Nanotechnology has been fostering excellent prospects in the development of enzymatic biosensors, since enzyme immobilization onto conductive nanostructures can improve characteristics that are crucial in biosensor transduction, such as surface-to-volume ratio, signal response, selectivity, sensitivity, conductivity, and biocatalytic activity, among others.
- biosensors
- nanomaterials
- enzyme immobilization
1. Nanomaterial-Based Biosensors
Nanobiosensors have become one of the main tools in the field of disease diagnosis, especially in the last decade [1][2][3]. Several industries have since incorporated nanomaterials in the production of biosensors that present refined sensitivity, selectivity, and specificity [4][5]. Industries such as the medical, food, electronic, enzymatic, and several others are the main ones responsible for their popularization. In this sense, the applications of several biosensors, according to the volume of publications across scientific databases, are discussed in the aforementioned sequence.
Four main types of nanomaterials can be highlighted due to their versatility: gold nanoparticles, graphene, carbon nanotubes, and photonic crystals [6][7][8]. Table 1 shows the nanomaterials most widely used in biosensing applications. Due to the growing demand for increasingly versatile and high value-added nanomaterials, universities, institutes, and laboratories have long been dedicating their research efforts to the improvement of nanobiosensor design technologies [9].
Table 1. Nanomaterial-based biosensors for different applications.
| Nature | Nanomaterial | Applications | Reference |
|---|---|---|---|
| Organic | Carbon nanotubes | Biomedical | [10] |
| Hybrid | GR-MWNTs/AuNP (1) | Biomedical | [11] |
| Hybrid | Au@PDMPAHCl (2) | Biomedical | [12] |
| Inorganic | Fe3O4 | Biomedical | [13] |
| Inorganic | Fe3O4-silica NPs (3) | Biomedical | [14] |
| Hybrid | CS/AuNPs-GNs (4) | Food and environmental | [15] |
| Inorganic | Ceria nanospheres | Food | [16] |
| Hybrid | MNP-PAMAM-PtNP/rGO-CMC (5) | Food | [17] |
| Organic | MnO2 modified MWCNTs * | Biomedical | [18] |
| Organic | Tobacco mosaic virus | Biomedical | [19] |
| Hybrid | Carbon ceramic | Biomedical | [20] |
| Organic | poly(l-aspartic acid)/MWCNT * | Food | [21] |
| Hybrid | Chi-Gr cry/PB/SPCE (6) | Uric acid detection | [22] |
| Hybrid | Titanim dioxide hybrid | Biomedical | [23] |
| Inorganic | Semiconductorquantum dots | Biomedical | [24] |
| Organic | Carbon black | Biomedical and environmental | [25] |
| Hybrid | Electrospun nanofibers | Biomedical | [26] |
(1) Gold nanoparticles prepared at graphene and multi-walled carbon nanotubes; (2) Core–shell gold nanoparticles stabilized with poly(3-dimethylammonium-1-propyne hydrochloride); (3) Magnetic nanoparticles–silica core shell; (4) Chitosan/gold nanoparticle−graphene nanosheets; (5) Poly(dopamine)-modified magnetic nanoparticles coated with four-generation ethylenediamine and core polyamidoamine G-4 dendrimers, all decorated with platinum nanoparticles on the surface of glassy carbon electrodes coated with graphene oxide and carboxymethylcellulose; (6) Porous cryogel platform of graphene-incorporated chitosan on top of a Prussian blue layer electrodeposited on a screen-printed carbon electrode. * Multi-walled carbon nanotubes (MWCNT).
The standard structure of biosensors is a classic system containing three fundamental elements: a bioreceptor, which is responsible for the selectivity of the device, a transducer that translates the physical or chemical change, leading to analyte recognition, and a signal processing unit [27]. The projection stage of the interaction between the biological and the transducer systems is fundamental for method design and possible test protocols for the nanosensor. In this phase, chemistry and computational biochemistry are essential elements to enable greater security to the method and generate considerable savings in reagents, human resources, and time.
Among medical applications, nanobiosensors are viable tools for detecting viruses or bacteria, which can cause potentially deadly diseases. Joshi et al. +developed a biocompatible, economically competitive, reduced graphene oxide (rGO) film. The film was obtained from shellac using a heat treatment (TrGO). After analysis, its relevant structural, chemical, and electrical properties were compared to similar films. After the heat treatment, the rGO (TrGO) film showed good crystallinity, low foil resistance, and high carbon content. From the TrGO, electrochemical immunosensors were produced without labels for the quantitative detection of the H1N1 influenza virus employing electrochemical impedance spectroscopy. These nanosensors exhibited high stability and reproducibility. The detection limits were of 26 and 33 plaque-forming units, respectively, in phosphate-buffered saline and diluted saliva. These low-cost TrGO-based sensors showed great potential as biosensors based on reliable and robust nanomaterials for general clinical applications [28].
Cancer diagnosis has become increasingly precise and selective, but errors are still common. Especially in this specific field, such errors must be minimized and procedures must reach high levels of accuracy. One of the most lethal types of cancer is ovary cancer, which is commonly diagnosed by biomarkers such as CA125, Mucin 1, HE4, and others, which may be present in the bloodstream [29]. However, there is a latent need for less expensive, more straightforward, and portable diagnostic tools for the timely diagnosis of ovarian as well as other types of cancer [30][31].
A possible solution is the employment of nanomaterial-based biosensors as tumor markers. In one of their pieces of research, Raghav et al. [32] presented an impedimetric immunosensor for free detection of the CA-125 marker. The CA125 immunosensor was produced using an electrode printed on a screen modified with Au–Ag NPs and functionalized with amine, which confers a larger surface area, besides providing the immobilization of antibodies in the correct orientation, that is, the formation of covalent amide bonds across the region antibody Fc. The functionalized immunosensor exhibited a linear response of up to 1–150 IU/mL (r2 = 0.994). The dynamic range of 1.0–1000 IU/mL reported in the literature makes it suitable for detecting CA125 without the need for any sample pretreatment, such as dilution, separation, or other adjacent processes. It is noteworthy that no significant interference was noticed from the chemical reagents or the serum proteins present in the blood. Considerable research is still being carried out to study the parameters that govern this linear response in more depth and whether they are dependent on nanoparticle size or on other particular properties of the nanomaterials used [32].
From this perspective, developing new sensing tools to identify tumor cells have evolved significantly due to the pressing need for less invasive and more accurate methods. These biosensors are mainly used to detect specific tumor biomarkers for different types of cancer. The literature already has a vast library of biomarkers but some methods are insufficiently selective. A classic example of a biomarker is the human mucin one protein (MUC1), which is the most common biomarker for monitoring metastatic breast tumors. In the study by Paimard et al. [33], the authors reported on an impedimetric assay for MUC1 identification using a gold nanocomposite with a nanofiber core shell on a multi-walled nanotube (MWCNT) that had been covalently modified with the MUC1 binding aptamer. MUC1 was detected by changing the surface resistance of the synthesized electrode. This nanobiosensor exhibited a high detection limit (LD) of 2.7 nM, good stability, and selectivity in the narrow region of 5–115 nM of MUC1. The assay was successfully applied to determine MUC1 in enriched serum samples and yielded satisfactory recoveries [33]. Despite the fact that this work is relatively recent, the laboratory tests were considered significant and indicated the possibility of their use on clinical trials from the first stage of the disease.
In the food industry, nanosensors are promising alternatives to solve several common problems in the sector [34]. The development of “smart tags” based on nanomaterials can evaluate product quality. These labels can interact with gases, microorganisms, and other by-products generated during food decomposition or adverse reactions with the packaging material. Many labels use a color change in the indicators present in the sensors as a means to alert consumers about the quality of a product [35][36]. This is in line with the concept of smart packaging (SP), which encompasses any type of container that provides specific functionality beyond their basic function of being a physical barrier between the food product and the surrounding environment [37]. Many packages are currently formulated with nanomaterials that provide improvements to their physical, chemical, and biochemical properties, imparting a longer shelf life for different products.
Practical applications in this area include the work of Faalnouri et al. [35], who developed surface plasma resonance (SPR) nanosensors to detect amoxicillin in milk samples using a molecular printing technique. Laboratory tests have shown that this nanosensor has a low detection limit and a high sensitivity and selectivity for identifying amoxicillin. As this study is very recent, the scientific community awaits for further tests before the product can be commercialized [35]. In addition, Xiang et al. [38] proposed the synthesis of a new nanosensor functionalized with black phosphorene (BP) in two-dimensional layers (2D). The tool was used to detect ochratoxin A (OTA) in grape juice and red wine samples. OTA is a toxic metabolite secreted by species of Aspergillus and Penicillium fungi, and it can cause severe nephrotoxic, immunotoxic, and carcinogenic effects [39]. In the literature, short reports on the electrochemical detection of this metabolite using a chemically modified electrode can be found. The BP-modified nanosensor exhibited an excellent linear electrochemical response to OTA in the concentration range of 0.3–10 μg/mL, with a detection limit of 0.18 μg/mL under ideal conditions. It was concluded that this electrochemical nanosensor showed good stability, superior anti-fouling property, and excellent sensitivity for OTA detection [38].
In summary, nanomaterials in the preparation and functionalization of biosensors have been proved to be a promising opportunity for solving several problems in the food sector, in agriculture, in applied medicine, in the enzyme preparation industry, among others, all of which are discussed in the next sections [3][40][41][42][43].
2. Enzymes
It has been observed that nanomaterial-based biosensors have elevated sensitivity and specificity [44]. These characteristics can be improved both by improving their conductivity and via the creation of a layer of nanomaterial on the surface of the transducer, onto where a wide variety of compounds can be immobilized, including biological materials [45][46]. It is important to highlight that materials with a high specific surface, such as NP, can increase the number of bioreceptor units within a reduced volume while still acting as a transduction element [25]. However, the variation in size, shape, and composition of nanoparticles, along with the general instability of their suspensions, can influence reaction performances and response times, potentially causing low reproducibility and negatively affecting their commercial interest [46].
Enzymatic biosensors, on the other hand, show less stability, lower signal intensity, higher cost, and in some cases, they require association with a mediating system [47]. However, they can be highly sensitive and selective [44]. Biomolecules can be immobilized and combined with other materials by surface modification through recombination or the introduction of binders [44]. Enzymatic biosensors are easy to use, sensible to very low concentrations, highly precise, and even when associated with NPS, they show great potential for miniaturization and real-time diagnostic capability, apart from requiring minimal sample preparation and promoting high yields [48].
Enzymes are biocatalysts that facilitate a plethora of reactions in biological systems, apart from being essential entities for sustaining life in several living organisms [49][50][51]. They are synthesized in animals, vegetables, fungi, and microorganisms [52][53], and their structure is composed of linear chains of amino acids that fold into complex, highly accurate tertiary structures with hydrophobic nuclei surrounded by hydrophilic layers [54][55]. The complexity of their three-dimensional structures provides the chemical environment necessary to catalyze a particular reaction mechanism, and they also present a defined region within their structure, called the active site, where catalysis takes place [56][57].
Several chemical-based transformation processes still employed in various industrial sectors show many disadvantages, from both a commercial and an environmental perspective, such as low yields, very high temperature, pressure, and acidity or alkalinity requirements, and high costs [58]. The environmental and economic impact imparted by using enzymes is greatly reduced by their potential of creation of more active variants than those found in nature [59]. Enzyme-assisted catalytic processes are highly efficient and advantageous due to the possibility of operation under mild conditions of reaction, high selectivity and specificity, lower environmental and physiological toxicity, and reduced costs and waste generation, all of which leads to more optimized production routes [60][61][62][63][64][65][66].
It is important to highlight that enzymes, when used in their free form, present limitations relating to their stability, efficiency, and specificity. Most of them are soluble in water, which makes it difficult to recover and reuse them [67]. Despite its excellent performance potential, industrial applications were made impossible due to these undesirable characteristics [68][69]. In this scenario, immobilization techniques stand out as alternatives to overcome these limitations, since they can offer better stability, increased activity and selectivity, excellent resistance, improvements in product separation and purification, and the possibility of enzyme reuse, rendering processes increasingly efficient [65][66][67][68][69][70][71][72][73][74][75][76][77][78][79].
Enzyme Immobilization
Enzymatic immobilization is based on the confinement of enzyme molecules on the surface of a reliable support that is different from that in which the substrate or products are present [75][76][77][78][79][80][81][82]. In contrast with their solubilized form, immobilized enzymes provide a large enzyme to substrate ratio, efficient digestion, and secure handling, in addition to showing more significant activity and the possibility of reuse for several cycles [70][83][84][85][86]. The stability of free enzymes is mainly dictated by its intrinsic structure, while the stability of their immobilized counterparts is highly dependent on several other factors, as shown in Figure 1. These factors are responsible for the stability of immobilized enzymes under different temperatures and storage conditions. The experimental variables can be expected to increase or decrease during the immobilization process [87][88][89][90].

Figure 1. Determining factors for the stability of immobilized enzymes.
According to the modes of interaction between enzymes and supports, immobilization methods can be classified into physical or chemical methods, as shown in the scheme in Figure 2 [53][91]. Physical methods show weaker monovalent interactions, such as hydrogen bonds, hydrophobic interactions, van der Waals forces, affinity or ionic bonding of enzymes to the support, or the mechanical containment of enzymes within the support [92][93][94][95]. In chemical methods, the formation of covalent bonds occurs from ether, thioether, amide, or carbamate bonds between the enzyme and the support material [96]. Each immobilization technique is applicable to a specific process, and choices are usually made based on the costs and sensitivity required [73][74][75][76][77][78][79][80][81][82][83][84][85][86][87][88][89][90][91][92][93][94][95][96][97].
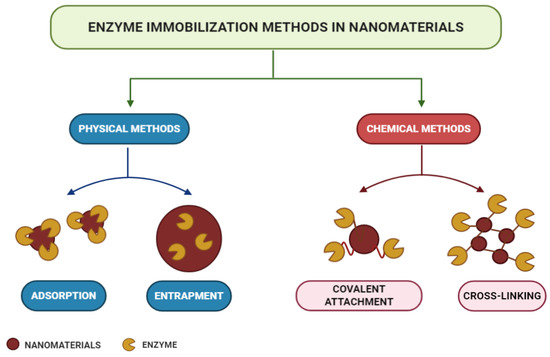
Figure 2. Enzyme immobilization methods.
Adsorption is a simple but efficient method of enzymatic immobilization [98]. Adsorption can be of physical, ionic, or affinity nature, with physical adsorption being the most commonly used method [53]. The latter is probably the fastest, most straightforward, and economical technique [99]. It is a reversible method in which enzymes are physically bound to the support material. It involves weak intermolecular interactions, such as Van der Waals forces, electrostatic forces, hydrophobic interactions, and hydrogen bonds [100][101]. With this technique, immobilized enzymes can be easily removed from the support, allowing its reuse in subsequent immobilization cycles [102]. In the study carried out by Lin et al. [103], FeO@C nanoparticles functionalized with amine were used as magnetic carriers for laccase immobilization by adsorption. As a result, the operational, pH, and storage stability of the immobilized laccase were significantly improved, and after 10 consecutive operations, it maintained its residual activity above 60%.
The trapping method is based on the incorporation of the enzyme into a polymeric network by covalent or non-covalent bonds that enable the passage of the substrate while retaining the enzyme [104][105]. In this method, enzymes are not bound to the support matrix, unlike in other methods [106]. Different strategies can be used for entrapment, such as the inclusion of the enzymes within a highly cross-linked polymeric matrix, their dissolution in a non-aqueous phase, or their separation through semipermeable microcapsules [80]. The entrapment to charged polymeric membranes appears as an alternative to the functionalization method, since this allows the immobilization of enzymes at high concentrations [102][103][104][105][106][107][108]. The technique presents several advantages, as it does not require many steps or extensive purification stages, apart from being fast and the reaction being able to run under moderate conditions [109][110]. However, difficulties regarding diffusion exist in the transport of substrate and product, when these have high molecular weight [111]. In the study by Xu et al. [112], an Aspergillus niger lipase was doubly immobilized via encapsulation in SiO2 nanoparticles in sol–gel powder prepared with tetramethoxysilane (TMOS) and methyltreimetotoxysilane (MTMS) catalyzed polymerization. The results indicated that under ideal conditions, the immobilized lipase retained 92% of its protein load and 94% of its total enzymatic activity, in addition to showing higher thermal and pH stability than its free form, confirming their great potential for industrial applications.
Enzyme crosslinking involves a bifunctional agent, usually glutaraldehyde [113], in the preparation of immobilized enzymes without the need for a carrier [114]. The advantages of this approach are high enzyme activity and low production costs due to the exclusion of additional expensive carriers [84]. The cross-linked enzyme aggregate (CLEA) method is an independent immobilization technique, which enables the production of recyclable and stable biocatalysts with high activity retention [115][116]. They can be applied to immobilize almost all enzymes and present many positive economic advantages and environmental benefits in industrial biocatalysis [110][117]. They are produced by the crosslinking of enzymatic aggregates resulting from the mixing of an aqueous protein solution with organic solvents, polymers, or anionic salts, or by crosslinking the bifunctional reagent, which generates a three-dimensional polymeric matrix [101][118]. Doraiswamy, Sarathi, and Pennathur [119] carried out a study using nanoparticles of magnetite (MGNP-CLEAs) and graphene oxide (GO-CLEAs), cross-linked with glutaraldehyde, as supports for the immobilization of the enzyme Staphylococcus spp through the CLEA method. As a result, MGNP-CLEAs have been shown to have better stability over a wide temperature and pH range, together with an increase in their reusability and storage stability.
Covalent bonding occurs through functional groups of enzymes that are not essential for its catalytic activity [120]. Nucleophilic functional groups of amino acid side chains, such as hydroxyl, amino, carboxylic, and thiol, are usually involved in the formation of these bonds [121]. Immobilization by covalent bonding is advantageous in that it forms strong bonds between the enzyme and the support, preventing enzyme leaching [122]. However, the amount of materials available commercially for covalent immobilization is low compared to immobilization by adsorption [123]. With the use of this technique, there is a slow release of enzymes and an improvement in its storage capacity and shelf life, so it is favorable for continuous applications at full scales [124]. Osuna et al. [125] studied the immobilization of Aspergillus niger lipases by covalent bonding on magnetic nanoparticles coated with chitosan (CMNP) and obtained by co-precipitation. The results showed high storage stability for 50 days in immobilized derivatives that maintained their initial activity above 80% after 15 hydrolytic cycles.
The characteristics of the support used for enzyme immobilization are fundamental factors to determine their performance [63][126]. An adequate support must present physical resistance to pressure and hydrophilicity, be readily available, and of low cost [127].
This entry is adapted from the peer-reviewed paper 10.3390/electrochem2010012
References
- Yang, W.; Ratinac, K.R.; Ringer, S.R.; Thordarson, P.; Gooding, J.J.; Braet, F. Carbon nanomaterials in biosensors: Should you use nanotubes or graphene. Angew. Chem. Int. Ed. 2010, 49, 2114–2138.
- Haes, A.J.; Van Duyne, R.P. A nanoscale optical biosensor: Sensitivity and selectivity of an approach based on the localized surface plasmon resonance spectroscopy of triangular silver nanoparticles. J. Am. Chem. Soc. 2002, 124, 10596–10604.
- Guo, L.J. Recent progress in nanoimprint technology and its applications. J. Phys. D Appl. Phys. 2004, 37, R123.
- Mokhtarzadeh, A.; Eivazzadeh-Keihan, R.; Pashazadeh, P.; Hejazi, M.; Gharaatifar, N.; Hasanzadeh, M.; Baradaran, B.; de la Guardia, M. Nanomaterial-based biosensors for detection of pathogenic virus. TrAC Trends Anal. Chem. 2017, 97, 445–457.
- Lan, L.; Yao, Y.; Ping, J.; Ying, Y. Recent advances in nanomaterial-based biosensors for antibiotics detection. Biosens. Bioelectron. 2017, 91, 504–514.
- Su, H.; Li, S.; Jin, Y.; Xian, Z.; Yang, D.; Zhou, W.; Mangaran, F.; Leung, F.; Sithamparanathan, G.; Kerman, K. Nanomaterial-based biosensors for biological detections. Adv. Health Care Technol. 2017, 3, 19–29.
- Ma, F.; Zhang, Q.; Zhang, C.Y. Nanomaterial-based biosensors for DNA methyltransferase assay. J. Mater. Chem. B 2020, 8, 3488–3501.
- Kuralay, F. Nanomaterials-Based Enzyme Biosensors for Electrochemical Applications: Recent Trends and Future Prospects. In New Developments in Nanosensors for Pharmaceutical Analysis; Elsevier: Amsterdam, The Netherlands, 2019; pp. 381–408. ISBN 9780128161449.
- Tiwari, J.N.; Vij, V.; Kemp, K.C.; Kim, K.S. Engineered carbon-nanomaterial-based electrochemical sensors for biomolecules. ACS Nano 2016, 10, 46–80.
- Kim, J.H.; Jun, S.A.; Kwon, Y.; Ha, S.; Sang, B.I.; Kim, J. Enhanced electrochemical sensitivity of enzyme precipitate coating (EPC)-based glucose oxidase biosensors with increased free CNT loadings. Bioelectrochemistry 2015, 101, 114–119.
- Devasenathipathy, R.; Mani, V.; Chen, S.M.; Huang, S.T.; Huang, T.T.; Lin, C.M.; Hwa, K.Y.; Chen, T.Y.; Chen, B.J. Glucose biosensor based on glucose oxidase immobilized at gold nanoparticles decorated graphene-carbon nanotubes. Enzym. Microb. Technol. 2015, 78, 40–45.
- Venditti, I.; Hassanein, T.F.; Fratoddi, I.; Fontana, L.; Battocchio, C.; Rinaldi, F.; Carafa, M.; Marianecci, C.; Diociaiuti, M.; Agostinelli, E.; et al. Bioconjugation of gold-polymer core-shell nanoparticles with bovine serum amine oxidase for biomedical applications. Colloids Surf. B Biointerfaces 2015, 134, 314–321.
- Hushiarian, R.; Yusof, N.A.; Abdullah, A.H.; Ahmad, S.A.A.; Dutse, S.W. Facilitating the indirect detection of genomic DNA in an electrochemical DNA biosensor using magnetic nanoparticles and DNA ligase. Anal. Chem. Res. 2015, 6, 17–25.
- Khaksarinejad, R.; Mohsenifar, A.; Rahmani-Cherati, T.; Karami, R.; Tabatabaei, M. An Organophosphorus Hydrolase-Based Biosensor for Direct Detection of Paraoxon Using Silica-Coated Magnetic Nanoparticles. Appl. Biochem. Biotechnol. 2015, 176, 359–371.
- Bao, J.; Hou, C.; Chen, M.; Li, J.; Huo, D.; Yang, M.; Luo, X.; Lei, Y. Plant Esterase-Chitosan/Gold Nanoparticles-Graphene Nanosheet Composite-Based Biosensor for the Ultrasensitive Detection of Organophosphate Pesticides. J. Agric. Food Chem. 2015, 63, 10319–10326.
- Gumpu, M.B.; Nesakumar, N.; Sethuraman, S.; Krishnan, U.M.; Rayappan, J.B.B. Determination of Putrescine in Tiger Prawn Using an Amperometric Biosensor Based on Immobilization of Diamine Oxidase onto Ceria Nanospheres. Food Bioprocess. Technol. 2016, 9, 717–724.
- Borisova, B.; Sánchez, A.; Jiménez-Falcao, S.; Martín, M.; Salazar, P.; Parrado, C.; Pingarrón, J.M.; Villalonga, R. Reduced graphene oxide-carboxymethylcellulose layered with platinum nanoparticles/PAMAM dendrimer/magnetic nanoparticles hybrids. Application to the preparation of enzyme electrochemical biosensors. Sens. Actuators B Chem. 2016, 232, 84–90.
- Aigner, M.; Kalcher, K.; Macheroux, P.; Lienhart, W.D.; Wallner, S.; Edmondson, D.; Ortner, A. Determination of Total Monoamines in Rat Brain via Nanotubes Based Human Monoamine Oxidase B Biosensor. Electroanalysis 2017, 29, 367–373.
- Bäcker, M.; Koch, C.; Eiben, S.; Geiger, F.; Eber, F.; Gliemann, H.; Poghossian, A.; Wege, C.; Schöning, M.J. Tobacco mosaic virus as enzyme nanocarrier for electrochemical biosensors. Sens. Actuators B Chem. 2017, 238, 716–722.
- Caldas, E.M.; Novatzky, D.; Deon, M.; de Menezes, E.W.; Hertz, P.F.; Costa, T.M.H.; Arenas, L.T.; Benvenutti, E.V. Pore size effect in the amount of immobilized enzyme for manufacturing carbon ceramic biosensor. Microporous Mesoporous Mater. 2017, 247, 95–102.
- Yazdanparast, S.; Benvidi, A.; Abbasi, S.; Rezaeinasab, M. Enzyme-based ultrasensitive electrochemical biosensor using poly(L-aspartic acid)/MWCNT bio-nanocomposite for xanthine detection: A meat freshness marker. Microchem. J. 2019, 149.
- Jirakunakorn, R.; Khumngern, S.; Choosang, J.; Thavarungkul, P.; Kanatharana, P.; Numnuam, A. Uric acid enzyme biosensor based on a screen-printed electrode coated with Prussian blue and modified with chitosan-graphene composite cryogel. Microchem. J. 2020, 154.
- Kadian, S.; Arya, B.D.; Kumar, S.; Sharma, S.N.; Chauhan, R.P.; Srivastava, A.; Chandra, P.; Singh, S.P. Synthesis and Application of PHT-TiO 2 Nanohybrid for Amperometric Glucose Detection in Human Saliva Sample. Electroanalysis 2018, 30, 2793–2802.
- Grinyte, R.; Barroso, J.; Möller, M.; Saa, L.; Pavlov, V. Microbead QD-ELISA: Microbead ELISA Using Biocatalytic Formation of Quantum Dots for Ultra High Sensitive Optical and Electrochemical Detection. ACS Appl. Mater. Interfaces 2016, 8, 29252–29260.
- Silva, T.A.; Moraes, F.C.; Janegitz, B.C.; Fatibello-Filho, O. Electrochemical Biosensors Based on Nanostructured Carbon Black: A Review. J. Nanomater. 2017, 2017, 1–14.
- Senthamizhan, A.; Balusamy, B.; Uyar, T. Glucose sensors based on electrospun nanofibers: A review. Anal. Bioanal. Chem. 2016, 408, 1285–1306.
- Cinti, S.; Arduini, F.; Moscone, D.; Palleschi, G.; Gonzalez-Macia, L.; Killard, A.J. Cholesterol biosensor based on inkjet-printed Prussian blue nanoparticle-modified screen-printed electrodes. Sens. Actuators B Chem. 2015, 221, 187–190.
- Joshi, S.R.; Sharma, A.; Kim, G.H.; Jang, J. Low cost synthesis of reduced graphene oxide using biopolymer for influenza virus sensor. Mater. Sci. Eng. C 2020, 108, 110465.
- Sha, R.; Badhulika, S. Recent advancements in fabrication of nanomaterial based biosensors for diagnosis of ovarian cancer: A comprehensive review. Microchim. Acta 2020, 187.
- Klein, T.; Wang, W.; Yu, L.; Wu, K.; Boylan, K.L.M.; Vogel, R.I.; Skubitz, A.P.N.; Wang, J.P. Development of a multiplexed giant magnetoresistive biosensor array prototype to quantify ovarian cancer biomarkers. Biosens. Bioelectron. 2019, 126, 301–307.
- Samadi Pakchin, P.; Ghanbari, H.; Saber, R.; Omidi, Y. Electrochemical immunosensor based on chitosan-gold nanoparticle/carbon nanotube as a platform and lactate oxidase as a label for detection of CA125 oncomarker. Biosens. Bioelectron. 2018, 122, 68–74.
- Raghav, R.; Srivastava, S. Core-shell gold-silver nanoparticles based impedimetric immunosensor for cancer antigen CA125. Sens. Actuators B Chem. 2015, 220, 557–564.
- Paimard, G.; Shahlaei, M.; Moradipour, P.; Karamali, V.; Arkan, E. Impedimetric aptamer based determination of the tumor marker MUC1 by using electrospun core-shell nanofibers. Microchim. Acta 2020, 187.
- Fuertes, G.; Soto, I.; Vargas, M.; Valencia, A.; Sabattin, J.; Carrasco, R. Nanosensors for a monitoring system in intelligent and active packaging. J. Sens. 2016.
- Faalnouri, S.; Çimen, D.; Bereli, N.; Denizli, A. Surface Plasmon Resonance Nanosensors for Detecting Amoxicillin in Milk Samples with Amoxicillin Imprinted Poly(hydroxyethyl methacrylate-N-methacryloyl-(L)- glutamic acid). ChemistrySelect 2020, 5, 4761–4769.
- Wang, H.; Li, B.; Ding, F.; Ma, T. Improvement of properties of smart ink via chitin nanofiber and application as freshness indicator. Prog. Org. Coat. 2020, 149, 105921.
- Huang, S.; Zhang, Q.; Yao, H.; Wang, W.; Zhang, J.R.; Zhu, J.J. Quantitative Detection and Imaging of Multiple Biological Molecules in Living Cells for Cell Screening. ACS Sens. 2020, 5, 1149–1157.
- Xiang, Y.; Camarada, M.B.; Wen, Y.; Wu, H.; Chen, J.; Li, M.; Liao, X. Simple voltammetric analyses of ochratoxin A in food samples using highly-stable and anti-fouling black phosphorene nanosensor. Electrochim. Acta 2018, 282, 490–498.
- Gooch, J.; Daniel, B.; Parkin, M.; Frascione, N. Developing aptasensors for forensic analysis. TrAC Trends Anal. Chem. 2017, 94, 150–160.
- Tasis, D.; Tagmatarchis, N.; Bianco, A.; Prato, M. Chemistry of carbon nanotubes. Chem. Rev. 2006, 106, 1105–1136.
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations and biological applications. Chem. Rev. 2008, 108, 2064–2110.
- Katz, E.; Willner, I. Integrated nanoparticle-biomolecule hybrid systems: Synthesis, properties, and applications. Angew. Chem. Int. Ed. 2004, 43, 6042–6108.
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of graphene: Covalent and non-covalent approaches, derivatives and applications. Chem. Rev. 2012, 112, 6156–6214.
- Kozitsina, A.; Svalova, T.; Malysheva, N.; Okhokhonin, A.; Vidrevich, M.; Brainina, K. Sensors Based on Bio and Biomimetic Receptors in Medical Diagnostic, Environment, and Food Analysis. Biosensors 2018, 8, 35.
- López-Gallego, F.; Jackson, E.; Betancor, L. Heterogeneous Systems Biocatalysis: The Path to the Fabrication of Self-Sufficient Artificial Metabolic Cells. Chem. A Eur. J. 2017, 23, 17841–17849.
- Tian, K.; Prestgard, M.; Tiwari, A. A review of recent advances in nonenzymatic glucose sensors. Mater. Sci. Eng. C 2014, 41, 100–118.
- Soleymani, L.; Li, F. Mechanistic Challenges and Advantages of Biosensor Miniaturization into the Nanoscale. ACS Sens. 2017, 2, 458–467.
- Fathi, M.; Karim, M.; Khoigani, S.R.; Mosayebi, V. Use of Nanotechnology for Immobilization and Entrapment of Food Applicable Enzymes. In Bioactive Molecules in Food; Springer: Cham, Switzerland, 2019; pp. 2037–2061.
- Singhania, R.R.; Patel, A.K.; Thomas, L.; Goswami, M.; Giri, B.S.; Pandey, A. Industrial Enzymes; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 9780444634535.
- Porter, J.L.; Rusli, R.A.; Ollis, D.L. Directed Evolution of Enzymes for Industrial Biocatalysis. ChemBioChem 2016, 17, 197–203.
- Conix, S. Enzyme classification and the entanglement of values and epistemic standards. Stud. Hist. Philos. Sci. Part. A 2020.
- Bhalla, T.C.; Kumar, V.; Kumar, V.; Thakur, N. Savitri Nitrile Metabolizing Enzymes in Biocatalysis and Biotransformation. Appl. Biochem. Biotechnol. 2018, 185, 925–946.
- Liu, D.M.; Chen, J.; Shi, Y.P. Advances on methods and easy separated support materials for enzymes immobilization. TrAC Trends Anal. Chem. 2018, 102, 332–342.
- Kuah, E.; Toh, S.; Yee, J.; Ma, Q.; Gao, Z. Enzyme Mimics: Advances and Applications. Chem. A Eur. J. 2016, 22, 8404–8430.
- Drout, R.J.; Robison, L.; Farha, O.K. Catalytic applications of enzymes encapsulated in metal–organic frameworks. Coord. Chem. Rev. 2019, 381, 151–160.
- Garske, A.L.; Kapp, G.; Mcauliffe, J.C. Handbook of Industrial Chemistry and Biotechnology; Springer: Cham, Switzerland, 2017; ISBN 9783319522876.
- Arsalan, A.; Younus, H. Enzymes and nanoparticles: Modulation of enzymatic activity via nanoparticles. Int. J. Biol. Macromol. 2018, 118, 1833–1847.
- Prasad, S.; Roy, I. Converting Enzymes into Tools of Industrial Importance. Recent Pat. Biotechnol. 2017, 12, 33–56.
- Hughes, G.; Lewis, J.C. Introduction: Biocatalysis in Industry. Chem. Rev. 2018, 118, 1–3.
- Singh, R.; Kumar, M.; Mittal, A.; Mehta, P.K. Microbial enzymes: Industrial progress in 21st century. 3 Biotech 2016, 6.
- Chapman, J.; Ismail, A.E.; Dinu, C.Z. Industrial applications of enzymes: Recent advances, techniques, and outlooks. Catalysts 2018, 8, 238.
- Sun, H.; Zhou, Y.; Ren, J.; Qu, X. Carbon Nanozymes: Enzymatic Properties, Catalytic Mechanism, and Applications. Angew. Chem. Int. Ed. 2018, 57, 9224–9237.
- Zdarta, J.; Meyer, A.; Jesionowski, T.; Pinelo, M. A General Overview of Support Materials for Enzyme Immobilization: Characteristics, Properties, Practical Utility. Catalysts 2018, 8, 92.
- Fernandes, K.V.; Papadaki, A.; da Silva, J.A.C.; Fernandez-Lafuente, R.; Koutinas, A.A.; Freire, D.M.G. Enzymatic esterification of palm fatty-acid distillate for the production of polyol esters with biolubricant properties. Ind. Crop. Prod. 2018, 116, 90–96.
- Monteiro, R.R.C.; Neto, D.M.A.; Fechine, P.B.A.; Lopes, A.A.S.; Gonçalves, L.R.B.; dos Santos, J.C.S.; de Souza, M.C.M.; Fernandez‐Lafuente, R. Ethyl Butyrate Synthesis Catalyzed by Lipases A and B from Candida antarctica Immobilized onto Magnetic Nanoparticles. Improvement of Biocatalysts’ Performance under Ultrasonic Irradiation. Int. J. Mol. Sci. 2019, 20, 5807.
- Cavalcante, F.T.T.; Neto, F.S.; Rafael de Aguiar Falcão, I.; Erick da Silva Souza, J.; de Moura Junior, L.S.; da Silva Sousa, P.; Rocha, T.G.; de Sousa, I.G.; de Lima Gomes, P.H.; de Souza, M.C.M.; et al. Opportunities for improving biodiesel production via lipase catalysis. Fuel 2020, 288, 119577.
- Rueda, N.; dos Santos, J.C.S.; Ortiz, C.; Torres, R.; Barbosa, O.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R. Chemical Modification in the Design of Immobilized Enzyme Biocatalysts: Drawbacks and Opportunities. Chem. Rec. 2016, 16, 1436–1455.
- Choi, J.M.; Han, S.S.; Kim, H.S. Industrial applications of enzyme biocatalysis: Current status and future aspects. Biotechnol. Adv. 2015, 33, 1443–1454.
- Da Fonseca, A.M.; Colares, R.P.; de Oliveira, M.M.; de Souza, M.C.M.; de Castro Monteiro, R.R.; dos Santos Araújo, R.; Amorim, A.V.; dos Santos, J.C.S.; Alcócer, J.C.A.; de Oliveira Pinto, O.R. Enzymatic Biocatalyst using enzymes from Pineapple (Ananas comosus) Peel Immobilized in Hydrogel Beads. Revista Eletrônica em Gestão Educação e Tecnologia Ambiental 2019, 23, 32.
- Da Moreira, K.S.; de Oliveira, A.L.B.; Lourembergue, S.d.M., Jr.; Monteiro, R.R.C.; da Rocha, T.N.; Menezes, F.L.; Fechine, L.M.U.D.; Denardin, J.C.; Michea, S.; Freire, R.M.; et al. Lipase from Rhizomucor miehei Immobilized on Magnetic Nanoparticles: Performance in Fatty Acid Ethyl Ester (FAEE) Optimized Production by the Taguchi Method. Front. Bioeng. Biotechnol. 2020, 8, 1–17.
- Bezerra, R.M.; Monteiro, R.R.C.; Neto, D.M.A.; da Silva, F.F.M.; de Paula, R.C.M.; de Lemos, T.L.G.; Fechine, P.B.A.; Correa, M.A.; Bohn, F.; Gonçalves, L.R.B.; et al. A new heterofunctional support for enzyme immobilization: PEI functionalized Fe3O4 MNPs activated with divinyl sulfone. Application in the immobilization of lipase from Thermomyces lanuginosus. Enzym. Microb. Technol. 2020, 138, 109560.
- De Souza, T.C.; de Sousa Fonseca, T.; de Sousa Silva, J.; Lima, P.J.M.; Neto, C.A.C.G.; Monteiro, R.R.C.; Rocha, M.V.P.; de Mattos, M.C.; dos Santos, J.C.S.; Gonçalves, L.R.B. Modulation of lipase B from Candida antarctica properties via covalent immobilization on eco-friendly support for enzymatic kinetic resolution of rac-indanyl acetate. Bioprocess. Biosyst. Eng. 2020, 43, 2253–2268.
- Eş, I.; Vieira, J.D.G.; Amaral, A.C. Principles, techniques, and applications of biocatalyst immobilization for industrial application. Appl. Microbiol. Biotechnol. 2015, 99, 2065–2082.
- Rueda, N.; Dos Santos, C.S.; Rodriguez, M.D.; Albuquerque, T.L.; Barbosa, O.; Torres, R.; Ortiz, C.; Fernandez-Lafuente, R. Reversible immobilization of lipases on octyl-glutamic agarose beads: A mixed adsorption that reinforces enzyme immobilization. J. Mol. Catal. B Enzym. 2016, 128, 10–18.
- Pinheiro, B.B.; Rios, N.S.; Rodríguez Aguado, E.; Fernandez-Lafuente, R.; Freire, T.M.; Fechine, P.B.A.; dos Santos, J.C.S.; Gonçalves, L.R.B. Chitosan activated with divinyl sulfone: A new heterofunctional support for enzyme immobilization. Application in the immobilization of lipase B from Candida antarctica. Int. J. Biol. Macromol. 2019, 130, 798–809.
- Rios, N.S.; Neto, D.M.A.; dos Santos, J.C.S.; Fechine, P.B.A.; Fernández-Lafuente, R.; Gonçalves, L.R.B. Comparison of the immobilization of lipase from Pseudomonas fluorescens on divinylsulfone or p-benzoquinone activated support. Int. J. Biol. Macromol. 2019, 134, 936–945.
- Rios, N.S.; Morais, E.G.; dos Santos Galvão, W.; Andrade Neto, D.M.; dos Santos, J.C.S.; Bohn, F.; Correa, M.A.; Fechine, P.B.A.; Fernandez-Lafuente, R.; Gonçalves, L.R.B. Further stabilization of lipase from Pseudomonas fluorescens immobilized on octyl coated nanoparticles via chemical modification with bifunctional agents. Int. J. Biol. Macromol. 2019, 141, 313–324.
- Pinheiro, M.P.; Monteiro, R.R.C.; Silva, F.F.M.; Lemos, T.L.G.; Fernandez-Lafuente, R.; Gonçalves, L.R.B.; dos Santos, J.C.S. Modulation of Lecitase properties via immobilization on differently activated Immobead-350: Stabilization and inversion of enantiospecificity. Process. Biochem. 2019, 87, 128–137.
- Moreira, K.S.; Moura, L.S.; Monteiro, R.R.C.; de Oliveira, A.L.B.; Valle, C.P.; Freire, T.M.; Fechine, P.B.A.; de Souza, M.C.M.; Fernandez-Lorente, G.; Guisan, J.M.; et al. Optimization of the Production of Enzymatic Biodiesel from Residual Babassu Oil (Orbignya sp.) via RSM. Catalysts 2020, 10, 414.
- Sirisha, V.L.; Jain, A.; Jain, A. Enzyme Immobilization: An Overview on Methods, Support Material, and Applications of Immobilized Enzymes, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 79.
- de Oliveira, U.M.F.; Lima de Matos, L.J.B.; de Souza, M.C.M.; Pinheiro, B.B.; dos Santos, J.C.S.; Gonçalves, L.R.B. Efficient biotechnological synthesis of flavor esters using a low-cost biocatalyst with immobilized Rhizomucor miehei lipase. Mol. Biol. Rep. 2019, 46, 597–608.
- dos Santos, J.C.S.; Garcia-Galan, C.; Rodrigues, R.C.; de Sant’Ana, H.B.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Stabilizing hyperactivated lecitase structures through physical treatment with ionic polymers. Process. Biochem. 2014, 49, 1511–1515.
- Mehta, J.; Bhardwaj, N.; Bhardwaj, S.K.; Kim, K.H.; Deep, A. Recent advances in enzyme immobilization techniques: Metal-organic frameworks as novel substrates. Coord. Chem. Rev. 2016, 322, 30–40.
- Vaghari, H.; Jafarizadeh-Malmiri, H.; Mohammadlou, M.; Berenjian, A.; Anarjan, N.; Jafari, N.; Nasiri, S. Application of magnetic nanoparticles in smart enzyme immobilization. Biotechnol. Lett. 2016, 38, 223–233.
- Boudrant, J.; Woodley, J.M.; Fernandez-Lafuente, R. Parameters necessary to define an immobilized enzyme preparation. Process. Biochem. 2020, 90, 66–80.
- Galvão, W.S.; Pinheiro, B.B.; Golçalves, L.R.B.; de Mattos, M.C.; Fonseca, T.S.; Regis, T.; Zampieri, D.; dos Santos, J.C.S.; Costa, L.S.; Correa, M.A.; et al. Novel nanohybrid biocatalyst: Application in the kinetic resolution of secondary alcohols. J. Mater. Sci. 2018, 53, 14121–14137.
- Meryam Sardar, R.A. Enzyme Immobilization: An Overview on Nanoparticles as Immobilization Matrix. Biochem. Anal. Biochem. 2015, 04.
- de Oliveira, U.M.F.; Lima de Matos, L.J.B.; de Souza, M.C.M.; Pinheiro, B.B.; dos Santos, J.C.S.; Gonçalves, L.R.B. Effect of the Presence of Surfactants and Immobilization Conditions on Catalysts’ Properties of Rhizomucor miehei Lipase onto Chitosan. Appl. Biochem. Biotechnol. 2018, 184, 1263–1285.
- Pinheiro, M.P.; Rios, N.S.; de Fonseca, T.S.; de Bezerra, F.A.; Rodríguez-Castellón, E.; Fernandez-Lafuente, R.; Carlos de Mattos, M.; dos Santos, J.C.S.; Gonçalves, L.R.B. Kinetic resolution of drug intermediates catalyzed by lipase B from Candida antarctica immobilized on immobead-350. Biotechnol. Prog. 2018, 34, 878–889.
- Melo, A.D.Q.; Silva, F.F.M.; Dos Santos, J.C.S.; Fernández-Lafuente, R.; Lemos, T.L.G.; Dias Filho, F.A. Synthesis of benzyl acetate catalyzed by lipase immobilized in nontoxic chitosan-polyphosphate beads. Molecules 2017, 22, 2165.
- Poorakbar, E.; Shafiee, A.; Saboury, A.A.; Rad, B.L.; Khoshnevisan, K.; Ma’mani, L.; Derakhshankhah, H.; Ganjali, M.R.; Hosseini, M. Synthesis of magnetic gold mesoporous silica nanoparticles core shell for cellulase enzyme immobilization: Improvement of enzymatic activity and thermal stability. Process. Biochem. 2018, 71, 92–100.
- Lima, G.V.; da Silva, M.R.; de Sousa Fonseca, T.; de Lima, L.B.; de Oliveira, M.d.C.F.; de Lemos, T.L.G.; Zampieri, D.; dos Santos, J.C.S.; Rios, N.S.; Gonçalves, L.R.B.; et al. Chemoenzymatic synthesis of (S)-Pindolol using lipases. Appl. Catal. A Gen. 2017, 546, 7–14.
- Dos Santos, J.C.S.; Bonazza, H.L.; de Matos, L.J.B.L.; Carneiro, E.A.; Barbosa, O.; Fernandez-Lafuente, R.; Gonçalves, L.R.B.; de Sant’ Ana, H.B.; Santiago-Aguiar, R.S. Immobilization of CALB on activated chitosan: Application to enzymatic synthesis in supercritical and near-critical carbon dioxide. Biotechnol. Rep. 2017, 14, 16–26.
- Rios, N.S.; Pinheiro, M.P.; dos Santos, J.C.S.; Fonseca, T.d.S.; Lima, L.D.; de Mattos, M.C.; Freire, D.M.G.; da Silva, I.J.; Rodríguez-Aguado, E.; Gonçalves, L.R.B. Strategies of covalent immobilization of a recombinant Candida antarctica lipase B on pore-expanded SBA-15 and its application in the kinetic resolution of (R,S)-Phenylethyl acetate. J. Mol. Catal. B Enzym. 2016, 133, 246–258.
- Bezerra, R.M.; Neto, D.M.A.; Galvão, W.S.; Rios, N.S.; Carvalho, A.C.L.d.M.; Correa, M.A.; Bohn, F.; Fernandez-Lafuente, R.; Fechine, P.B.A.; de Mattos, M.C.; et al. Design of a lipase-nano particle biocatalysts and its use in the kinetic resolution of medicament precursors. Biochem. Eng. J. 2017, 125, 104–115.
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220.
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. Developments in support materials for immobilization of oxidoreductases: A comprehensive review. Adv. Colloid Interface Sci. 2018, 258, 1–20.
- Virgen-Ortíz, J.J.; Dos Santos, J.C.S.; Berenguer-Murcia, Á.; Barbosa, O.; Rodrigues, R.C.; Fernandez-Lafuente, R. Polyethylenimine: A very useful ionic polymer in the design of immobilized enzyme biocatalysts. J. Mater. Chem. B 2017, 5, 7461–7490.
- Nadar, S.S.; Rathod, V.K. Magnetic-metal organic framework (magnetic-MOF): A novel platform for enzyme immobilization and nanozyme applications. Int. J. Biol. Macromol. 2018, 120, 2293–2302.
- Santos, M.P.F.; Brito, M.J.P.; Junior, E.C.S.; Bonomo, R.C.F.; Veloso, C.M. Pepsin immobilization on biochar by adsorption and covalent binding, and its application for hydrolysis of bovine casein. J. Chem. Technol. Biotechnol. 2019, 94, 1982–1990.
- Reis, C.L.B.; de Sousa, E.Y.A.; de França Serpa, J.; Oliveira, R.C.; Dos Santos, J.C.S. Design of immobilized enzyme biocatalysts: Drawbacks and opportunities. Quim. Nova 2019, 42, 768–783.
- Fopase, R.; Paramasivam, S.; Kale, P.; Paramasivan, B. Strategies, challenges and opportunities of enzyme immobilization on porous silicon for biosensing applications. J. Environ. Chem. Eng. 2020, 8, 104266.
- Lin, J.; Wen, Q.; Chen, S.; Le, X.; Zhou, X.; Huang, L. Synthesis of amine-functionalized nanoparticles for laccase immobilization. Int. J. Biol. Macromol. 2017, 96, 377–383.
- Sneha, H.P.; Beulah, K.C.; Murthy, P.S. Enzyme Immobilization Methods and Applications in the Food Industry; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128132807.
- Bashir, N.; Sood, M.; Bandral, J.D. Enzyme immobilization and its applications in food processing: A review. Int. J. Chem. Stud. 2020, 8, 254–261.
- Thangaraj, B.; Solomon, P.R. Immobilization of Lipases—A Review. Part I: Enzyme Immobilization. ChemBioEng Rev. 2019, 6, 157–166.
- Strakosas, X.; Huerta, M.; Donahue, M.J.; Hama, A.; Pappa, A.M.; Ferro, M.; Ramuz, M.; Rivnay, J.; Owens, R.M. Catalytically enhanced organic transistors for in vitro toxicology monitoring through hydrogel entrapment of enzymes. J. Appl. Polym. Sci. 2017, 134, 1–7.
- Grollmisch, A.; Kragl, U.; Großeheilmann, J. Enzyme Immobilization in Polymerized Ionic Liquids-based Hydrogels for Active and Reusable Biocatalysts. SynOpen 2018, 02, 0192–0199.
- Adnan, M.; Li, K.; Xu, L.; Yan, Y. X-shaped zif-8 for immobilization Rhizomucor miehei lipase via encapsulation and its application toward biodiesel production. Catalysts 2018, 8, 96.
- Cacicedo, M.L.; Manzo, R.M.; Municoy, S.; Bonazza, H.L.; Islan, G.A.; Desimone, M.; Bellino, M.; Mammarella, E.J.; Castro, G.R. Immobilized Enzymes and Their Applications. In Advances in Enzyme Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 169–200. ISBN 9780444641144.
- Muguruma, H. Biosensors: Enzyme Immobilization Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780128098943.
- Xu, L.; Ke, C.; Huang, Y.; Yan, Y. Immobilized Aspergillus niger lipase with SiO2 nanoparticles in sol-gel materials. Catalysts 2016, 6, 149.
- Chung, Y.; Christwardana, M.; Tannia, D.C.; Kim, K.J.; Kwon, Y. Biocatalyst including porous enzyme cluster composite immobilized by two-step crosslinking and its utilization as enzymatic biofuel cell. J. Power Sources 2017, 360, 172–179.
- Thompson, M.P.; Peñafiel, I.; Cosgrove, S.C.; Turner, N.J. Biocatalysis Using Immobilized Enzymes in Continuous Flow for the Synthesis of Fine Chemicals. Org. Process. Res. Dev. 2019, 23, 9–18.
- Velasco-Lozano, S.; López-Gallego, F.; Mateos-Díaz, J.C.; Favela-Torres, E. Cross-linked enzyme aggregates (CLEA) in enzyme improvement—A review. Biocatalysis 2016, 1, 166–177.
- Voběrková, S.; Solčány, V.; Vršanská, M.; Adam, V. Immobilization of ligninolytic enzymes from white-rot fungi in cross-linked aggregates. Chemosphere 2018, 202, 694–707.
- Wang, S.; Zheng, D.; Yin, L.; Wang, F. Preparation, activity and structure of cross-linked enzyme aggregates (CLEAs) with nanoparticle. Enzym. Microb. Technol. 2017, 107, 22–31.
- Tavernini, L.; Ottone, C.; Illanes, A.; Wilson, L. Entrapment of enzyme aggregates in chitosan beads for aroma release in white wines. Int. J. Biol. Macromol. 2020, 154, 1082–1090.
- Doraiswamy, N.; Sarathi, M.; Pennathur, G. Cross-linked esterase aggregates (CLEAs) using nanoparticles as immobilization matrix. Prep. Biochem. Biotechnol. 2019, 49, 270–278.
- Bilal, M.; Asgher, M.; Cheng, H.; Yan, Y.; Iqbal, H.M.N. Multi-point enzyme immobilization, surface chemistry, and novel platforms: A paradigm shift in biocatalyst design. Crit. Rev. Biotechnol. 2019, 39, 202–219.
- Asal, M.; Özen, Ö.; Şahinler, M.; Baysal, H.T.; Polatoğlu, İ. An overview of biomolecules, immobilization methods and support materials of biosensors. Sens. Rev. 2019, 39, 377–386.
- Mehrasbi, M.R.; Mohammadi, J.; Peyda, M.; Mohammadi, M. Covalent immobilization of Candida antarctica lipase on core-shell magnetic nanoparticles for production of biodiesel from waste cooking oil. Renew. Energy 2017, 101, 593–602.
- Carvalho, Y.; Almeida, J.M.A.R.; Romano, P.N.; Farrance, K.; Demma Carà, P.; Pereira, N.; Lopez-Sanchez, J.A.; Sousa-Aguiar, E.F. Nanosilicalites as Support for β-Glucosidases Covalent Immobilization. Appl. Biochem. Biotechnol. 2017, 182, 1619–1629.
- Lonappan, L.; Liu, Y.; Rouissi, T.; Pourcel, F.; Brar, S.K.; Verma, M.; Surampalli, R.Y. Covalent immobilization of laccase on citric acid functionalized micro-biochars derived from different feedstock and removal of diclofenac. Chem. Eng. J. 2018, 351, 985–994.
- Osuna, Y.; Sandoval, J.; Saade, H.; López, R.G.; Martinez, J.L.; Colunga, E.M.; de la Cruz, G.; Segura, E.P.; Arévalo, F.J.; Zon, M.A.; et al. Immobilization of Aspergillus niger lipase on chitosan-coated magnetic nanoparticles using two covalent-binding methods. Bioprocess. Biosyst. Eng. 2015, 38.
- Dos Santos, J.C.S.; Rueda, N.; Barbosa, O.; Fernández-Sánchez, J.F.; Medina-Castillo, A.L.; Ramón-Márquez, T.; Arias-Martos, M.C.; Millán-Linares, M.C.; Pedroche, J.; Yust, M.D.M.; et al. Characterization of supports activated with divinyl sulfone as a tool to immobilize and stabilize enzymes via multipoint covalent attachment. Application to chymotrypsin. RSC Adv. 2015, 5, 20639–20649.
- Dos Santos, J.C.S.; Barbosa, O.; Ortiz, C.; Berenguer-Murcia, A.; Rodrigues, R.C.; Fernandez-Lafuente, R. Importance of the Support Properties for Immobilization or Purification of Enzymes. ChemCatChem 2015, 7, 2413–2432.
