DNA replication, repair, and recombination in the cell play a significant role in the regulation of the inheritance, maintenance, and transfer of genetic information. To elucidate the biomolecular mechanism in the cell, some molecular models of DNA replication, repair, and recombination have been proposed. These biological studies have been conducted using bulk assays, such as gel electrophoresis. Because in bulk assays, several millions of biomolecules are subjected to analysis, the results of the biological analysis only reveal the average behavior of a large number of biomolecules. Therefore, revealing the elementary biological processes of a protein acting on DNA (e.g., the binding of protein to DNA, DNA synthesis, the pause of DNA synthesis, and the release of protein from DNA) is difficult. Single-molecule imaging allows the analysis of the dynamic behaviors of individual biomolecules that are hidden during bulk experiments. Thus, the methods for single-molecule imaging have provided new insights into almost all of the aspects of the elementary processes of DNA replication, repair, and recombination. However, in an aqueous solution, DNA molecules are in a randomly coiled state. Thus, the manipulation of the physical form of the single DNA molecules is important.
- DNA
- single-molecule
- DNA manipulation
- fluorescence microscope
1. Introduction
Single-molecule imaging and DNA manipulation provide new analytical methods in molecular biology and biochemistry[1][2][3][4][5]. The advancements in microscope and fluorescence imaging technology for biomolecules enabled the direct observation of DNA and proteins at the single-molecule level. Unlike electron microscopy (EM)[6] and atomic force microscopy (AFM)[7], fluorescence single-molecule imaging technologies allow direct observation of fluorescently labeled biomolecules in an aqueous solution. This makes the determination of the dynamic behavior of individual biomolecules possible[2][3]. Information obtained from single-molecule imaging and DNA manipulation contains not only the average behavior of biomolecules but also the distribution of the dynamic behavior of individual biomolecules. For the past two decades, single-molecule imaging and DNA manipulation have provided insights into biological processes based on the dynamic behavior of individual biomolecules. Here, we describe the features and measurements of single-molecule imaging and DNA manipulation.
2. DNA Manipulation
The conformation of DNA molecules in an aqueous solution flexibly fluctuates due to Brownian motion. The DNA molecules behave as a wormlike chain, and voluntarily shorten the distance between the ends as their entropies increase. Thus, fluorescently stained DNA molecules appear as bright spots under a fluorescence microscopic field. Therefore, measuring the full length of the single DNA molecules under the relaxed state is difficult. Even though bright spots are detected, it is difficult to determine the contour lengths between the bright spots of the protein bound to DNA and the DNA terminus in randomly coiled DNA molecules. For this reason, the manipulation of the physical form of the DNA molecules is necessary for obtaining information on the binding sites of proteins.
2.1. DNA Manipulation by Orientation Forces
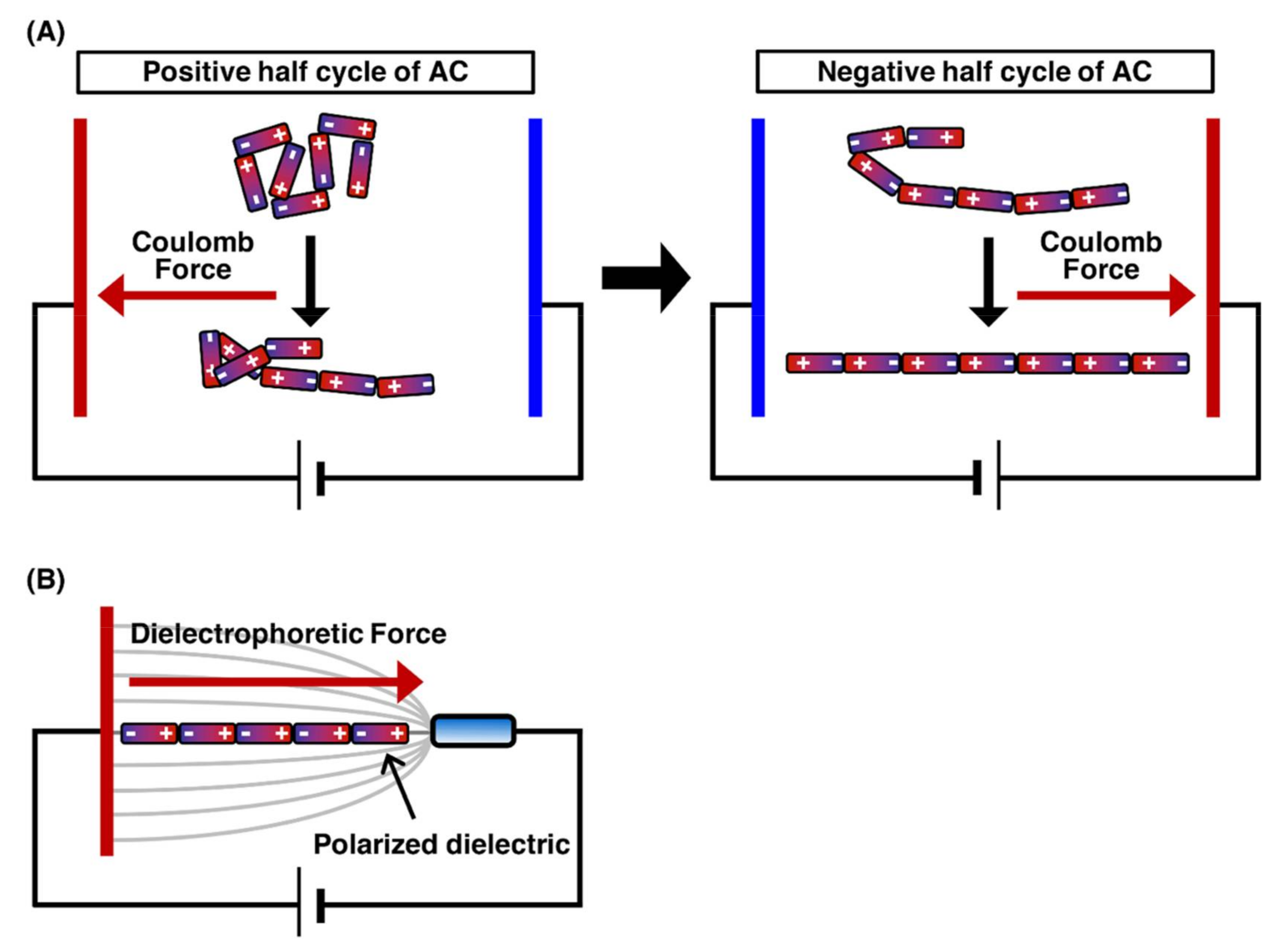
The application of an electric field to linear DNA molecules results in the local induction of charges opposite to the closer electrode on the DNA molecules. Figure 1 presents the orientation force exerted on DNA molecules. Each end of the charged DNA molecules is attracted by the Coulomb force toward the near electrode, which results in the stretching of the DNA molecules toward each electrode[8][9]. However, when a direct current (DC) electric field is applied, fluid flow is generated by bubbles with the electrode reactions, thus preventing the manipulation of DNA molecules. Conversely, when an alternating current (AC) electric field with cycling for the positive half-cycle and negative half-cycle is applied, the fluid flow generation by bubbles with the electrode reactions is significantly suppressed. This is because the electrode reactions generated during the positive half-cycle can be canceled by those generated during the negative half-cycle. Therefore, the application of an AC electric field is suitable for DNA manipulation by orientation forces (Figure 1A)[10][11]. The dielectrophoretic force (the interaction between an induced dipole on the molecule and a nonuniform electric field) is generated according to the dielectric properties of the molecule and medium. In a positive dielectrophoretic force, the molecule, which is more polarizable than the medium, is attracted in a direction toward the region of high electric field gradients. On the other hand, in a negative dielectrophoretic force, the molecule, which is less polarizable than the medium, is attracted in a direction away from the region of high electric field gradients. By controlling the dielectrophoresis force, single DNA molecules were manipulated by the application of an AC electric field with a frequency of 1 MHz at a field strength of 1 MV/m (Figure 1B)[12]. The application of an AC electric field enables the efficient alignment of hundreds of individual DNA molecules under fluorescence microscopic fields. The field strength required for DNA manipulation, i.e., 1 MV/m, is quite high; therefore, electrode miniaturization is necessary to generate a sufficient electric field. Electrode miniaturization also suppresses heat generation by Joule heating, thus hindering the manipulation of DNA molecules. For this reason, it should be noted that a higher salt concentration causes significant heat generation and electrode reactions. For DNA manipulation by the electric fields, it is important to keep the solution under a low ionic strength condition in the concentration range 1–100 µM for multivalent cations, such as calcium ions, magnesium ions, zinc ions and aluminum ions, that reduce polarization by binding to the DNA backbone[10].
Figure 1. Outline of the stretching of the DNA molecule by orientation manipulation. The charge is induced on the single DNA molecule by the electric field. The Coulomb forces are exerted on the induced charges, and then, the DNA molecule is stretched by the Coulomb forces. (A) The stretching process for DNA molecule by the application of AC electric field. (B) The stretching of DNA molecule by orientational force.
2.2. DNA Manipulation by Optical Tweezers
The physical principles of laser trapping were discovered by Arthur Ashkin in 1970[13][14] . In laser trapping, an extremely bright and squeezed laser beam is refracted by a particle at the focal point. The photons that have momentum exert a force on the microparticle during refraction. Due to this power, particles are trapped without physical contact around the focal point of the laser beam. The manipulation technology for the particle position is called optical tweezers[15][16]. However, the manipulation of randomly coiled DNA molecules by optical tweezers is extremely difficult. Thus, by attachment to a particle at one end of the linear DNA molecule, the single DNA molecule is indirectly manipulated by the trapped particle by optical tweezers. The indirectly manipulated single DNA molecules can be stretched by the fluid flow or an electric field[17][18][19] . However, the fluid flow cannot uniformly stretch the trapped single DNA molecules. To enable the uniform stretching of single DNA molecules, they need to be physically stretched from one end to the other end using particles via laser trapping. Figure 2 demonstrates that one end of a single DNA molecule is immobilized on the surface, whereas the other end is attached to a particle. The manipulation of the particle under this condition enables the uniform stretching of the trapped single DNA molecules[20][21]. The use of dual-beam optical tweezers can successfully stretch DNA molecules whose termini are attached to different particles and control their tension based on the particle position[22][23] . The development of optical tweezers has made it possible to determine the force in the order of piconewtons to nanonewtons, which facilitates progressive analyses of the dynamic interaction between DNA and protein at a single-molecule level[24].
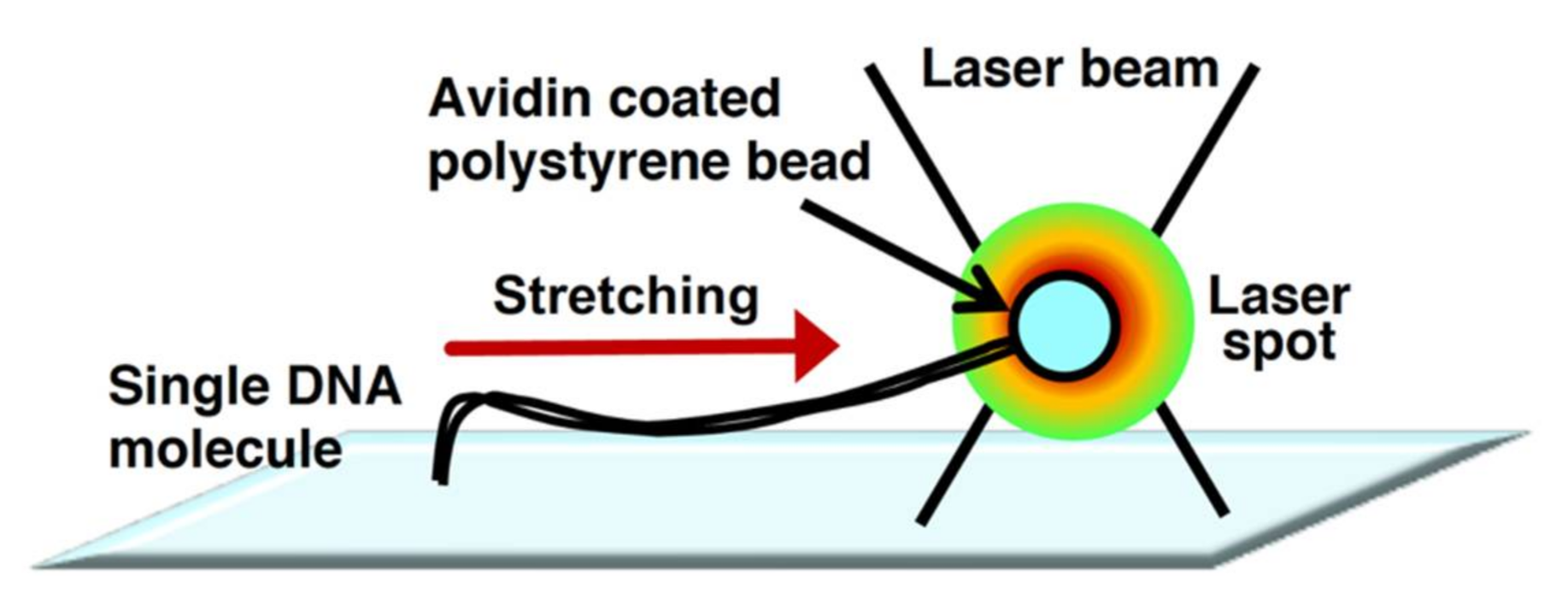
Figure 2. Physical manipulation of a single DNA molecule attached to microbeads using optical tweezers. Conceptual diagram of DNA manipulation by optical tweezers.
2.3. DNA Manipulation by Electric Field and Fluid Flow
In a microchannel device, individual linear DNA molecules with one end immobilized on a glass surface can be stretched by the fluid flow or an electric field generated using miniaturized electrodes (Figure 3A,B). The application of a DC electric field at 10 V/cm enabled the stretching of single DNA molecules without a fluid flow in a microchannel[25][26]. However, the single DNA molecules were not stretched without both the fluid flow and DC electric field, which resulted in the randomly coiled state[26]. This indicates that the negative charge of the phosphate backbone of DNA strands was attracted toward the anode upon applying a DC electric field. In terms of the fluid flow, the individual linear DNA molecules can be stretched by manipulating the fluid flow using a microsyringe pump in a microchannel (Figure 3B)[27]. This is because the fluid flow generates an external force to transform the physical form of the single DNA molecules from the randomly coiled state to the stretched state. The solution can be controlled at the reaction field by exchanging with the solution that is newly injected into the microchannel device. Upon applying the fluid flow and electric field, several dozens of stretched single DNA molecules can be observed under the same fluorescence microscopic fields. This enables an efficient analysis of the dynamic interaction between DNA and protein in a high-throughput manner. In particular, by using a simple micro- or nanofabricated glass surface with lipid bilayer coating, a single-molecule technique, called DNA curtains, has been developed to align the arbitrary patterns of thousands of single DNA molecules in a microchannel[28][29][30][31][32]. This technique has provided a powerful experimental platform for the concurrent observation of hundreds or thousands of dynamic interactions between DNA and protein[5].
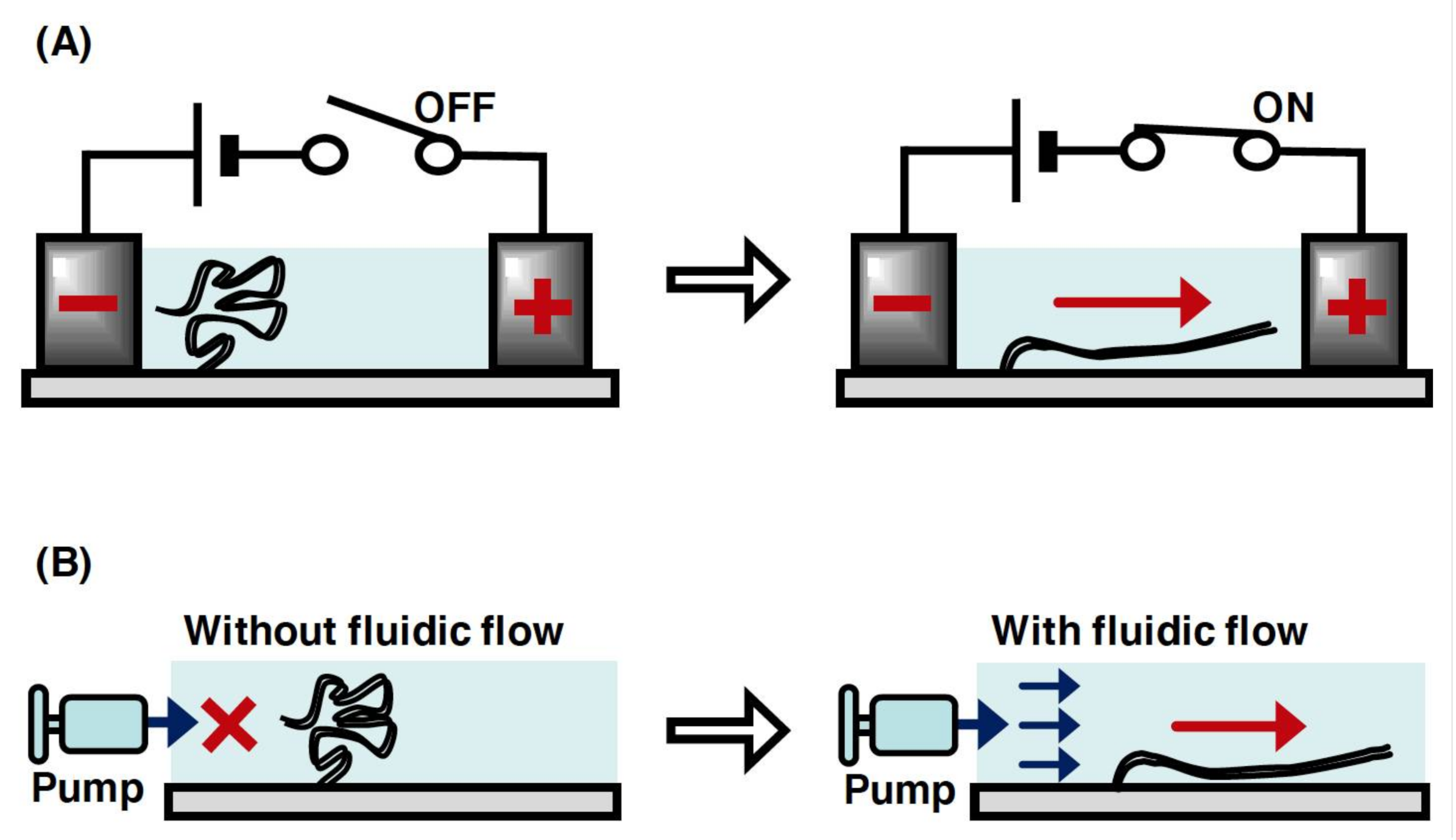
Figure 3. Conceptual diagram of the manipulation of single DNA molecules with one end immobilized on a surface. (A) Stretching manipulation of single DNA molecules by a DC electric field. The DNA molecule is relaxed without the application of electric field but stretched with the application of it. (B) Stretching of single DNA molecule by the fluid flow. The DNA molecule is relaxed without the fluid flow but stretched with the fluid flow.
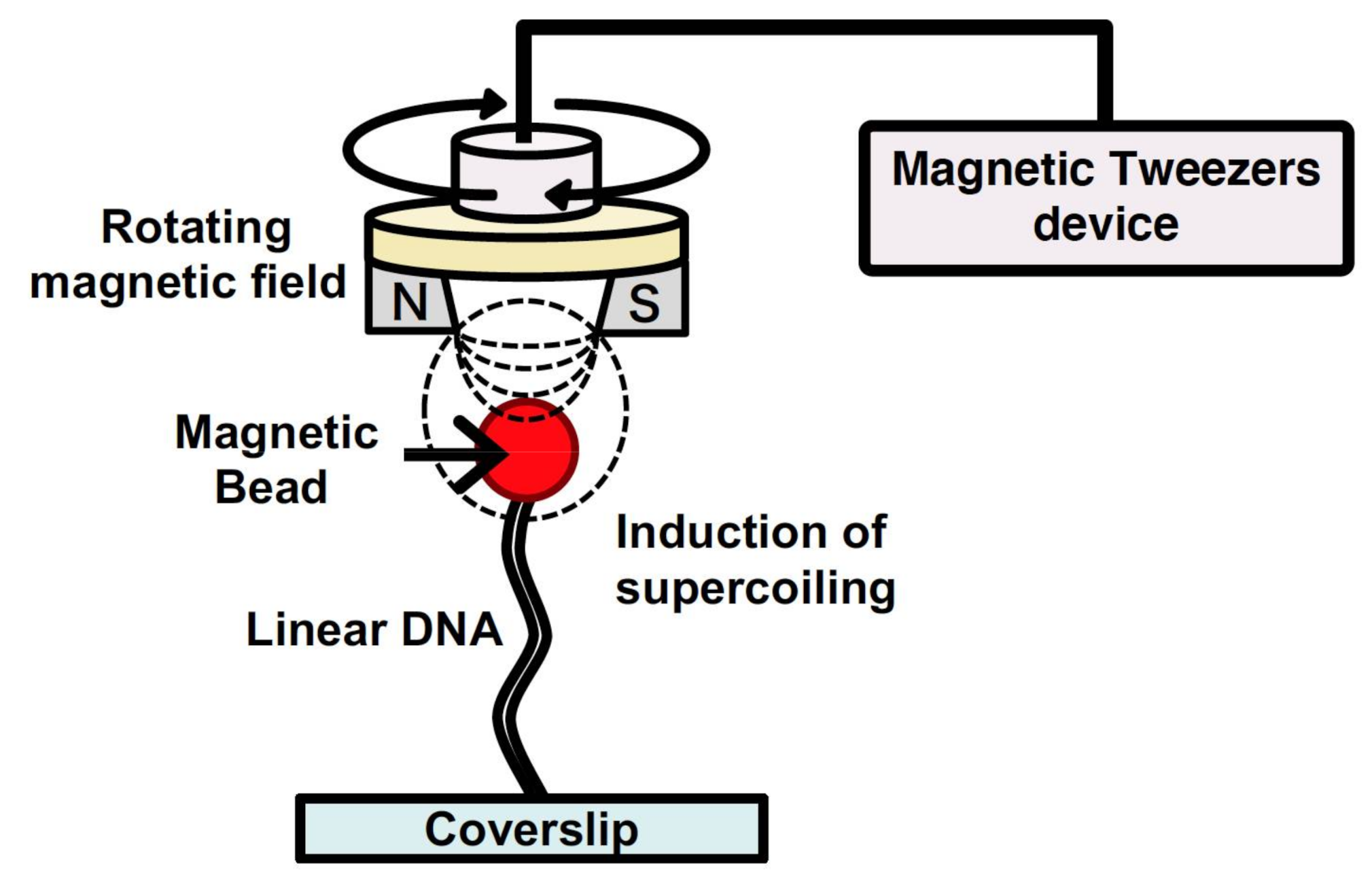
2.4. DNA Manipulation by Magnetic Tweezers
Magnetic tweezers have been utilized to manipulate single DNA molecules and to conduct analysis of the mechanical properties of the interaction between DNA and protein at a single-molecule level[33][34]. A single DNA molecule is tethered to a surface at one end and attached to a magnetic particle at the other end. By generating a magnetic field using external magnets, a force is exerted on the single DNA molecule bound to the magnetic particle. The force applied to the single DNA molecule can be determined from both the applied magnetic force and fluctuations of the magnetic particle position. Thus, by determining the applied force, the force of the action and behavior of protein with the single DNA molecule can be determined by tracking the magnetic particle[33][34]. In addition, magnetic tweezers can generate a rotating magnetic field via a magnet rotation; therefore, they can induce supercoiling of a specified density in the single DNA molecule (Figure 4). For these reasons, magnetic tweezers can be used to analyze the mechanical properties of the various physical forms of DNA, which change in response to DNA-binding protein and enzyme activity[35][36][37][38]. However, tracking the behavior of magnetic beads makes it difficult to analyze the position of protein bound to a single DNA molecule and that of the non-B DNA structures induced by supercoiling. By using a fluorescence microscope equipped with magnetic tweezers in a microchannel, a single-molecule manipulation system has been developed[39][40]. This system can be used to control the supercoiling density of single DNA molecules. Single DNA molecules are supercoiled by rotating the magnetic field generated by rotating a magnet above the microscope stage. By placing the magnet downstream of the microflow channel, the supercoiled single DNA molecules are stretched, and this can be observed under a microscope. By using magnetic tweezers, the full length of the supercoiled single DNA molecules can be directly observed under a fluorescence microscopic field.
3. Single-Molecule Imaging for Biological Processes
By manipulating DNA, the randomly coiled state of a single DNA molecule can be controlled toward stretched and supercoiled states. Unlike in the randomly coiled state, the position of a protein bound to single DNA molecules under the stretched state can be determined from the length information of the DNA molecules. Thus, single-molecule techniques combining DNA manipulation and single-molecule imaging have successfully analyzed the dynamic interaction between DNA and protein, thus providing a new insight into the elementary processes of DNA replication, repair, recombination, and others.
3.1. Single-Molecule Imaging by Single-Molecule FRET
Förster resonance energy transfer (FRET) has been used as a powerful tool to quantify putative interactions between neighboring biomolecules, such as protein–protein interactions, protein–DNA interactions, and also protein conformational changes[41][42]. For monitoring the interaction between two biomolecules, one of the biomolecules is fluorescently labeled as a donor fluorophore, and the other, as an acceptor one. When the donor and the acceptor biomolecules are separated by less than approximately 10 nm, the excitation energy of the donor, which reaches an excited electronic state, is transferred to the acceptor through the electronic resonance of molecular orbitals[41][42]. By nonradiative energy transfer between a donor and an acceptor molecule, thus, the FRET is able to identify interactions between the labeled complexes.
Single-molecule FRET (smFRET) facilitates measurement for the dynamic interactions and behavior between a fluorescently labeled donor and an acceptor, such as protein–protein and protein–DNA interactions[43][44][45][46][47]. In addition, by labeling with a donor and an acceptor at different sites on the same single molecule, the conformation changes within a single molecule are allowed to be observed[48][49]. However, it is necessary to understand the overall structure of the molecule or complex of molecules and the site-specific attachment of fluorophores and color of the fluorophores.
SmFRET has been applied to the analysis of the DNA-unwinding mechanisms of various DNA helicases acting on single DNA molecules. With one smFRET-based approach, the DNA-unwinding mechanisms of Mcm2–7 helicase in eukaryotic DNA replication have been analyzed[50][51][52]. Based on the closed Mcm2–7 ring structure, the Mcm2 and Mcm5 were fluorescently labeled with a donor and an acceptor, respectively[52]. This is because ATP binding at the Mcm2–Mcm5 interface is purported to close the Mcm2–7 ring by cryo-EM and negative-stain EM studies. For this reason, the opening and closing of S. cerevisiae ring-shaped Mcm2–7 DNA helicases were monitored during the recruitment of the pre-replicative complex (pre-RC) to the origin of replication. The application of smFRET provided new insights into the mechanism of eukaryotic DNA replication. Through another smFRET-based approach, the DNA-unwinding mechanisms of the WRN helicase of Werner syndrome (WRN), which is caused by mutations in the WRN gene encoding WRN helicase, have been analyzed[53]. To evaluate the DNA-unwinding properties of WRN helicase, the substrates of different DNA structures, including forked DNA, overhanging DNA, and G-quadruplex-containing DNA, were fluorescently labeled as a donor and an acceptor, respectively. The DNA unwinding of WRN helicase was not caused by complete dissociation from and rebinding to substrates or by strand switching, but by the reciprocating of WRN moving along the same ssDNA substrates. The repetitive movements were shown to behave differently for each DNA substrate, such as forked DNA, 3′/5′-overhanging DNA, and G-quadruplex-containing DNA. These functions of WRN helicase assist the access of other enzymes acting during biological processes, such as DNA replication, repair, and recombination, resulting in it being helpful for the inheritance and maintenance of genome stability.
3.2. Single-Molecule Imaging for ssDNA Molecules by Fluorescent ssDNA-Binding Protein
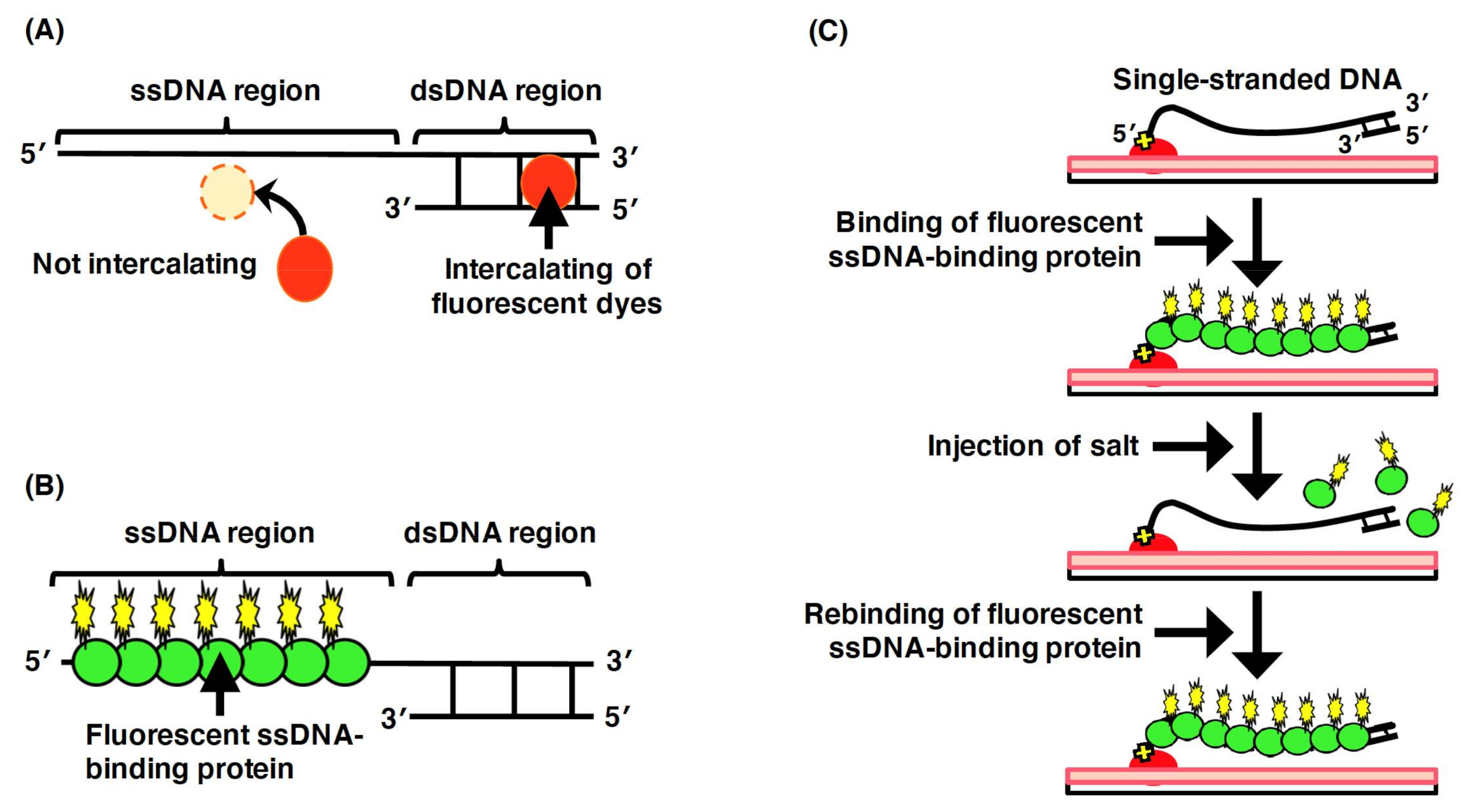
To directly observe DNA molecules under a fluorescence microscopic field, the biomolecules need to be labeled using a fluorescent chemical compound. Intercalating dyes (e.g., YOYO-1 and SYTOX Orange), which are intercalated between the base pairs of dsDNA, can be utilized to directly observe the double-stranded regions of DNA molecules (Figure 5A). However, fluorescent intercalating dyes are not applicable for the direct observation of the single-stranded regions of DNA molecules. However, in the elementary processes of DNA replication, repair, and recombination, single-stranded regions of DNA molecules are generated by proteins acting on DNA, such as DNA helicase and DNA exonuclease. Thus, to analyze the elementary biological processes, the single-stranded regions of the DNA molecules need to be directly observed.
To enable the direct observation of the single-stranded regions of DNA molecules, ssDNA-binding proteins are used (Figure 5B)[54][55]. In particular, eukaryotic replication protein A (RPA), which is composed of the heterotrimeric subunits of 70, 32, and 14 kDa, is an ssDNA-binding protein that binds to the phosphate group of ssDNA[56][57]. Using a fusion protein between the ssDNA-binding domain of RPA and a fluorescent protein, the single-stranded region of a single DNA molecule has been directly observed under a fluorescence microscopic field[54][55]. In single-molecule imaging using a microchannel device, ssDNA molecules fluorescently labeled with ssDNA-binding proteins are stretched with the fluid flow, whereas are they randomly coiled without the fluid flow[58]. As an effect of the salt, the fluorescent ssDNA-binding protein, RPA–YFP molecules bound to the ssDNA molecules are released by a high concentration of sodium chloride, and then, the RPA–YFP molecules are rebound to the ssDNA molecules under a salt-free condition[58] (Figure 5C). In the presence of free DNA-binding protein (e.g., RPA, SSB, and Rad51) in the solution, the RPA molecules dissociate from the single-stranded regions of DNA molecules, which results in a rapid exchange between the free and bound states of RPA molecules[59]. Thus, single-molecule imaging has revealed that the ssDNA-binding affinity of RPA is not strong but that RPA is reversibly bound to ssDNA. These results indicate that RPA protects ssDNA. However, RPA is easily displaced from ssDNA; therefore, other proteins acting on DNA are allowed to access the ssDNA. Therefore, fluorescent ssDNA-binding protein is highly effective for the single-molecule imaging of the elementary process of DNA replication, repair, and recombination and others.
Figure 5. Labeling of the single-stranded regions of single DNA molecules with fluorescent ssDNA-binding proteins. (A) Staining the double-stranded region of a DNA molecule with an intercalating dye. The single-stranded region is not stained with an intercalating dye. (B) Labeling of the single-stranded regions of DNA molecules with fluorescent ssDNA-binding proteins. (C) Schematic illustration of the dynamic behavior of the single-stranded DNA molecules labeled with fluorescent ssDNA-binding protein. One end of ssDNA molecule was specifically immobilized on modified glass surface, and then, fluorescent RPA molecules were injected into the flow cell, resulting in the binding of fluorescent RPA to the ssDNA molecules. The dynamic behavior of the physical form of ssDNA molecule was monitored with and without fluid flow. When salt was added at more than 200 mM, the fluorescent RPA molecules were released from the ssDNA molecule. The ssDNA was restained with the fluorescent RPA after removing the salt. Refer to [58] for details.
3.3. Single-Molecule Imaging for Supercoiled DNA and DNA Secondary Structures
It has been strongly suggested that the physical form of DNA affects the activity of enzymes acting on DNA[21][23][60][61][62]. This indicates that the physical forms of DNA, such as supercoiling and supercoiling-induced non-B-DNA structures, may play significant roles in the regulation of DNA replication, repair, recombination, and others. To induce supercoiling of a specified density in a single DNA molecule, magnetic tweezers have been used. The superhelicity of single DNA molecules has been controlled using magnetic tweezers under a fluorescence microscopic field[39][40]. Single-molecule imaging has revealed the following: (i) plectonemes were induced by supercoiling; (ii) plectonemes were moved along a single DNA molecule by diffusion; (iii) new plectonemes were induced at a distant position via a fast hopping process. It has been suggested that the dynamic behavior of plectonemes may enhance protein binding and gene expression as follows[63]. In single-molecule imaging, the plectonemes on single DNA molecules were simply observed by the intercalating of SYTOX Orange between base pairs of DNA. It was shown that plectonemes were induced upstream of promoters in several prokaryotic genomes.
It has been proposed that DNA looping with DNA supercoiling plays critical roles in the spatial organization of chromosomes. Structural maintenance of chromosome (SMC) protein complexes such as condensin and cohesion play key roles in restructuring genomes during the cell cycle[64][65]. The formation and processive extension of DNA loops by yeast condensin on single DNA molecules was directly observed via single-molecule imaging[66]. The elegant single-molecule experiment demonstrated that by the action of a condensin complex, tens of kilobase pairs of DNA were extruded as a loop structure, at a force-dependent speed of up to 1500 base pairs per second. Single-molecule imaging has shown that the induction of DNA loop extrusion by SMC complexes may be the key principle for the organization of genome architecture.
In single-molecule imaging for DNA secondary structures induced by supercoiling, local structure denaturation of single DNA molecules was frequently induced under a high negative supercoiling density but was almost not induced under a low negative supercoiling density[40]. Local structure denaturation was often observed at the initiation region for DNA replication on single DNA molecules under a high supercoiling density. These results indicate that negative supercoiling enhances the initiation of DNA replication.
3.4. Single-Molecule Imaging for the Initiation of DNA Replication
DNA replication is initiated from replication origin[67]. The consensus sequence of replication origin is highly conserved among bacteria and budding yeast; however, it is not completely conserved in some eukaryotes, such as flies, frogs, and mammals[67][68][69][70][71]. However, in bacteria and eukaryotes, the initiation of DNA replication is promoted by the negative supercoiling of DNA[72][73][74][75][76]. It has been suggested that the physical form of DNA plays a significant role in regulating the initiation of DNA replication. The large T antigen (Tag) of Simian virus 40 (SV40) has been utilized as a model of DNA replication in eukaryotic cells. SV40 Tag is crucial for SV40 DNA replication and is the only replication factor encoded by a viral gene. The multifunctional protein SV40 Tag acts as a replication initiator and simultaneously exhibits a DNA helicase activity during DNA replication. Thus, it is assembled at the SV40 replication origin and unwinds duplex DNA[77].
The initiation of DNA replication using SV40 Tag has been analyzed via single-molecule imaging[78]. In the single-molecule technique, the unwound DNA on the single DNA molecules was labeled by fluorescent ssDNA-binding protein, whereas the double-stranded regions of the single DNA molecules were labeled by fluorescent intercalating dyes. SV40 Tag assembled on the SV40 origin efficiently unwound DNA as a single hexamer translocated on the ssDNA of the lagging strand in the 3′-to-5′ direction. The translocation of SV40 Tag is the same as that of the Mcm2-7 helicase[79][80][81]. These results indicate that the replicative DNA helicase in eukaryotes unwinds a ssDNA on a lagging strand in the 3′-to-5′ direction. The effect of negative supercoiling on the initiation of DNA replication initiated by SV40 Tag has been analyzed by combining DNA manipulation and single-molecule imaging[82]. The increase in the negative supercoiling density stimulated the DNA unwinding more strongly. Furthermore, negative supercoiling was associated with an increased probability, from the assembly of Tag on the SV40 origin, of DNA unwinding. These results indicate that negative superhelicity facilitates the initiation of DNA replication.
4. Conclusions
Single-molecule techniques are maximally utilized through integration with microfluidic devices. Microdevices used in single-molecule analysis have many advantages in terms of reduced sample volumes and the reaction field to be controlled. In particular, the detection frequencies for the biological events between DNA and protein are significantly improved by the reduced distance of molecular diffusion. The further improvement of DNA manipulation and single-molecule imaging techniques will significantly enhance the dynamic analysis of the behavior of and interaction between biomolecules and provide new insights into the mechanisms of elementary biological processes such as DNA replication, repair, and recombination. A knowledge of DNA replication, repair, recombination, and others at a single-molecule level is helpful for not only predicting behaviors at the cellular level but also understanding the inheritance and maintenance of genome stability and diseases associated with DNA replication, repair, and recombination (e.g., aging and cancer). Thus, these single-molecule studies will play important roles as bridges to close the gaps between the research at the molecular level and the research at the cellular level in the fields of biochemistry and molecular biology.
This entry is adapted from the peer-reviewed paper 10.3390/molecules26041050
References
- Nils G. Walter; Cheng-Yen Huang; Anthony J. Manzo; Mohamed A. Sobhy; Do-it-yourself guide: how to use the modern single-molecule toolkit. Nature Methods 2008, 5, 475-489, 10.1038/nmeth.1215.
- Antoine M van Oijen; Single-molecule approaches to characterizing kinetics of biomolecular interactions. Current Opinion in Biotechnology 2011, 22, 75-80, 10.1016/j.copbio.2010.10.002.
- Keir C. Neuman; Attila Nagy; Single-molecule force spectroscopy: optical tweezers, magnetic tweezers and atomic force microscopy. Nature Methods 2008, 5, 491-505, 10.1038/nmeth.1218.
- Gurleen Kaur; Jacob S. Lewis; Antoine M. Van Oijen; Shining a Spotlight on DNA: Single-Molecule Methods to Visualise DNA. Molecules 2019, 24, 491, 10.3390/molecules24030491.
- Daniel Duzdevich; Sy Redding; Eric C. Greene; DNA Dynamics and Single-Molecule Biology. Chemical Reviews 2014, 114, 3072-3086, 10.1021/cr4004117.
- Jack Griffith; Joel A. Huberman; Arthur Kornberg; Electron microscopy of DNA polymerase bound to DNA. Journal of Molecular Biology 1971, 55, 209-IN15, 10.1016/0022-2836(71)90192-6.
- Stephen W. Carmichael; Atomic Force Microscopy for Biologists. Microscopy Today 1997, 5, 3-4, 10.1017/s1551929500060193.
- M. Washizu; O. Kurosawa; Electrostatic manipulation of DNA in microfabricated structures. IEEE Transactions on Industry Applications 1990, 26, 1165-1172, 10.1109/28.62403.
- Masao Washizu; Osamu Kurosawa; I. Arai; Seiichi Suzuki; Nobuo Shimamoto; Applications of electrostatic stretch-and-positioning of DNA. IEEE Transactions on Industry Applications 1995, 31, 447-456, 10.1109/28.382102.
- S. Suzuki; T. Yamanashi; S. Tazawa; O. Kurosawa; M. Washizu; Quantitative analysis of DNA orientation in stationary AC electric fields using fluorescence anisotropy. IEEE Transactions on Industry Applications 1998, 34, 75-83, 10.1109/28.658723.
- H Kabata; O Kurosawa; I Arai; M Washizu; S A Margarson; R E Glass; N Shimamoto; Visualization of single molecules of RNA polymerase sliding along DNA. Science 1993, 262, 1561-1563, 10.1126/science.8248804.
- Kyung Eun Sung; Mark A. Burns; Optimization of Dielectrophoretic DNA Stretching in Microfabricated Devices. Analytical Chemistry 2006, 78, 2939-2947, 10.1021/ac051662f.
- A. Ashkin; J. M. Dziedzic; J. E. Bjorkholm; Steven Chu; Observation of a single-beam gradient force optical trap for dielectric particles. Optics Letters 1986, 11, 288-290, 10.1364/ol.11.000288.
- A. Ashkin; Forces of a single-beam gradient laser trap on a dielectric sphere in the ray optics regime. Biophysical Journal 1992, 61, 569-582, 10.1016/s0006-3495(92)81860-x.
- Jeffrey R. Moffitt; Yann R. Chemla; Steven B. Smith; Carlos Bustamante; Recent Advances in Optical Tweezers. Annual Review of Biochemistry 2008, 77, 205-228, 10.1146/annurev.biochem.77.043007.090225.
- Keir C. Neuman; Steven M. Block; Optical trapping. Review of Scientific Instruments 2004, 75, 2787-2809, 10.1063/1.1785844.
- Laurence R. Brewer; Michele Corzett; Rod Balhorn; Protamine-Induced Condensation and Decondensation of the Same DNA Molecule. Science 1999, 286, 120-123, 10.1126/science.286.5437.120.
- Piero R. Bianco; Laurence R. Brewer; Michele Corzett; Rod Balhorn; Yin Yeh; Stephen C. Kowalczykowski; Ronald J. Baskin; Processive translocation and DNA unwinding by individual RecBCD enzyme molecules. Nature 2001, 409, 374-378, 10.1038/35053131.
- Maria Spies; Piero R. Bianco; Mark S. Dillingham; Naofumi Handa; Ronald J. Baskin; Stephen C. Kowalczykowski; A Molecular Throttle. Cell 2003, 114, 647-654, 10.1016/s0092-8674(03)00681-0.
- Hirofumi Kurita; Hachiro Yasuda; Kazunori Takashima; Shinji Katsura; Akira Mizuno; Physical manipulation of single-molecule DNA using microbead and its application to analysis of DNA–protein interaction. Journal of Magnetism and Magnetic Materials 2009, 321, 655-658, 10.1016/j.jmmm.2008.11.018.
- Hong Yin; Michelle D. Wang; Karel Svoboda; Robert Landick; Steven M. Block; Jeff Gelles; Transcription Against an Applied Force. Science 1995, 270, 1653-1657, 10.1126/science.270.5242.1653.
- Joshua W. Shaevitz; Elio A. Abbondanzieri; Robert Landick; Steven M. Block; Backtracking by single RNA polymerase molecules observed at near-base-pair resolution. Nature 2003, 426, 684-687, 10.1038/nature02191.
- Bram Van Den Broek; Maarten C. Noom; Gijs J. L. Wuite; DNA-tension dependence of restriction enzyme activity reveals mechanochemical properties of the reaction pathway. Nucleic Acids Research 2005, 33, 2676-2684, 10.1093/nar/gki565.
- Iddo Heller; Tjalle P. Hoekstra; Graeme A. King; Erwin J. G. Peterman; Gijs J. L. Wuite; Optical Tweezers Analysis of DNA–Protein Complexes. Chemical Reviews 2014, 114, 3087-3119, 10.1021/cr4003006.
- Shun-Ichi Matsuura; Jun Komatsu; Ken Hirano; Hachiro Yasuda; Kazunori Takashima; Shinji Katsura; Akira Mizuno; Real-time observation of a single DNA digestion by lambda exonuclease under a fluorescence microscope field. Nucleic Acids Research 2001, 29, 79e-79, 10.1093/nar/29.16.e79.
- Shun-Ichi Matsuura; Hirofumi Kurita; Michihiko Nakano; Jun Komatsu; Kazunori Takashima; Shinji Katsura; Akira Mizuno; One-End Immobilization of Individual DNA Molecules on a Functional Hydrophobic Glass Surface. Journal of Biomolecular Structure and Dynamics 2002, 20, 429-436, 10.1080/07391102.2002.10506861.
- Annette Granéli; Caitlyn C. Yeykal; Tekkatte Krishnamurthy Prasad; Eric C. Greene; Organized Arrays of Individual DNA Molecules Tethered to Supported Lipid Bilayers. Langmuir 2006, 22, 292-299, 10.1021/la051944a.
- Teresa Fazio; Mari-Liis Visnapuu; Shalom Wind; Eric C. Greene; DNA Curtains and Nanoscale Curtain Rods: High-Throughput Tools for Single Molecule Imaging. Langmuir 2008, 24, 10524-10531, 10.1021/la801762h.
- Eric C. Greene; Shalom Wind; Teresa Fazio; Jason Gorman; Mari-Liis Visnapuu; DNA Curtains for High-Throughput Single-Molecule Optical Imaging. Methods in Enzymology 2010, 472, 293-315, 10.1016/s0076-6879(10)72006-1.
- Ilya J. Finkelstein; Eric C. Greene; Supported Lipid Bilayers and DNA Curtains for High-Throughput Single-Molecule Studies. Methods in Molecular Biology 2011, 745, 447-461, 10.1007/978-1-61779-129-1_26.
- Aaron D. Robison; Ilya J. Finkelstein; Rapid Prototyping of Multichannel Microfluidic Devices for Single-Molecule DNA Curtain Imaging. Analytical Chemistry 2014, 86, 4157-4163, 10.1021/ac500267v.
- Ignacio F. Gallardo; Praveenkumar Pasupathy; Megan Sonntag Brown; Carol M. Manhart; Dean P. Neikirk; Eric Alani; Ilya J. Finkelstein; High-Throughput Universal DNA Curtain Arrays for Single-Molecule Fluorescence Imaging. Langmuir 2015, 31, 10310-10317, 10.1021/acs.langmuir.5b02416.
- T. R. Strick; J.-F. Allemand; D. Bensimon; V. Croquette; The Elasticity of a Single Supercoiled DNA Molecule. Science 1996, 271, 1835-1837, 10.1126/science.271.5257.1835.
- T.R. Strick; J.-F. Allemand; D. Bensimon; V. Croquette; Behavior of Supercoiled DNA. Biophysical Journal 1998, 74, 2016-2028, 10.1016/s0006-3495(98)77908-1.
- Terence R. Strick; Vincent Croquette; David Bensimon; Single-molecule analysis of DNA uncoiling by a type II topoisomerase. Nature 2000, 404, 901-904, 10.1038/35009144.
- Daniel A. Koster; Vincent Croquette; Cees Dekker; Stewart Shuman; Nynke H. Dekker; Friction and torque govern the relaxation of DNA supercoils by eukaryotic topoisomerase IB. Nature 2005, 434, 671-674, 10.1038/nature03395.
- Jeff Gore; Zev Bryant; Michael D. Stone; Marcelo Nöllmann; Nicholas R. Cozzarelli; Carlos Bustamante; Mechanochemical analysis of DNA gyrase using rotor bead tracking. Nature 2006, 439, 100-104, 10.1038/nature04319.
- Daniel A. Koster; Aurélien Crut; Stewart Shuman; Mary-Ann Bjornsti; Nynke H. Dekker; Cellular Strategies for Regulating DNA Supercoiling: A Single-Molecule Perspective. Cell 2010, 142, 519-530, 10.1016/j.cell.2010.08.001.
- M. T. J. Van Loenhout; M. V. De Grunt; C. Dekker; Dynamics of DNA Supercoils. Science 2012, 338, 94-97, 10.1126/science.1225810.
- Shunsuke Takahashi; Shinya Motooka; Tomohiro Usui; Shohei Kawasaki; Hidefumi Miyata; Hirofumi Kurita; Takeshi Mizuno; Shun-Ichi Matsuura; Akira Mizuno; Masahiko Oshige; et al. Direct Single-Molecule Observations of Local Denaturation of a DNA Double Helix under a Negative Supercoil State. Analytical Chemistry 2015, 87, 3490-3497, 10.1021/acs.analchem.5b00044.
- P R Selvin; The renaissance of fluorescence resonance energy transfer.. Nature Structural & Molecular Biology 2000, 7, 730-734, 10.1038/78948.
- Robert M Clegg; Fluorescence resonance energy transfer. Current Opinion in Biotechnology 1995, 6, 103-110, 10.1016/0958-1669(95)80016-6.
- Rahul Roy; Sungchul Hohng; Taekjip Ha; A practical guide to single-molecule FRET. Nature Methods 2008, 5, 507-516, 10.1038/nmeth.1208.
- Sungchul Hohng; Sanghwa Lee; Jinwoo Lee; Myung Hyun Jo; Maximizing information content of single-molecule FRET experiments: multi-color FRET and FRET combined with force or torque. Chemical Society Reviews 2014, 43, 1007-1013, 10.1039/c3cs60184f.
- Emil Marklund; Brad Van Oosten; Guanzhong Mao; Elias Amselem; Kalle Kipper; Anton Sabantsev; Andrew Emmerich; Daniel Globisch; Xuan Zheng; Laura C. Lehmann; et al. DNA surface exploration and operator bypassing during target search. Nature 2020, 583, 858-861, 10.1038/s41586-020-2413-7.
- Carel Fijen; Alejandro Montón Silva; Alejandro Hochkoeppler; Johannes Hohlbein; A single-molecule FRET sensor for monitoring DNA synthesis in real time.. Physical Chemistry Chemical Physics 2017, 19, 4222-4230, 10.1039/c6cp05919h.
- Jill M. Grimme; Masayoshi Honda; Rebecca Wright; Yusuke Okuno; Eli Rothenberg; Alexander V. Mazin; Taekjip Ha; Maria Spies; Human Rad52 binds and wraps single-stranded DNA and mediates annealing via two hRad52–ssDNA complexes. Nucleic Acids Research 2010, 38, 2917-2930, 10.1093/nar/gkp1249.
- Mina Lee; Sook Ho Kim; Seok-Cheol Hong; Minute negative superhelicity is sufficient to induce the B-Z transition in the presence of low tension. Proceedings of the National Academy of Sciences 2010, 107, 4985-4990, 10.1073/pnas.0911528107.
- Xi Long; Joseph W. Parks; Clive R. Bagshaw; Michael D. Stone; Mechanical unfolding of human telomere G-quadruplex DNA probed by integrated fluorescence and magnetic tweezers spectroscopy. Nucleic Acids Research 2013, 41, 2746-2755, 10.1093/nar/gks1341.
- Brian R Chapados; David J Hosfield; Seungil Han; Junzhuan Qiu; Biana Yelent; Binghui Shen; John A Tainer; Structural Basis for FEN-1 Substrate Specificity and PCNA-Mediated Activation in DNA Replication and Repair. Cell 2004, 116, 39-50, 10.1016/s0092-8674(03)01036-5.
- Tammy R. L. Collins; Gordon G. Hammes; Tao-Shih Hsieh; Analysis of the eukaryotic topoisomerase II DNA gate: a single-molecule FRET and structural perspective. Nucleic Acids Research 2009, 37, 712-720, 10.1093/nar/gkn1059.
- Simina Ticau; Larry J. Friedman; Kanokwan Champasa; Ivan R. Corrêa; Larry J Friedman Jeff Gelles; Simina Ticau Kanokwan Champasa Stephen P Bell; Mechanism and timing of Mcm2–7 ring closure during DNA replication origin licensing. Nature Structural & Molecular Biology 2017, 24, 309-315, 10.1038/nsmb.3375.
- Wen-Qiang Wu; Xi-Miao Hou; Bo Zhang; Philippe Fossé; Brigitte René; Olivier Mauffret; Ming Li; Shuo-Xing Dou; Xu-Guang Xi; Single-molecule studies reveal reciprocating of WRN helicase core along ssDNA during DNA unwinding. Scientific Reports 2017, 7, 43954, 10.1038/srep43954.
- Masahiko Oshige; Shohei Kawasaki; Hiroki Takano; Kouji Yamaguchi; Hirofumi Kurita; Takeshi Mizuno; Shun-Ichi Matsuura; Akira Mizuno; Shinji Katsura; Direct Observation Method of Individual Single-Stranded DNA Molecules Using Fluorescent Replication Protein A. Journal of Fluorescence 2011, 21, 1189-1194, 10.1007/s10895-010-0797-8.
- Bryan Gibb; Tim D. Silverstein; Ilya J. Finkelstein; Eric C. Greene; Single-Stranded DNA Curtains for Real-Time Single-Molecule Visualization of Protein–Nucleic Acid Interactions. Analytical Chemistry 2012, 84, 7607-7612, 10.1021/ac302117z.
- Kengo Sakaguchi; Toyotaka Ishibashi; Yukinobu Uchiyama; Kazuki Iwabata; The multi-replication protein A (RPA) system - a new perspective. The FEBS Journal 2009, 276, 943-963, 10.1111/j.1742-4658.2008.06841.x.
- Ellen Fanning; Vitaly Klimovich; Andrew R. Nager; A dynamic model for replication protein A (RPA) function in DNA processing pathways. Nucleic Acids Research 2006, 34, 4126-4137, 10.1093/nar/gkl550.
- Shunsuke Takahashi; Shohei Kawasaki; Koji Yamaguchi; Hidefumi Miyata; Hirofumi Kurita; Takeshi Mizuno; Shun-Ichi Matsuura; Akira Mizuno; Masahiko Oshige; Shinji Katsura; et al. Direct Observation of Fluorescently Labeled Single-stranded λDNA Molecules in a Micro-Flow Channel. Journal of Fluorescence 2013, 23, 635-640, 10.1007/s10895-013-1210-1.
- Bryan Gibb; Ling F. Ye; Stephanie C. Gergoudis; Youngho Kwon; Hengyao Niu; Patrick Sung; Eric C. Greene; Concentration-Dependent Exchange of Replication Protein A on Single-Stranded DNA Revealed by Single-Molecule Imaging. PLOS ONE 2014, 9, e87922, 10.1371/journal.pone.0087922.
- Hirofumi Kurita; Ken Torii; Hachiro Yasuda; Kazunori Takashima; Shinji Katsura; Akira Mizuno; The Effect of Physical Form of DNA on ExonucleaseIII Activity Revealed by Single-molecule Observations. Journal of Fluorescence 2008, 19, 33-40, 10.1007/s10895-008-0376-4.
- Shunsuke Takahashi; Shohei Kawasaki; Hidefumi Miyata; Hirofumi Kurita; Takeshi Mizuno; Shun-Ichi Matsuura; Akira Mizuno; Masahiko Oshige; Shinji Katsura; A New Direct Single-Molecule Observation Method for DNA Synthesis Reaction Using Fluorescent Replication Protein A. Sensors 2014, 14, 5174-5182, 10.3390/s140305174.
- Tension-dependent DNA cleavage by restriction endonucleases: Two-site enzymes are . , , , .
- Sung Hyun Kim; Mahipal Ganji; Eugene Kim; Jaco Van Der Torre; Elio Abbondanzieri; Cees Dekker; DNA sequence encodes the position of DNA supercoils. eLife 2018, 7, e36557, 10.7554/elife.36557.
- Kristian Jeppsson; Takaharu Kanno; Katsuhiko Shirahige; Camilla Sjögren; The maintenance of chromosome structure: positioning and functioning of SMC complexes. Nature Reviews Molecular Cell Biology 2014, 15, 601-614, 10.1038/nrm3857.
- Sumanjit Datta; Léa LeComte; Christian H Haering; Structural insights into DNA loop extrusion by SMC protein complexes. Current Opinion in Structural Biology 2020, 65, 102-109, 10.1016/j.sbi.2020.06.009.
- Mahipal Ganji; Indra A. Shaltiel; Shveta Bisht; Eugene Kim; Ana Kalichava; Christian H. Haering; Cees Dekker; Real-time imaging of DNA loop extrusion by condensin. Science 2018, 360, 102-105, 10.1126/science.aar7831.
- David Bramhill; Arthur Kornberg; A model for initiation at origins of DNA replication. Cell 1988, 54, 915-918, 10.1016/0092-8674(88)90102-x.
- E. Crooke; D.S. Hwang; K. Skarstad; B. Thöny; A. Kornberg; E. coli minichromosome replication: regulation of initiation at oriC. Research in Microbiology 1991, 142, 127-130, 10.1016/0923-2508(91)90019-7.
- Hisao Masai; Seiji Matsumoto; Zhiying You; Naoko Yoshizawa-Sugata; Masako Oda; Eukaryotic Chromosome DNA Replication: Where, When, and How?. Annual Review of Biochemistry 2010, 79, 89-130, 10.1146/annurev.biochem.052308.103205.
- Marcel Méchali; Eukaryotic DNA replication origins: many choices for appropriate answers. Nature Reviews Molecular Cell Biology 2010, 11, 728-738, 10.1038/nrm2976.
- R. S. Fuller; A. Kornberg; Purified dnaA protein in initiation of replication at the Escherichia coli chromosomal origin of replication.. Proceedings of the National Academy of Sciences 1983, 80, 5817-5821, 10.1073/pnas.80.19.5817.
- B.E. Funnell; T.A. Baker; A. Kornberg; In vitro assembly of a prepriming complex at the origin of the Escherichia coli chromosome.. Journal of Biological Chemistry 1987, 262, 10327-10334, 10.1016/s0021-9258(18)61116-0.
- Tsuneaki Asai; Chi-Pien Chen; Toshio Nagata; Mitsuru Takanami; Mutsuo Imai; Transcription in vivo within the replication origin of the Escherichia coli chromosome: a mechanism for activating initiation of replication. Molecular Genetics and Genomics 1992, 231, 169-178, 10.1007/bf00279788.
- Samuel Corless; Nick Gilbert; Effects of DNA supercoiling on chromatin architecture. Biophysical Reviews 2016, 8, 51-64, 10.1007/s12551-016-0242-6.
- E. Rampakakis; C. Gkogkas; D. Di Paola; M. Zannis-Hadjopoulos; Replication initiation and DNA topology: The twisted life of the origin. Journal of Cellular Biochemistry 2010, 110, 35-43, 10.1002/jcb.22557.
- Joaquim Roca; The torsional state of DNA within the chromosome. Chromosoma 2011, 120, 323-334, 10.1007/s00412-011-0324-y.
- Ellen Fanning; Kun Zhao; SV40 DNA replication: From the A gene to a nanomachine. Virology 2009, 384, 352-359, 10.1016/j.virol.2008.11.038.
- Hasan Yardimci; Xindan Wang; Anna B. Loveland; Inger Tappin; David Z. Rudner; Jerard Hurwitz; Antoine M. Van Oijen; Johannes C. Walter; Bypass of a protein barrier by a replicative DNA helicase. Nature 2012, 492, 205-209, 10.1038/nature11730.
- Max E. Douglas; Ferdos Abid Ali; Alessandro Costa; John F. X. Diffley; The mechanism of eukaryotic CMG helicase activation. Nature 2018, 555, 265-268, 10.1038/nature25787.
- Hazal B. Kose; Nicolai B. Larsen; Julien P. Duxin; Hasan Yardimci; Dynamics of the Eukaryotic Replicative Helicase at Lagging-Strand Protein Barriers Support the Steric Exclusion Model. Cell Reports 2019, 26, 2113-2125.e6, 10.1016/j.celrep.2019.01.086.
- Daniel R. Burnham; Hazal B. Kose; Rebecca B. Hoyle; Hasan Yardimci; The mechanism of DNA unwinding by the eukaryotic replicative helicase. Nature Communications 2019, 10, 2159, 10.1038/s41467-019-09896-2.
- Shunsuke Takahashi; Shinya Motooka; Shohei Kawasaki; Hirofumi Kurita; Takeshi Mizuno; Shun-Ichi Matsuura; Fumio Hanaoka; Akira Mizuno; Masahiko Oshige; Shinji Katsura; et al. Direct single-molecule observations of DNA unwinding by SV40 large tumor antigen under a negative DNA supercoil state. Journal of Biomolecular Structure and Dynamics 2017, 36, 32-44, 10.1080/07391102.2016.1269689.