The SLC25A20 transporter, also known as carnitine acyl-carnitine carrier (CAC), catalyzes the transport of short, medium and long carbon chain acyl-carnitines across the mitochondrial inner membrane in exchange for carnitine. The 30-year story of the protein responsible for this function started with its purification from rat liver mitochondria.
- carnitine
- carnitine acyl-carnitine carrier
- carnitine acyl-carnitine translocase
- membrane transport
- mitochondria
- mitochondrial carrier
The mitochondrial carnitine acyl-carnitine carrier (CAC) is the member A20 of the SLC25 protein family, including 53 solute transporters in humans, the majority of which are localized in the inner mitochondrial membrane. Until now, only one family member has been found in the peroxisomal membrane. Furthermore, approximately one-third of them are still orphans, i.e., their transported substrates are unknown. This family members share a peculiar structural fold of six transmembrane segments characterized by 3-fold repeated couples of hydrophobic α-helices. Each couple is connected by a hydrophilic loop and contains the SLC25 sequence motif PX[D/E]XX[K/R] at about the boundary of the odd α-helix and the loop. The structural information on the SLC25 proteins derives mainly from the ADP/ATP carrier, which has been crystallized in both the outwards and inward open conformations. All the other carrier structures have been predicted by homology modeling, including CAC, whose structure has been corroborated by site-directed mutagenesis and chemical targeting approaches. CAC is a key component of the carnitine shuttle, which is crucial for the mitochondrial β-oxidation pathway. In this shuttle (Figure 1), fatty acids are activated by the cytosolic acyl-CoA synthetase (ACSL) to fatty acyl-CoAs thioesters. Since the mitochondrial inner membrane is not permeable to acyl-CoAs, acyl groups are transferred from CoA to carnitine by the action of “carnitine palmitoyltransferase-1a and b” (CPT-1a; CPT-1b), an integral outer membrane enzyme. The acyl-carnitines cross the outer mitochondrial membrane through an almost unspecific pore constituted by the voltage-dependent anion channel (VDAC) and, then, are specifically translocated across the inner mitochondrial membrane by the action of CAC. In the mitochondrial matrix, the enzyme carnitine palmitoyltransferase 2 (CPT-2) catalyzes the trans-esterification of the acyl groups from carnitine to mitochondrial CoA with the release of free carnitine, thereby providing acyl-CoA substrates for fatty acid β-oxidation. CAC and CPT-2 form a supramolecular complex in the inner mitochondrial membrane, devoted to acyl-carnitine channeling from the carrier to the enzyme (Figure 1). The carnitine released in this reaction is translocated backward to the cytosol by the same carrier via an acyl-carnitine/carnitine antiport reaction. The β-oxidation pathway is active in many tissues, especially those characterized by higher metabolic expenditure. It provides a large portion of the energy required by heart muscle, kidneys and also skeletal muscle, when glycogen has been consumed. This pathway is also active in hepatocytes where fatty acid oxidation provides acetyl-CoA for ketone body synthesis during prolonged fasting conditions, in which glycogen stores have been depleted. Neurons also perform fatty acid oxidation even though at a very low rate. Indeed, CAC also has been described in brain. The crucial role of CAC in energy metabolism was demonstrated by the discovery of inherited defects of its gene SLC25A20 causing secondary carnitine deficiency, a syndrome that arises in the very first stage of life as a life-threatening pathology. In this altered metabolic condition, acyl-carnitines fail to reach the mitochondrial matrix with consequent strong impairment of the β-oxidation. This syndrome is more severe than the primary carnitine deficiency caused by defects of the plasma membrane transporter OCTN2 (SLC22A5). Recent findings have correlated alterations of CAC expression or regulation with diabetes.
Unlike most mitochondrial carriers, which are obligatory antiporters, CAC can catalyze, besides the antiport reaction, also a unidirectional transport of substrates event though at a rate about one order of magnitude lower than the antiport. Interestingly CAC is not only operating in animals but also yeast and plants. The Saccharomyces cerevisiae and the Aspergillus nidulans CACs share 29% and 42% identity with the human CAC, respectively. The main function of these transporters, in contrast to that of mammalian CACs, is to transport acetylcarnitine rather than medium- and long-chain acyl-carnitines into mitochondria. The plant CAC ortholog, identified based on the 37% sequence identity with the human counterpart, most probably plays a different role, that is, the transport of glutamate. It is still not clear if CAC also operates in peroxisomes, where very long, branched-chain, and medium-chain fatty acids are imported.
The history of CAC started with the detection of an acyl-carnitine uptake into mitochondria, which was saturable, stereospecific, inhibitable, and temperature-dependent. Then, the availability of methodologies capable of handling hydrophobic membrane proteins allowed us to purify the protein responsible for the observed transport phenomena. In 1990, a classical approach based on chromatography fractionation of a rat liver mitochondrial extract and on transport assay of the fractions by proteoliposome technology was adopted. The purified protein was used for the first functional characterization. Later, CAC was identified at a molecular level and obtained on a large-scale by overexpression in Escherichia coli by a procedure introduced in our laboratory for the bacterial overexpression of the oxoglutarate carrier and recently named the expression, purification, reconstitution assay (EPRA) method. The recombinant purified CAC was employed in studies of structure/function relationships, interaction with drugs and xenobiotics, and post-translational modifications that modulate its transport function.
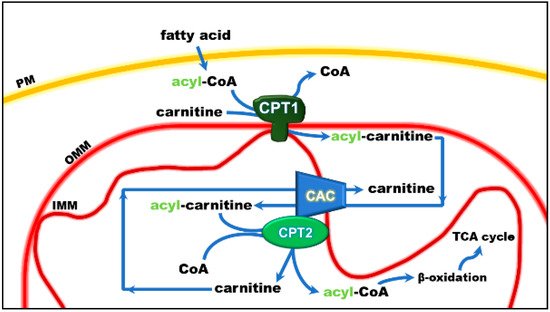
Figure 1. Role of the carnitine shuttle in the mitochondrial β-oxidation pathway. The shuttle is constituted by carnitine palmitoyltransferase 1 (CPT1) that converts acyl-CoAs into acyl-carnitines; carnitine/acyl-carnitine carrier (CAC) that allows the uptake of acyl-carnitines in the mitochondrial matrix in exchange with free carnitine, and carnitine palmitoyltransferase 2 (CPT2) that converts acyl-carnitines back to acyl-CoAs and releases free carnitine, which is ready to be translocated back to the cytosol by CAC. Once in the matrix, acyl-CoA undergoes β-oxidation with the production of acetyl-CoA that enters the tricarboxylic acid cycle (TCA). Other abbreviations: IMM, inner mitochondrial membrane; OMM, outer mitochondrial membrane; PM, plasma membrane.
This entry is adapted from the peer-reviewed paper 10.3390/biom11040521
