Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Computer Science, Information Systems
Computer-navigated surgery has been used in neurosurgery since the 1980s, where improved accuracy in resections for cancer was achieved by mapping brain tumours pre-operatively and using this to plan surgical resection. It is only at the turn of the 21st century that it has been adopted within the orthopaedic community, principally in the field of spinal surgery. A technology that provides real-time feedback within a field that has a small margin for error has an obvious home in the specialty of orthopaedic oncology.
Computer navigation encompasses all techniques using computing to augment surgical procedures. The two main types of navigation currently used in orthopaedic surgery are “image-based” and “patient-specific instrumentation and reconstruction”.
- Computer Navigation
1. Patient-Specific Instrumentation and Reconstruction
In recent years, CATS has been developed along with 3D printing technology, which represents the possibility to personalize reconstruction with custom-made prostheses [18,19,20] and improve accuracy in bone cutting using patient-specific instruments (PSI) [21,22].
Manufacturing of 3D-printed PSI jigs and custom-made prostheses is based upon the principle of rapid prototyping (RP). RP is a process that directly produces a physical object with a defined structure and shape on the basis of virtual/mathematical model data. Rapid prototyping was first used in the late 1980s and was developed to apply the precision and functionality of computer-assisted design (CAD) to manufacturing. With this technology, a prototype could be quickly produced and accurately represents the engineers draft [23,24]. Electron beam melting (EMB) is the optimal technology to fabricate metallic components with complex shapes and porous structures and has a pivotal role either in the PSI technology or prosthesis manufacturing. EBM involves the generation of an electron beam focused on a powder layer that is added to a previous one. Each layer (50–100 µm thickness) is pre-heated, and using an electron beam a high temperature is generated (up to 600–800 °C), allowing the fusion of the powder according to the 3D CAD project. This process is repeated layer by layer, building the model in a vacuum chamber [25].
Traditional methods of manufacturing orthopaedic implants used subtractive machining, where the material is removed from a metal block until the planned shape has been achieved. The process of RP is different to this and uses the process of additive manufacturing. This is when a construct is made based upon a digital model by plastic or metal being deposited in layers. This provides versatility when constructing complex geometric shapes. Changes in design require no new equipment; therefore, it is ideally suited to low volume patient-specific instrumentation or implants [25,26,27].
-
Anatomical models.
-
Patient-specific instrumentation (PSI).
-
Custom-made implants and devices.
2. Anatomical Models
Anatomical model fabrication is the most common use of 3D printing in surgery.
In the planning stage, 3D models are used to represent the anatomy, simulate surgical procedure and test surgical tools (Figure 1 and Figure 2). Studying a patient’s anatomy with a replication of the structures gives a better comprehension compared to 2D images on a computer screen [28,29]. The 3D-printed model can be shown to patients in order to explain the pathology, surgical planning and assist the consenting process [30,31].
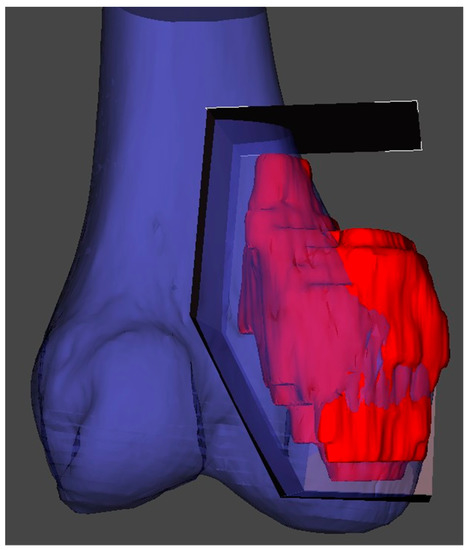
Figure 1. 3D reconstruction and planning of a resection for a distal femoral sarcoma.
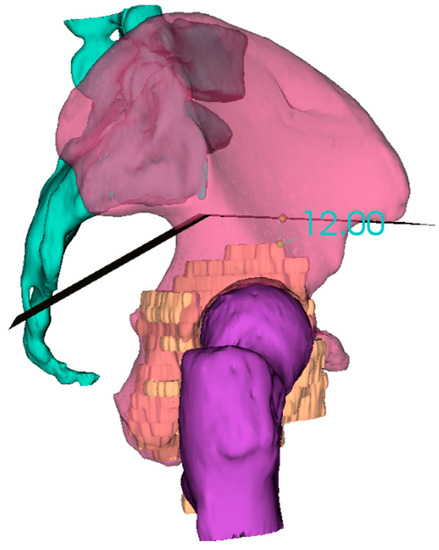
Figure 2. 3D reconstruction and planning of a resection for a pelvic sarcoma.
3. PSI (Patient-Specific Instrumentation)
PSI technology is the most popular medical application of 3D printing and has been developed as an alternative to intraoperative navigation. PSI is designed on a 3D model based on CT and MRI fusion according to the surgical approach, desired resection margins and reconstruction method. The unique anatomy of the patient and the PSI shape allows to place the guide only in a pre-defined position. These contact surfaces must be defined by both surgeon and engineer, considering the surgical approach, the bone exposure and tumour extension. These features make the application of PSI more useful for bone deformities and tumours where the anatomy is often abnormal. The final PSI is commonly printed in nylon or polyamide and provided to the surgeon sterile or to be sterilized (Figure 3). Thus, this method reproduces surgical planning with more accuracy than a free-hand approach. Furthermore, it encourages surgeons to perform a “virtual” surgery in advance, leading to a better comprehension of the possible pitfalls and to a tailored treatment for each patient. This complex procedure in some way has changed surgeons’ mindset due to the network that must be established with a team of engineers.
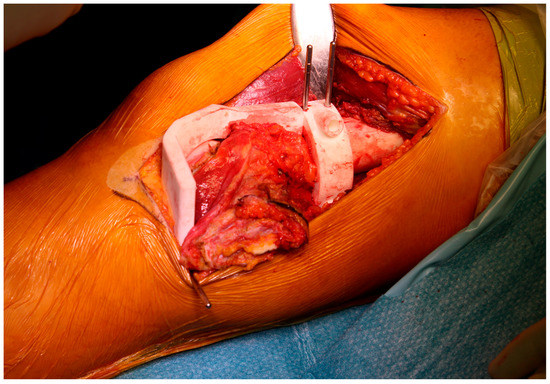
Figure 3. Final patient-specific instrumentation (PSI) printed in nylon and secured intraoperatively.
Limb salvage surgery has become the rule for most patients with bone tumours. However, resection of these tumours needs to achieve wide margins to limit local recurrence and at the same time preserve function [32,33]. In pelvic tumours, surgery is more difficult due to the complex three-dimensional (3D) anatomy of the pelvic bone and the presence of neurovascular structures as well as viscera. This situation leads to a higher local recurrence and complication rate in the pelvis compared with long bones [21,34,35]. In long bones, the use of PSI is most useful in joint preserving resection, in particular in children or young patients in which a reconstruction with a bone allograft can be carried out [32,33].
The performance and accuracy of PSI has been demonstrated; it improves alignment and surgical and operating theatre times [36,37,38,39,40,41,42,43], as well as reducing the risk of contamination and wasting of instrument trays, even if some trials have not displayed significant advantages [44]. Nonetheless, in tumour surgery, the possibility to reduce surgical time whilst performing a more precise cut on the bone and reproducing it on an allograft (or a prosthesis) may improve the outcome of the oncological patient [45].
4. Custom-Made Implants and Devices
Custom-made implants have been used since the 1950s, when a complex reconstruction was performed in a limb salvage procedure [46]. The physical properties of titanium alloy are the most adaptable to bone defect reconstruction. Titanium has high mechanical resistance similar to stainless steel, a low density (50% less than stainless steel) and a low elasticity Young’s modulus, giving the necessary flexibility associated with a high mechanical capacity [47,48,49]. Furthermore, the superficial part of titanium is covered by a free oxygen layer that increase the biocompatibility and biological integration with the surrounding tissues [50,51,52,53].
Many studies have shown that the porosity of titanium is one of the most relevant features influencing bone ingrowth; Frosch et al. has demonstrated that 300–600 µm is the better porosity for osteoconduction [54].
A more recent development is a particular configuration of titanium called trabecular titanium; the main advantage of the trabecular structure is the rough surface that amplifies the contact with host bone, increasing the integration.
This entry is adapted from the peer-reviewed paper 10.3390/cells10020195
This entry is offline, you can click here to edit this entry!
