Periodontal diseases, in the light of the new classification, have been divided into three general categories: gum disease, periodontitis, and other conditions affecting the periodontium.
- bacterial complexes
- inflammation
- periodontitis
- oxidative stress
1. Introduction
The oral cavity is the place where a person’s outer world meets his inner world. It has very diverse microbiological conditions that are constantly changing. If we do not take care of proper hygiene of this site, lesions can appear in the oral cavity, which over time contribute to pathological phenomena throughout the body-systemic diseases, and vice versa-all general diseases are reflected in the oral cavity. Physical, chemical and immunohistochemical factors also influence the bacterial environment in the mouth [1]. These are temperature, redox potential (Eh) [2], concentration of hydrogen ions, availability of nutrients, colonization with microflora, pH of saliva [3][4][5][6][7], order of oral cavity colonization or resistance to it [8][9][10][11], conditions of bacterial adhesion to plaque [12][8][13][14][15]. These conditions are constantly changing as food is introduced into the oral cavity, which lowers the pH, favoring the development of carious lesions in the tooth tissues.
Contemporary periodontics and implantology pose many therapeutic challenges due to the multitude of disease processes affecting the structure of periodontal tissues. Bleeding caused by gentle examination with a periodontal probe is a sign of gingivitis. Additionally, the high frequency of replacing missing teeth with dental implants necessitates increased control and periodontal care in both healthy patients and those with reduced periodontium. At the 2018 annual FDI World Dental Federation Congress organized by the American Academy of Periodontology (AAP) and the European Federation of Periodontology (EFP) in San Francisco (CA, USA), a new classification of periodontal diseases and implant-related diseases was presented to help dentists treat them more effectively [16]. The principles of dealing not only with inflammatory forms of periodontal diseases but also with deformations of the gingival mucosa and periodontal diseases are presented. In addition, the most important disease entities from the surgical point of view, which affect the achievement of the best and longest therapeutic effect, were analyzed, regardless of whether in non-surgical or surgical treatment of periodontitis and tissue around the implant [16]. Periodontal diseases, in the light of the new classification, have been divided into three general categories: gum disease, periodontitis, and other conditions affecting the periodontium. The periodontitis category includes:
- -periodontitis
- -necrotic periodontal diseases
- -periodontitis as a symptom of systemic diseases.
Before making a diagnosis, the dentist should consider the patient’s overall health and some of the risk factors, particularly:
- -smoking
- -variety of diet
- -nutritional deficiencies, e.g., vitamin C deficiency
- -hormonal changes such as maturation
- -diabetes.
The classification includes new categories based on disease severity, extent, rate of development, and treatment complexity. Thus, periodontitis as a symptom of systemic diseases is divided into four categories taking into account the underlying general disease, according to the International Statistical Classification of Diseases and Related Health Problems (ICD) code:
Stage I: Initial periodontitis
Stage II: Moderate periodontitis
Stage III: Severe periodontitis with possible additional tooth loss
Stage IV: Severe periodontitis with possible loss of dentition
There are three grades based on the patient’s overall health, risk factors, indications or risk of rapid progression, and expected response to treatment:
Grade A: Slow progression
Grade B: Average rate of progression
Grade C: Fast rate of progression
Although the stage of periodontitis will remain unchanged, its severity can be increased after periodontal treatment in combination with reliable patient cooperation and effective control of risk factors [16].
For the first time, diseases developing around implants (peri-implantitis) were classified. The importance of examination with a periodontal probe and frequent checkups, which should be the basic conduct of a doctor after diagnosis, was emphasized [16]. However, diseases and conditions around the implant are divided into four categories:
-
-No changes around the implant,
-
-Inflammation of the mucosa around the implant,
-
-Peri-implantitis,
-
-The loss of soft and hard tissues around the implant.
-
2. Diet and Oral Health
The outside world with its flora and fauna is essential to human life. It is used to obtain food that provides many stimuli to enjoy, and it is also the fuel needed to live/be-for the development of the physical side of a person-his body, metabolic processes and life energy. The oral cavity is a special place in the human body. It is there that you can taste the food that you can enjoy more or less, it is where the first digestive processes take place, which give rise to further processes necessary for the functioning of the macroorganism. It is there that pathological conditions arise, which in many situations generate systemic health problems. Pathological conditions in the oral cavity are the result of ineffective removal of food residues after meals and the active activity of the bacterial microflora inhabiting there. All living micro- and macro-organisms need food to survive, and the oral micro-world needs food scraps to thrive. Its development is unfortunately unfavorable for the macroorganism—its host. It damages the dental apparatus, as a result of which the host cannot use it effectively, which causes a cascade of adverse events. First, food that is not “worked out” properly in the mouth will not be able to be fully used by the body as it passes through the gastrointestinal tract [11]. Each section of the digestive tract has its own tasks. In the mouth, food is broken down by the teeth, softened by saliva, and thanks to amylase (an enzyme contained in saliva) it starts the process of digesting sugars. Amylase breaks down starch and other polysaccharides into simple sugars. The next stage of digestion takes place in the stomach and further in the subsequent sections of the digestive tract, until it is saturated with what the body will be able to obtain from it, and its remnants will be excreted outside. The human body is an very specific organism, equipped with defense mechanisms (specific and non-specific) that are triggered in emergency situations. These mechanisms work effectively when the macro-organism is healthy, nourishes properly and regularly uses proper oral hygiene. The antibodies make it difficult for bacteria to colonize the tissues and block their metabolism, but there are microbes that can destroy them. Other defense mechanisms are the continuity of the enamel and mucosa (constituting a natural barrier and protection of tissues against the penetration of microorganisms), exfoliation of the epithelium and bacteria deposited on them, the presence of bacterial flora (preventing the deposition of bacteria), movements of the tongue, cheeks and saliva (cleaning the surface of the teeth). As we know, saliva hinders the colonization of microorganisms and contains bactericidal substances (lysozyme, lactoferrin, histatin, staterin, apolactoferrin, bacteriocins and the sialoperoxidase system). In a situation where the body is subjected to ultraviolet radiation, ionizing radiation, ultrasonic waves, xenobiotics (along with food), oxygen consumption (which in 5% undergoes an unfavorable transformation, resulting in free radicals), oxidative stress will arise [17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50]. Oxidative stress is the result of disturbed homeostasis in the body, which can lead to irreversible changes. Low and high levels of oxidative stress mobilize cellular antioxidant mechanisms and stimulate the inflammatory response of cells, but very high levels of oxidative stress contribute to cell death (apoptosis and necrosis). A positive thing in this situation is the fact that maintaining an appropriate level of oxidative stress significantly influences the treatment of many inflammatory diseases [51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70][71][72][73][74][75][76][77][78][79][80][81][82][83][84][85][86][87][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50].
We propose the use of “protocols” of four diets containing individual nutrients that should reduce inflammation and the formation of pathogenic bacteria in the mouth:
-
Diet F including meals, containing proteins, carbohydrates-sugars, fats, vegetables.
-
Diet B. Mainly targeted at protein products. You can eat other foods as well, but end each meal with a sugar-free protein product such as kefir, yoghurt, cheese, etc.
-
Diet W. Mainly oriented towards vegetables and other foods can also be eaten, but each meal should end with vegetables, such as radish, watercress, kale, broccoli, kohlrabi, etc.
-
Diet T. Mainly targeted at foods containing Omega-3 fatty acids, can also eat other foods, but each meal should be finished with food containing Omega-3 fats, e.g., fish-especially salmon, herring, mackerel, sardines, seafood, sushi, rapeseed oil, linseed, soybean oil, soy products, nuts, almonds, pumpkin seeds.
Collagen is the backbone of the organic matrix in the deposition of phosphate and calcium crystals and bone mineralization [54][82][83][84][85][86][87] (Figure 4).
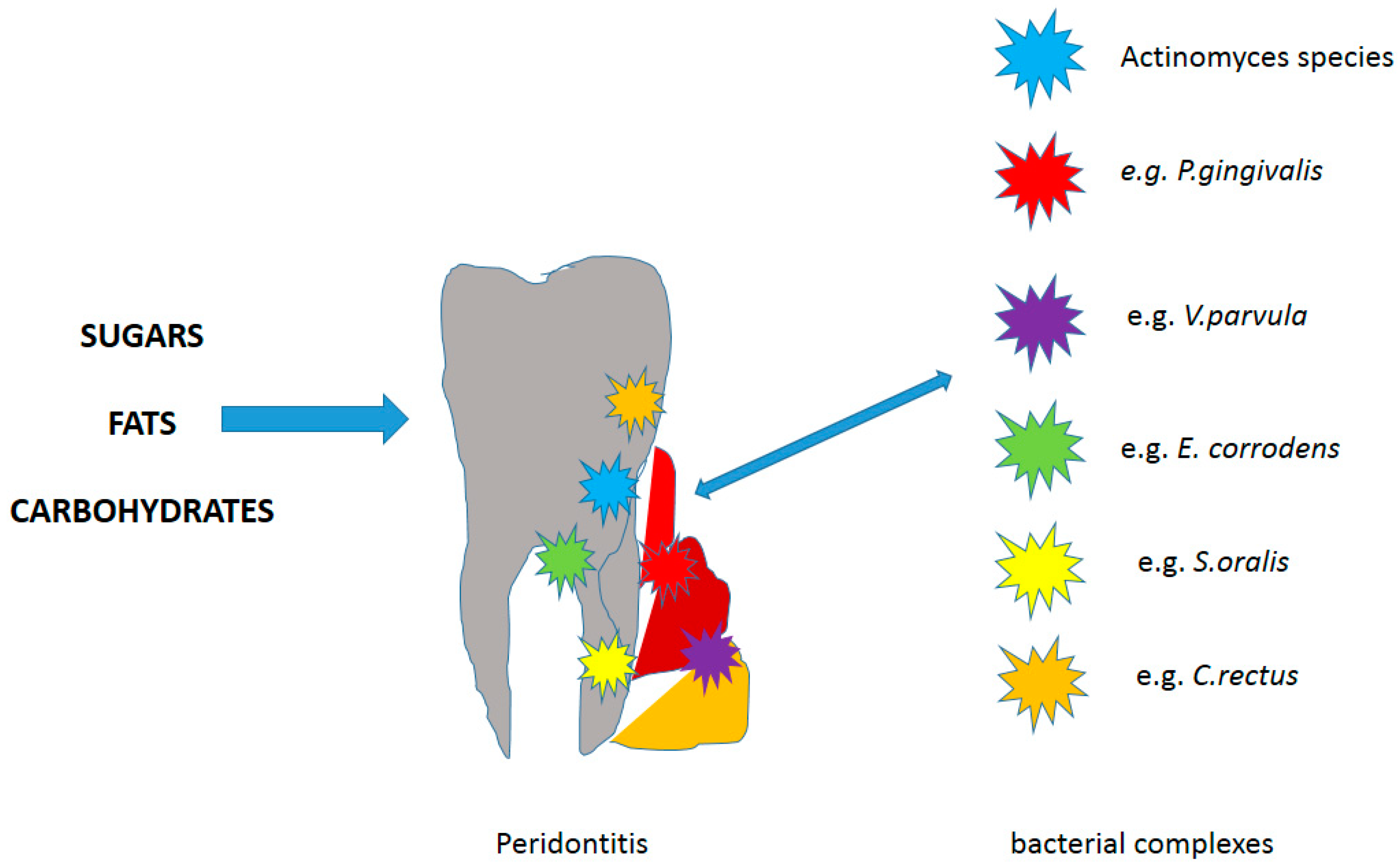
Figure 4. The role of nutrients in the body according to [88].
3. Conclusions
Periodontal diseases are still one of the major health problems in Poland. Statistics show that out of 10 people, nine have periodontal problems [32]. The development of the disease is a consequence of an imbalance between the potentially pathological bacteria found in the oral cavity (supra- and subgingival plaque) and the host’s immune response. In the oral cavity, homeostasis can be modified by a number of constant (congenital) and variable (acquired) factors that are risk factors for developing disease. The most important congenital risk factors include age, genotypes (genetic factors), gender, and race. The second group includes variable determinants, i.e., improper oral hygiene, local factors of plaque accumulation (gingival areas, inadequately placed fillings, tooth crowding, cervical areas of crowns, bridges), unfavorable composition of bacterial biofilm present on the teeth, use of tobacco (smoking pipes, cigarettes, cigars, the use of non-flammable tobacco-chewing snuff), nutritional deficiencies (lack of vitamin C, calcium), diseases (e.g., diabetes, alcoholism, osteoporosis) and long-term exposure to stressors [82][83][84][85][86][87][89][90][91][92][93][94][95][96][97][98][99][100][101][102],
Considering the cariogenic factor, the diet should be rich in calcium, phosphorus, fluorine and vitamin D products, as well as nutrients involved in the growth and mineralization of teeth. Based on the quoted literature data, recommendations can be made regarding the change of eating habits in the diet to reduce the risk of oxidative stress and inflammation that may contribute to the development of pathogenic microflora in the oral cavity. One should definitely limit the consumption of carbohydrates, especially those with a viscous consistency, sweetened drinks, sour drinks, carbonated drinks and juices. People with healthy temporomandibular joints may be advised to chew sugar-free chewing gum after a meal (but for about 5–10 min) when they cannot brush their teeth. Hygiene that is appropriate to the age and situation in the mouth is very important. Considering the erosive factors, in addition to the above, avoid drinking beverages lowering the pH below 4.5, it is recommended to drink them through a straw. It is advisable to end your meals with foods that neutralize the pH of the oral cavity (e.g., cheese, dairy products, milk). It is recommended to drink water, and if you drink acidic drinks, you should sweeten them a little before drinking (then the pH becomes neutral), do not drink between meals and in the evening. Allow 1–2 h breaks between meals to facilitate the remineralization of hard tissues. It is recommended not to brush your teeth immediately after consuming acidic foods or liquids so as not to exacerbate erosive changes. By following the above recommendations, we can hope to avoid serious inflammation, even leading to tooth loss, through chronic inflammation of the tissues of the attachment apparatus. We hope that our review article will be a compendium of knowledge on the recommendations for choosing the right diet for daily oral hygiene. This study highlights a different relationship between gum disease and systemic health, and confirms the need for continuous, systematic dental care for periodontal-prone people and for strong periodontal prophylaxis for the entire population. Periodontal diseases affect up to 50 percent of all adults around the world and they are recognized by various dental associations and federations as a disease of civilization.
-
This entry is adapted from the peer-reviewed paper 10.3390/ma14061444
References
- Khomich, O.A.; Kochetkov, S.N.; Bartosch, B.; Ivanov, A.V. Redox Biology of Respiratory Viral Infections. Viruses 2018, 10, 392.
- Oliveira, F.A.F.; Forte, F.C.P.; Silva, P.G.B.; Lopes, C.B.; Montenegro, R.C.; Dos Santos, Â.K.C.R.; Mota, M.R.L.; Sousa, F.B.; Alves, A.P.N.N. Relationship of Streptococcus mutans with valvar cardiac tissue: A molecular and immunohistochemical study. J. Oral Pathol. Med. 2019, 48, 745–753.
- Lamy, E.; Capela-Silva, F.; Tvarijonaviciute, A. Research on Saliva Secretion and Composition. Biomed. Res. Int. 2018, 2018, 7406312.
- Pandit, P.; Cooper-White, J.; Punyadeera, C. High-yield RNA-extraction method for saliva. Clin Chem. 2013, 59, 1118–1122.
- Canon, F.; Neiers, F.; Guichard, E. Saliva and Flavor Perception: Perspectives. J. Agric. Food Chem. 2018, 66, 7873–7879.
- Lexner, M.O.; Blomqvist, S.; Dahlén, G.; Twetman, S. Microbiological profiles in saliva and supragingival plaque from caries-active adolescents before and after a short-term daily intake of milk supplemented with probiotic bacteria—A pilot study. Oral Health Prev. Dent. 2010, 8, 383–388.
- Marsh, P.D.; Do, T.; Beighton, D.; Devine, D.A. Influence of saliva on the oral microbiota. Periodontol 2000 2016, 70, 80–92.
- Gibbons, R.J. Role of adhesion in microbial colonization of host tissues: A contribution of oral microbiology. J. Dent. Res. 1996, 75, 866–870.
- Velusamy, S.K.; Sampathkumar, V.; Ramasubbu, N.; Paster, B.J.; Fine, D.H. Aggregatibacter actinomycetemcomitans colonization and persistence in a primate model. Proc. Natl. Acad. Sci. USA 2019, 116, 22307–22313.
- Leonard, A.C.; Petrie, L.E.; Cox, G. Bacterial Anti-adhesives: Inhibition of Staphylococcus aureus Nasal Colonization. ACS Infect. Dis. 2019, 5, 1668–1681.
- Jeziorek, M.; Frej-Mądrzak, M.; Choroszy-Król, I. The influence of diet on gastrointestinal Candida spp. colonization and the susceptibility of Candida spp. to antifungal drugs. Rocz. Panstw. Zakl. Hig. 2019, 70, 195–200.
- Sterzenbach, T.; Helbig, R.; Hannig, C.; Hannig, M. Bioadhesion in the oral cavity and approaches for biofilm management by surface modifications. Clin. Oral Investig. 2020, 24, 4237–4260.
- Almaguer-Flores, A.; Ximénez-Fyvie, L.A.; Rodil, S.E. Oral bacterial adhesion on amorphous carbon and titanium films: Effect of surface roughness and culture media. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 92, 196–204.
- Haukioja, A.; Yli-Knuuttila, H.; Loimaranta, V.; Kari, K.; Ouwehand, A.C.; Meurman, J.H.; Tenovuo, J. Oral adhesion and survival of probiotic and other lactobacilli and bifidobacteria in vitro. Oral Microbiol. Immunol. 2006, 21, 326–332.
- Sheets, S.M.; Potempa, J.; Travis, J.; Casiano, C.A.; Fletcher, H.M. Gingipains from Porphyromonas gingivalis W83 induce cell adhesion molecule cleavage and apoptosis in endothelial cells. Infect. Immun. 2005, 73, 1543–1552.
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S1–S8.
- Magán-Fernández, A.; Papay-Ramírez, L.; Tomás, J.; Marfil-Álvarez, R.; Rizzo, M.; Bravo, M.; Mesa, F. Association of simvastatin and hyperlipidemia with periodontal status and bone metabolism markers. J. Periodontol. 2014, 85, 1408–1415.
- Bartold, P.M. Turnover in periodontal connective tissues: Dynamic homeostasis of cells, collagen and ground substances. Oral Dis. 1995, 1, 238–253.
- Manthena, S.; Rao, M.V.; Penubolu, L.P.; Putcha, M.; Harsha, A.V. Effectiveness of CoQ10 Oral Supplements as an Adjunct to Scaling and Root Planing in Improving Periodontal Health. J. Clin. Diagn. Res. 2015, 9, ZC26–ZC28.
- Yelins’ka, A.M.; Liashenko, L.I.; Kostenko, V.O. Quercetin potentiates antiradical properties of epigallocatechin-3-gallate in periodontium of rats under systemic and local administration of lipopolisaccharide of salmonella typhi. Wiad. Lek. 2019, 72, 1499–1503.
- Shanmugam, T.; Selvaraj, M.; Poomalai, S. Epigallocatechin gallate potentially abrogates fluoride induced lung oxidative stress, inflammation via Nrf2/Keap1 signaling pathway in rats: An in-vivo and in-silico study. Int. Immunopharmacol. 2016, 39, 128–139.
- Sriram, N.; Kalayarasan, S.; Sudhandiran, G. Epigallocatechin-3-gallate augments antioxidant activities and inhibits inflammation during bleomycin-induced experimental pulmonary fibrosis through Nrf2-Keap1 signaling. Pulm. Pharmacol. Ther. 2009, 22, 221–236.
- Kim, H.S.; Quon, M.J.; Kim, J.A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014, 2, 187–195.
- Chapple, I.L.C.; Bouchard, P.; Cagetti, M.G.; Campus, G.; Carra, M.C.; Cocco, F.; Nibali, L.; Hujoel, P.; Laine, M.L.; Lingstrom, P.; et al. Interaction of lifestyle, behaviour or systemic diseases with dental caries and periodontal diseases: Consensus report of group 2 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J. Clin. Periodontol. 2017, 44, S39–S51.
- Dodington, D.W.; Fritz, P.C.; Sullivan, P.J.; Ward, W.E. Higher Intakes of Fruits and Vegetables, beta-Carotene, Vitamin C, alpha-Tocopherol, EPA, and DHA Are Positively Associated with Periodontal Healing after Nonsurgical Periodontal Therapy in Nonsmokers but Not in Smokers. J. Nutr. 2015, 145, 2512–2519.
- Woelber, J.P.; Bremer, K.; Vach, K.; König, D.; Hellwig, E.; Ratka-Krüger, P.; Al-Ahmad, A.; Tennert, C. An oral health optimized diet can reduce gingival and periodontal inflammation in humans—A randomized controlled pilot study. BMC Oral Health 2016, 17, 28.
- Woelber, J.P.; Gärtner, M.; Breuninger, L.; Anderson, A.; König, D.; Hellwig, E.; Al-Ahmad, A.; Vach, K.; Dötsch, A.; Ratka-Krüger, P.; et al. The influence of an anti-inflammatory diet on gingivitis. A randomized controlled trial. J. Clin. Periodontol. 2019, 46, 481–490.
- Teshome, A.; Yitayeh, A. The effect of periodontal therapy on glycemic control and fasting plasma glucose level in type 2 diabetic patients: Systematic review and meta-analysis. BMC Oral Health 2016, 17, 31.
- Belstrøm, D. The salivary microbiota in health and disease. J. Oral Microbiol. 2020, 12, 1723975.
- Woo, H.D.; Park, S.; Oh, K.; Kim, H.J.; Shin, H.R.; Moon, H.K.; Kim, J. Diet and cancer risk in the Korean population: A meta- analysis. Asian Pac. J. Cancer Prev. 2014, 15, 8509–8519.
- Li, J.; Zou, L.; Chen, W.; Zhu, B.; Shen, N.; Ke, J.; Lou, J.; Song, R.; Zhong, R.; Miao, X. Dietary mushroom intake may reduce the risk of breast cancer: Evidence from a meta-analysis of observational studies. PLoS ONE 2014, 9, e93437.
- Jenssen, H.; Hancock, R.E. Antimicrobial properties of lactoferrin. Biochimie 2009, 91, 19–29.
- Allaker, R.P.; Stephen, A.S. Use of Probiotics and Oral Health. Curr. Oral Health Rep. 2017, 4, 309–318.
- Baker, H.M.; Baker, E.N. Lactoferrin and iron: Structural and dynamic aspects of binding and release. Biometals 2004, 17, 209–216.
- Berlutti, F.; Pilloni, A.; Pietropaoli, M.; Polimeni, A.; Valenti, P. Lactoferrin and oral diseases: Current status and perspective in periodontitis. Ann. Stomatol. 2011, 2, 10–18.
- Haffajee, A.D.; Socransky, S.S.; Patel, M.R.; Song, X. Microbial complexes in supragingival plaque. Oral Microbiol. Immunol. 2008, 23, 196–205.
- Jayaram, P.; Chatterjee, A.; Raghunathan, V. Probiotics in the treatment of periodontal disease: A systematic review. J. Indian Soc. Periodontol. 2016, 20, 488–495.
- Food and Agriculture Organization, World Health Organization. Guidelines for the Evaluation of Probiotics in Food—Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food; Food and Agriculture Organization: Rome, Italy; World Health Organization: Geneva, Switzerland, 2002.
- Stamatova, I.; Meurman, J.H. Probiotics and periodontal disease. Periodontology 2009, 51, 141–151.
- Yeo, S.; Lee, S.; Park, H.; Shin, H.; Holzapfel, W.; Huh, C.S. Development of putative probiotics as feed additives: Validation in a porcine-specific gas-trointestinal tract model. Appl. Microbiol. Biotechnol. 2016, 100, 10043–10054.
- Kaur, G.; Kathariya, R.; Bansal, S.; Singh, A.; Shahakar, D. Dietary antioxidants and their indispensable role in periodontal health. J. Food Drug Anal. 2016, 24, 239–246.
- Bravi, F.; Bosetti, C.; Filomeno, M.; Levi, F.; Garavello, W.; Galimberti, S.; Negri, E.; La Vecchia, C. Foods, nutrients and the risk of oral and pharyngeal cancer. Br. J. Cancer 2013, 109, 2904–2910.
- Garavello, W.; Lucenteforte, E.; Bosetti, C.; La Vecchia, C. The role of foods and nutrients on oral and pharyngeal cancer risk. Minerva Stomatol. 2009, 58, 25–34.
- Moynihan, P.J. The role of diet and nutrition in the etiology and prevention of oral diseases. Bull World Health Organ. 2005, 83, 694–699.
- Lavin, J.H.; French, S.J.; Ruxton, C.H.; Read, N.W. An investigation of the role of oro-sensory stimulation in sugar satiety? Int. J. Obes. Relat. Metab. Disord. 2002, 26, 384–388.
- Biesalski, H.K. (Ed.) Pocket Atlas of Nutrition, 1st ed.; Grimm Medical Books; Georg Thieme Verlag: Stuttgart, Germany, 2004; ISBN-13: 978-3131354815, ISBN-10: 9783131354815.
- Konopka, T.; Dembowska, E.; Pietruska, M.; Dymalski, P.; Górska, R. Periodontal status and selected parameters of oral condition of Poles aged 65 to 74 years. Przegl. Epidemiol. 2015, 69, 537–542.
- Lee, W.H.; Chen, H.M.; Yang, S.F.; Liang, C.; Peng, C.Y.; Lin, F.M.; Tsai, L.L.; Wu, B.C.; Hsin, C.H.; Chuang, C.Y.; et al. Bacterial alterations in salivary microbiota and their association in oral cancer. Sci. Rep. 2017, 7, 16540.
- De Angelis, M.; Garruti, G.; Minervini, F.; Bonfrate, L.; Portincasa, P.; Gobbetti, M. The Food-gut Human Axis: The Effects of Diet on Gut Microbiota and Metabolome. Curr. Med. Chem. 2019, 26, 3567–3583.
- Rachid, R.; Chatila, T.A. The role of the gut microbiota in food allergy. Curr. Opin. Pediatr. 2016, 28, 748–753.
- Zhang, H.; Yan, A.; Liu, X.; Ma, Y.; Zhao, F.; Wang, M.; Loor, J.J.; Wang, H. Melatonin ameliorates ochratoxin A induced liver inflammation, oxidative stress and mitophagy in mice involving in intestinal microbiota and restoring the intestinal barrier function. J. Hazard. Mater. 2021, 407, 124489.
- Miggiano, G.A.; Gagliardi, L. Diet, nutrition and rheumatoid arthritis. Clin. Ter. 2005, 156, 115–123.
- Watad, A.; Quaresma, M.; Bragazzi, N.L.; Cervera, R.; Tervaert, J.W.C.; Amital, H.; Shoenfeld, Y. The autoimmune/inflammatory syndrome induced by adjuvants (ASIA)/Shoenfeld’s syndrome: Descriptive analysis of 300 patients from the international ASIA syndrome registry. Clin. Rheumatol. 2018, 37, 483–493.
- Dzidic, M.; Collado, M.C.; Abrahamsson, T.; Artacho, A.; Stensson, M.; Jenmalm, M.C.; Mira, A. Oral microbiome development during childhood: An ecological succession influenced by postnatal factors and associated with tooth decay. ISME J. 2018, 12, 2292–2306.
- O’Toole, S.; Mullan, F. The role of the diet in tooth wear. Br. Dent. J. 2018, 224, 379–383.
- Jimenez, M.; Giovannucci, E.; Krall Kaye, E.; Joshipura, K.J.; Dietrich, T. Predicted vitamin D status and incidence of tooth loss and periodontitis. Public Health Nutr. 2014, 17, 844–852.
- Simpson, T.C.; Weldon, J.C.; Worthington, H.V.; Needleman, I.; Wild, S.H.; Moles, D.R.; Stevenson, B.; Furness, S.; Iheozor-Ejiofor, Z. Treatment of periodontal disease for glycaemic control in people with diabetes mellitus. Cochrane Database Syst Rev. 2015, 11, CD004714.
- Winning, L.; Linden, G.J. Periodontitis and systemic disease. BDJ Team 2015, 2.
- Kim, J.; Amar, S. Odontology. Periodontal disease and systemic conditions: A bidirectional relationship. Odontology 2006, 94, 10–21.
- Arigbede, A.O.; Babatope, B.O.; Bamidele, M.K. Periodontitis and systemic diseases: A literature review. J. Indian Soc. Periodontol. 2012, 16, 487–491.
- Nędzi-Góra, M.; Kowalski, J.; Krajewski, J.; Górska, R. Microbiological analysis of deep periodontal pockets in people with chronic periodontitis by PCR. Stomatol. J. 2007, 11, 717–725.
- Sbordone, L.; DiGenio, M.; Bortolaia, C. Bacterial virulence in the etiology of periodontal diseases. Minerva Stomatol. 2000, 49, 485–500.
- Sela, M.N. Role of Treponema denticola in periodontal diseases. Crit. Rev. Oral Biol. Med. 2001, 12, 399–413.
- Albandar, J.M. Aggressive and acute periodontal diseases. Periodontology 2000 2014, 65, 7–12.
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primers 2017, 3, 17038.
- Colombo, A.V.; Silva, C.M.; Haffajee, A.; Colombo, A.P.V. Identification of oral bacteria associated with crevicular epithelial cells from chronic periodontitis lesions. J. Med. Microbiol. 2006, 55, 609–615.
- Kaye, E.K.; Valencia, A.; Baba, N.; Spiro, A.; Dietrich, T.; Garcia, R.I. Tooth loss and periodontal disease predict poor cognitive function in older men. J. Am. Geriatr. Soc. 2010, 58, 713–718.
- Ishida, N.; Ishihara, Y.; Ishida, K.; Tada, H.; Funaki-Kato, Y.; Hagiwara, M.; Ferdous, T.; Abdullah, M.; Mitani, A.; Michikawa, M.; et al. Periodontitis induced by bacterial infection exacerbates features of Alzheimer’s disease in transgenic mice. NPJ Aging Mech. Dis. 2017, 6, 3–15.
- Curtis, M.A.; Diaz, P.I.; Van Dyke, T.E. The role of the microbiota in periodontal disease. Periodontol. 2000 2020, 83, 14–25.
- Park, S.-Y.; Kim, S.-H.; Kang, S.-H.; Yoon, C.-H.; Lee, H.-J.; Yun, P.-Y.; Youn, T.-J.; Chae, I.-H. Improved oral hygiene care attenuates the cardiovascular risk of oral health disease: A population-based study from Korea. Eur. Heart J. 2019, 40, 1138–1145.
- Hu, Y.; Chen, D.; Zheng, P.; Yu, J.; He, J.; Mao, X.; Yu, B. The Bidirectional Interactions between Resveratrol and Gut Microbiota: An Insight into Oxidative Stress and Inflammatory Bowel Disease Therapy. Biomed. Res. Int. 2019, 2019, 5403761.
- Dursun, E.; Akalin, F.A.; Genc, T.; Cinar, N.; Erel, O.; Yildiz, B.O. Oxidative Stress and Periodontal Disease in Obesity. Medicine 2016, 95, e3136.
- Buranasin, P.; Mizutani, K.; Iwasaki, K.; Pawaputanon, N.; Mahasarakham, C.; Kido, D.; Takeda, K.; Izumi, Y. High glucose-induced oxidative stress impairs proliferation and migration of human gingival fibroblasts. PLoS ONE 2018, 13, e0201855.
- Żukowski, P.; Maciejczyk, M.; Waszkiel, D. Sources of free radicals and oxidative stress in the oral cavity. Arch. Oral Biol. 2018, 92, 8–17.
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014, 224, 164–175.
- Rams, T.E.; Sautter, J.D.; van Winkelhoff, A.J. Comparative In Vitro Resistance of Human Periodontal Bacterial Pathogens to Tinidazole and Four Other Antibiotics. Antibiotics 2020, 9, 68.
- Fan, C.; Guo, L.; Gu, H.; Huo, Y.; Lin, H. Alterations in Oral-Nasal-Pharyngeal Microbiota and Salivary Proteins in Mouth-Breathing Children. Front. Microbiol. 2020, 11, 575550.
- Huang, C.; Shi, G. Smoking and microbiome in oral, airway, gut and some systemic diseases. J. Transl. Med. 2019, 17, 225.
- Yang, Y.; Zheng, W.; Cai, Q.Y.; Shrubsole, M.J.; Pei, Z.; Brucker, R.; Steinwandel, M.D.; Bordenstein, S.R.; Li, Z.; Blot, W.J.; et al. Cigarette smoking and oral microbiota in low-income and African-American populations. J. Epidemiol. Community Health 2019, 73, 1108–1115.
- Yu, G.; Phillips, S.; Gail, M.H.; Goedert, J.J.; Humphrys, M.S.; Ravel, J.; Ren, Y.; Caporaso, N.E. The effect of cigarette smoking on the oral and nasal microbiota. Microbiome 2017, 5, 3.
- Grine, G.; Terrer, E.; Boualam, M.A.; Aboudharam, G.; Chaudet, H.; Ruimy, R.; Drancourt, M. Tobacco-smoking-related prevalence of methanogens in the oral fluid microbiota. Sci. Rep. 2018, 8, 9197.
- Boqué, N.; Campión, J.; Milagro, F.I.; Moreno-Aliaga, M.J.; Martinez, J.A. Some cyclin-dependent kinase inhibitors-related genes are regulated by vitamin C in a model of diet-induced obesity. Biol. Pharm. Bull. 2009, 32, 1462–1468.
- Giacaman, R.A. Sugars and beyond. The role of sugars and the other nutrients and their potential impact on caries. Oral Dis. 2018, 24, 1185–1197.
- Ponziani, F.R.; Pompili, M.; Di Stasio, E.; Zocco, M.A.; Gasbarrini, A.; Flore, R. Subclinical atherosclerosis is linked to small intestinal bacterial overgrowth via vitamin K2-dependent mechanisms. World J. Gastroenterol. 2017, 23, 1241–1249.
- Charoenngam, N.; Shirvani, A.; Kalajian, T.A.; Song, A.; Holick, M.F. The Effect of Various Doses of Oral Vitamin D(3) Supplementation on Gut Microbiota in Healthy Adults: A Randomized, Double-blinded, Dose-response Study. Anticancer Res. 2020, 40, 551–556.
- Singh, V.N.; Gaby, S.K. Premalignant lesions: Role of antioxidant vitamins and beta-carotene in risk reduction and prevention of malignant transformation. Am. J. Clin. Nutr. 1991, 53, 386S–390S.
- Vollmer, D.L.; West, V.A.; Lephart, E.D. Enhancing Skin Health: By Oral Administration of Natural Compounds and Minerals with Implications to the Dermal Microbiome. Int. J. Mol. Sci. 2018, 19, 3059.
- Kumar, V.; Farell, G.; Yu, S.; Harrington, S.; Fitzpatrick, L.; Rzewuska, E.; Miller, V.M.; Lieske, J.C. Cell biology of pathologic renal calcification: Contribution of crystal transcytosis, cell-mediated calcification, and nanoparticles. J. Investig. Med. 2006, 54, 412–424.
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659.
- Jia, G.; Zhi, A.; Lai, P.F.H.; Wang, G.; Xia, Y.; Xiong, Z.; Zhang, H.; Che, N.; Ai, L. The oral microbiota—A mechanistic role for systemic diseases. Br. Dent. J. 2018, 224, 447–455.
- Wade, W.G. The oral microbiome in health and disease. Pharmacol. Res. 2013, 69, 137–143.
- Servin, A.L. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol. Rev. 2004, 28, 405–440.
- Vanhatalo, A.; Blackwell, J.R.; L’Heureux, J.E.; Williams, D.W.; Smith, A.; van der Giezen, M.; Winyard, P.G.; Kelly, J.; Jones, A.M. Nitrate-responsive oral microbiome modulates nitric oxide homeostasis and blood pressure in humans. Free Radic. Biol. Med. 2018, 124, 21–30.
- Lai, S.; Molfino, A.; Testorio, M.; Perrotta, A.M.; Currado, A.; Pintus, G.; Pietrucci, D.; Unida, V.; La Rocca, D.; Biocca, S.; et al. Effect of Low-Protein Diet and Inulin on Microbiota and Clinical Parameters in Patients with Chronic Kidney Disease. Nutrients 2019, 11, 3006.
- Esberg, A.; Haworth, S.; Hasslöf, P.; Lif Holgerson, P.; Johansson, I. Oral Microbiota Profile Associates with Sugar Intake and Taste Preference Genes. Nutrients 2020, 12, 681.
- Kato, I.; Vasquez, A.; Moyerbrailean, G.; Land, S.; Djuric, Z.; Sun, J.; Lin, H.-L.; Ram, J.L. Nutritional Correlates of Human Oral Microbiome. J. Am. Coll. Nutr. 2017, 36, 88–98.
- Lenhart, A.; Chey, W.D. A Systematic Review of the Effects of Polyols on Gastrointestinal Health and Irritable Bowel Syndrome. Adv. Nutr. 2017, 8, 587–596.
- Chen, Z.; Han, S.; Zhou, D.; Zhou, S.; Jia, G. Effects of oral exposure to titanium dioxide nanoparticles on gut microbiota and gut-associated metabolism in vivo. Nanoscale 2019, 11, 22398–22412.
- Marquis, R.E. Applied and ecological aspects of oxidative-stress damage to bacterial spores and to oral microbes. Sci. Prog. 2004, 87 Pt 3, 153–177.
- Dixon, S.J.; Stockwell, B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014, 10, 9–17.
- Yang, B.; Chen, Y.; Shi, J. Reactive Oxygen Species (ROS)-Based Nanomedicine. J. Chem. Rev. 2019, 119, 4881–4985.
- Sarniak, A.; Lipińska, J.; Tytman, K.; Lipińska, S. Endogenous mechanisms of reactive oxygen species (ROS) generation. Postepy Hig. Med. Dosw. 2016, 70, 1150–1165.
