several types of mechanical forces including stretch, strain, compression, and shear stress [23]. Stem cells react to flow effects, mainly caused by shear stress, not only through changes in growth behavior up to cell death, but also, among other, by a shear induced differentiation. Flow-induced shear stress can be both stimulating and harmful to the cells. A large number of studies have dealt with the question of how a targeted “lineage”-specific differentiation of stem cells can be induced [24][25][26][27]. For the expansion of stem cells, an undesired “pre-differentiation” during cultivation is to be regarded as disadvantageous. This requires the least possible variation of the lineage-specific differentiation, while the genetic and epigenetic stability of the cells must be kept under control [28]. To ensure this, the micro-environment that affects the cells must be characterized in a bioreactor and kept as similar as possible during the “up-scaling” of the process [29][30].
In the complex, mostly turbulent environment e.g., in a stirred bioreactor, the local shear rate varies within the vessel and it therefore is very difficult to associate cellular effects (cell damage, differentiation, etc.) with the magnitude of the prevailing shear rate or the associated shear stress.
Flow-induced shear stress can be detrimental or stimulating to the behavior of tissue cells. Cell damage in microcarrier cultures was studied very intensively for permanent or established cell lines in the 1980s and 1990s. Especially for stem cells, it was shown that these cells are affected by shear stress below damaging levels. With respect to cell expansion, preferably without significant unwanted differentiation, a narrow band of shear stress levels is essential. Turbulent flow in stirred bioreactors, however, represents a scenario that is not characterized by a narrow band of shear stress levels. The effect of broadband stresses associated with the turbulence in stirred bioreactors on preservation of the physiological state of stem cells is still poorly understood. Although these can be easily described using modern CFD methods, a reliable link between flow-related data and cellular effects is still missing, especially a detailed examination of the micro-environment affecting the cells (flow, oxygen concentration, etc.) (reviewed by [23]).
A further severe problem with the expansion of stem cells on microcarriers can be seen in the formation of aggregates that can be several millimeters in size (Note: Diameter of the microcarrier is approximately 200 µm) [31]. It is obvious that cells in these aggregates are only very poorly supplied mainly with oxygen, so that, in extreme cases, necrotic zones develop with massive cell death. In addition, the influence of large oxygen gradients on the physiological state of the cells has to be considered [32]. Oxygen limitation in stem cell aggregates in stirred suspension cultures is described by Wu et al. [33] using a time-dependent reaction-diffusion model that was linked to a “population balance” model. Again, a link to cellular effects is still missing.
5.2. Bioreactors for Liver Tissue Engineering
Using the example of bioreactors intended as bioartificial liver to develop enhanced bioartificial liver support systems to study liver cell metabolism or as an alternative to animal tests for drug screening or toxicity assessment in vitro [34][35][36], it can be shown how mathematical modelling can help to improve understanding, design, and operation of the system. The ideal biological liver substitute should perform most or all of the liver-specific detoxification, synthetic, and biotransformation functions, many of which are hardly or not at all known. The design of liver-bioreactors poses special technical challenges with respect to liver cell physical-chemical requirements and the scale, as the liver is a highly structured organ with many distinct cell sub-populations and a sophisticated network of blood vessels. Specific phenomena such as “liver zonation”, apparently regulated by gradients of oxygen, hormones, and ECM composition, and the structure function relationship, among others.
Typically, liver constructs are engineered in vitro by culturing liver (or liver-like) cells in/on synthetic scaffolds, which provide the template for cell adhesion, re-arrangement, proliferation, and development. To meet the specifications discussed above, it has been suggested to pattern the biological substitutes after the liver micro-architecture. This comprehensive approach has been realized for organs-on-a-chip´s (see chapter 2.3) on a small scale. However, as for extracorporal liver support systems, at least 500g cells are supposed to be required, and most culture techniques used in this respect focus just on maintaining the hepatic functions relevant to stop the progression of acute liver failure [37][38]. Most bioreactors used as a bioartificial liver support system are membrane bioreactors (for review compare [39][36][38][40]), mainly to prevent a cellular immune response. These mostly polymeric permselective membranes act as selective barriers that compartmentalize the cells in an immuno-privileged compartment (i.e., the cell compartment) by preventing immune-competent cells and proteins from crossing their wall, yet they are freely permeable to the nutrients and metabolites necessary for cell survival and metabolism. Engineering challenges addressed by mathematical modelling comprise mainly nutrient and oxygen supply to cells, and the control of biochemical signals gradients and concentrations. Special attention is made to the transport of oxygen, often limiting cell functions and tissue growth in vitro. Just by using mass transfer equations for the mostly diffusive external, transmembrane, and internal transport, linked to cell metabolism, optimal conditions with respect to the composition of gas phase, membrane properties (especially membrane thickness), flow velocities, an internal distance of membranes, etc., it is possible to provide an optimal design of the complex bioreactor system, as shown by the groups of Gerlach and Catapano [38].
5.3. Permeation and Diffusion in Cultivated 3D Skin Models
Characterization of native skin or cultured 3D skin models with respect to permeability plays an important role in the development and testing of pharmaceuticals and cosmetics. Extensive efforts have been dedicated to determining the key parameters describing permeability and diffusion. Whereas respective methods are well established for native skin biopsies [41], only few are available for 3D skin models. The need to evaluate skin permeation, test cosmetic products, and screen topically applied compounds is evident. Since animal experiments are under massive debate, ethical and regulatory issues, but also severe differences between animal and human data, pushed the development and commercialization of diverse in vitro skin models [42]. Human skin equivalents (HSEs) can be categorized into two main groups: The epidermis-only and full-thickness models. Even if huge progress has been made over the years and a number of skin models are commercially available [43], the barrier function between artificial and human skin can especially differ, so permeation and diffusion investigation in this area are indispensable.
A number of methods for the determination of diffusion and permeability parameters have been developed for large biopsies (e.g., the Franz diffusion cell, Fluorescence recovery after photobleaching (FRAP), Fourier-transform-infrared (FTIR) spectroscopy, tape stripping [44]). These together provide a detailed characterization of diffusion and permeation effects within the skin. However, most of them can hardly be adapted to skin tissue models used in drug and substance testing. Here, usually small culture devices, e.g., 12- or 96-well plates or even Multi-organ-chips are preferred, as they allow for handling of a large number of samples in parallel or investigation of multi-organ interactions. Furthermore, most of the methods mentioned above require treatment of the sample in one or the other way. Therefore, they do not allow to determine the time-depending changes of diffusion and permeation in vitro.
Hsu et al. [45][44][46] developed an alternative method based on skin tissue cultures in a membrane insert system. By this, the permeation coefficient of substances, especially permeation of substances with a high molecular weight, through a skin-tissue barrier can be determined. The diffusion coefficient is estimated via parameter optimization performed in COMSOL Multiphysics. This method can be used to investigate changes in the permeation behavior of the skin model during the cultivation or it can also be adapted for other systems, which use membrane insert Systems, e.g., Multi-Organ-Chips [47]. Furthermore, different diffusion mechanisms, e.g., diffusion described by Fick’s law or abnormal diffusion, can be included. Furthermore, the simulation is an attractive tool to support the experiments. On the one hand, it can be used to understand physical phenomena and to reduce experimental effort. On the other hand, it is modular and can be integrated into a more complex system to support permeation studies.
5.4. An in Silico Strategy for 3D Тumor Мodels
Identification of new drug targets and biomarker profiles for innovative, personalized new treatment strategies against cancer require an in-depth understanding of signaling networks and their changes upon drug response. Tissue culture techniques offer tumor models mimicking the tumor microenvironment and providing multiple read-out options to reflect the clinical situation. The group of Walles et al. [48][49] combined a 3D in vitro model for lung carcinoma based on a decellularized tissue matrix to provide a complex microenvironment for cell growth and an in silico model to identify dependencies in signaling networks involved in proliferation, apoptosis, and invasion. The combined in vitro and in silico model represents allow to identify biomarker profiles for targeted therapies.
5.5. Digital Strategies from Bench to Bedside
Digital, model-based strategies have already been developed for a variety of tissue engineering processes. This is illustrated using the example of the extensive work of the working group of Geris et al. (reviewed in [14][50]). Using skeletal tissue engineering as a case study, a number of mathematical models at various stages of use between bench and bedside are discussed and ranging from pure data-driven models to models built on known mechanisms and first principles. Generally, in all these models, the aim is firstly to understand the biological process at hand and secondly to design strategies in silico to enhance the desired in vitro or in vivo behavior. The shown examples cover the cellular regulation of the growth plate, a system approach to the design of new biomaterials, 3D neotissue growth modelling for understanding and optimization of the bioreactor process, and on-line monitoring techniques to determine critical quality attributes. This comprehensive approach underpins that the tools toward a digital transformation of development and manufacturing of tissue engineering products as ATMP are at hand and the main goal for the future will be to link these in a comprehensive, digital strategy.
This entry is adapted from the peer-reviewed paper 10.3390/pr9030447
References
- Pörtner, R.; Parida, S.K.; Schaffer, C.; Hoffmeister, H. Landscape of Manufacturing Process of ATMP Cell Therapy Products for Unmet Clinical Needs. In Stem Cells in Clinical Practice and Tissue Engineering; Sharma, R., Ed.; InTechOpen: London, UK, 2018.
- Brunner, M.; Braun, P.; Doppler, P.; Posch, C.; Behrens, D.; Herwig, C.; Fricke, J. The impact of pH inhomogeneities on CHO cell physiology and fed-batch process performance—Two-compartment scale-down modelling and intracellular pH excursion. Biotechnol. J. 2017, 12.
- Gao, Y.; Ray, S.; Dai, S.; Ivanov, A.R.; Abu-Absi, N.R.; Lewis, A.M.; Huang, Z.; Xing, Z.; Borys, M.C.; Li, Z.J.; et al. Combined metabolomics and proteomics reveals hypoxia as a cause of lower productivity on scale-up to a 5000-liter CHO bioprocess. Biotechnol. J. 2016, 11, 1190–1200.
- Schmitz, J.; Noll, T.; Grünberger, A. Heterogeneity Studies of Mammalian Cells for Bioproduction: From Tools to Application. Trends Biotechnol. 2019, 37, 645–660.
- Lovett, M.; Rockwood, D.; Baryshyan, A.; Kaplan, D.L. Simple modular bioreactors for tissue engineering: A system for characterization of oxygen gradients, human mesenchymal stem cell differentiation, and prevascularization. Tissue Eng. Part C Methods 2010, 16, 1565–1573.
- Jossen, V.; Pörtner, R.; Kaiser, S.C.; Kraume, M.; Eibl, D.; Eibl, R. Mass Production of Mesenchymal Stem Cells—Impact of Bioreactor Design and Flow Conditions on Proliferation and Differentiation. In Cells and Biomaterials in Regenerative Medicine; Eberli, D., Ed.; InTech: London, UK, 2014.
- Burdick, J.A.; Vunjak-Novakovic, G. Engineered microenvironments for controlled stem cell differentiation. Tissue Eng. Part A 2009, 15, 205–219.
- Anderson, E.J.; Kaliyamoorthy, S.; Iwan, J.; Alexander, D.; Knothe, T.M.L. Nano-microscale models of periosteocytic flow show differences in stresses imparted to cell body and processes. Ann. Biomed. Eng. 2005, 33.
- Zhang, H.; Dai, S.; Bi, J.; Liu, K.-K. Biomimetic three-dimensional microenvironment for controlling stem cell fate. Interface Focus 2011, 1, 792–803.
- Titmarsh, D.; Hidalgo, A.; Turner, J.; Wolvetang, E.; Cooper-White, J. Optimization of flowrate for expansion of human embryonic stem cells in perfusion microbioreactors. Biotechnol. Bioeng. 2011, 108, 2894–2904.
- Liovic, P.; Šutalo, I.D.; Stewart, R.; Glattauer, V.; Meagher, L. Fluid flow and stresses on microcarriers in spinner flask bioreactors. In Proceedings of the Ninth International Conference on CFD in the Minerals and Process Industries, Melbourne, Australia, 10–12 December 2012.
- Bratt-Leal, A.M.; Carpenedo, R.L.; McDevitt, T.C. Engineering the embryoid body microenvironment to direct embryonic stem cell differentiation. Biotechnol. Prog. 2009, 25, 43–51.
- Chen, A.; Ting, S.; Seow, J.; Reuveny, S.; Oh, S. Considerations in designing systems for large scale production of human cardiomyocytes from pluripotent stem cells. Stem Cell Res. Ther. 2014, 5, 12.
- Jossen, V.; Schirmer, C.; Mostafa Sindi, D.; Eibl, R.; Kraume, M.; Pörtner, R.; Eibl, D. Theoretical and Practical Issues That Are Relevant When Scaling Up hMSC Microcarrier Production Processes. Stem Cells Int. 2016, 2016, 4760414.
- Sart, S.; Agathos, S.N.; Li, Y. Process engineering of stem cell metabolism for large scale expansion and differentiation in bioreactors. Biochem. Eng. J. 2014, 84, 74–82.
- Wu, J.; Rostami, M.R.; Cadavid Olaya, D.P.; Tzanakakis, E.S. Oxygen transport and stem cell aggregation in stirred-suspension bioreactor cultures. PLoS ONE 2014, 9, e102486.
- García Martínez, J.J.; Bendjelid, K. Artificial liver support systems: What is new over the last decade? Ann. Intensive Care 2018, 8.
- Tandon, R.; Froghi, S. Artificial liver support systems. J. Gastroenterol. Hepatol. 2020.
- He, Y.-T.; Qi, Y.-N.; Zhang, B.-Q.; Li, J.-B.; Bao, J. Bioartificial liver support systems for acute liver failure: A systematic review and meta-analysis of the clinical and preclinical literature. World J. Gastroenterol. 2019, 25, 3634–3648.
- Catapano, G. Bioreactors for Bioartificial Organs. In Cell and Tissue Reaction Engineering; Eibl, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 279–313.
- Catapano, G.; Gerlach, J.C. Bioreactors for Liver Tissue Engineering. Topics in Tissue Engineering. 2007, Volume 3. Ashammakhi, N., Reis, R., Chiellini, E., Eds. Available online: (accessed on 1 March 2021).
- Zhao, L.-F.; Pan, X.-P.; Li, L.-J. Key challenges to the development of extracorporeal bioartificial liver support systems. Hepatobiliary Pancreat. Dis. Int. 2012, 11, 243–249.
- Struecker, B.; Raschzok, N.; Sauer, I.M. Liver support strategies: Cutting-edge technologies. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 166–176.
- Meyer, S.; Peters, N.; Mann, T.; Wolber, R.; Pörtner, R.; Nierle, J. In vitro efficacy and release study with anti-inflammatory drugs incorporated in adhesive transdermal drug delivery systems. J. Pharm. Sci. 2014, 103, 1142–1148.
- Basketter, D.; Jírova, D.; Kandárová, H. Review of skin irritation/corrosion Hazards on the basis of human data: A regulatory perspective. Interdiscip. Toxicol. 2012, 5, 98–104.
- Mathes, S.H.; Ruffner, H.; Graf-Hausner, U. The use of skin models in drug development. Adv. Drug Deliv. Rev. 2014, 69–70, 81–102.
- Hsu, H.-H.; Schimek, K.; Marx, U.; Pörtner, R. Measurement and Simulation of Permeation and Diffusion in Native and Cultivated Tissue Constructs. In Biomaterials in Regenerative Medicine; Dobrzański, L.A., Ed.; IntechOpen: London, UK, 2018.
- Hsu, H.-H.; Kracht, J.-K.; Harder, L.E.; Rudnik, K.; Lindner, G.; Schimek, K.; Marx, U.; Pörtner, R. A Method for Determination and Simulation of Permeability and Diffusion in a 3D Tissue Model in a Membrane Insert System for Multi-well Plates. J. Vis. Exp. 2018.
- Hsu, H.-H. Charakterisierung und Numerische Simulation an Hautmodellen in Einem Multi-Organ-Chip. Ph.D. Thesis, Technische Universität Hamburg, Hamburg, Germany, 2019.
- Schimek, K.; Hsu, H.-H.; Boehme, M.; Kornet, J.J.; Marx, U.; Lauster, R.; Pörtner, R.; Lindner, G. Bioengineering of a Full-Thickness Skin Equivalent in a 96-Well Insert Format for Substance Permeation Studies and Organ-On-A-Chip Applications. Bioengineering 2018, 5, 43.
- Göttlich, C.; Müller, L.C.; Kunz, M.; Schmitt, F.; Walles, H.; Walles, T.; Dandekar, T.; Dandekar, G.; Nietzer, S.L. A Combined 3D Tissue Engineered In Vitro/In Silico Lung Tumor Model for Predicting Drug Effectiveness in Specific Mutational Backgrounds. J. Vis. Exp. 2016, e53885.
- Stratmann, A.T.; Fecher, D.; Wangorsch, G.; Göttlich, C.; Walles, T.; Walles, H.; Dandekar, T.; Dandekar, G.; Nietzer, S.L. Establishment of a human 3D lung cancer model based on a biological tissue matrix combined with a Boolean in silico model. Mol. Oncol. 2014, 8, 351–365.
- Geris, L.; Lambrechts, T.; Carlier, A.; Papantoniou, I. The future is digital: In silico tissue engineering. Curr. Opin. Biomed. Eng. 2018, 6, 92–98.
- Geris, L. Computational Modeling in Tissue Engineering; Springer: Berlin/Heidelberg, Germany, 2013.