Bladder cancer (BCa) is the most prevalent neoplasia of the urinary tract.
- bladder cancer
- liquid biopsy
- exosome
1. Current Landscape
Over the last twenty years, the use of liquid biopsies as a noninvasive way to characterize the genomic landscape of cancer patients has been increasing steadily. The term “liquid biopsy” includes the sampling and analysis of biological fluids such as blood, plasma, urine, pleural liquid, cerebrospinal fluid, and saliva (Figure 1) [1][2]. The main focus of this research has been on circulating tumor cells (CTC) and circulating cell-free tumor DNA (ctDNA), but additional biomarkers present in the blood or urine, such as exosomes, could also be used for early cancer detection. The presence of cell-free DNA and RNA in human blood was first demonstrated in 1948 [3]; however, only a few liquid biopsies are currently approved for clinical use. The identification of suitable biomarkers for the presence and prognosis of a disease, as well as for monitoring the effect of certain treatments, is still a hot topic in cancer research.
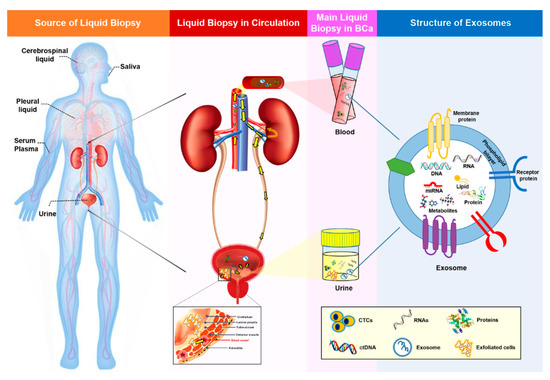
Figure 1. Summary of liquid biopsy and liquid biopsy biomarkers. Liquid biopsy samples represent all body fluids, including urine, serum, plasma, saliva, cerebrospinal fluid, and pleural fluid. In BCa, urine (which intimately contacts the tumor), serum, and plasma are used widely for the detection and surveillance of the disease. Genetic information from tumor cells is spread through the circulation (yellow arrows in the second image indicate the circulation in the urinary system), thereby favoring the detection of liquid biopsy biomarkers in the blood and urine. Common biomarkers present in liquid biopsies include CTCs, ctDNA, and exosomes. Moreover, exfoliated cells derived from a tumor can also be found in urine. Tumor cells continuously influence surrounding or distal sites by transmission of cancerous signals. Exosomes act as important mediators of cell-to-cell communication by transferring their contents, including DNA, RNA, miRNA, lipids, proteins, and metabolites. Due to their lipid bilayer structure, exosomes are extremely stable and can resist degradation by enzymes such as RNases. BCa, bladder cancer; CTC, circulating tumor cell; and ctDNA, circulating cell-free tumor DNA.
2. Exosomes and Exosomal miRNAs in Cancer
Numerous studies have shown that exosomal contents, such as double-stranded DNA, various RNA species, and proteins, can be used as predictive biomarkers for cancer diagnosis and prognosis. Studies regarding the biomarkers have revealed that several RNAs possess advantages over DNA, due to their dynamic and fluctuating expression pattern (such as lncRNAs) corresponding with the internal needs of cells, thereby indicating that those RNAs have a high tissue- or disease-state-specific feature [4]. Furthermore, paradoxically, the DNAs existing in exosomes vary along with the different detection methods [5][6], which makes an elusive whether genomic DNA exists in exosomes. Among exosomal RNA species, miRNAs, a class of small, single-stranded, noncoding RNA molecules, play a role in virtually all biological pathways, including cell growth, proliferation, and differentiation, as well as immune responses, apoptosis, metabolism, and tumorigenesis. Exosomes provide a protective and highly stable source of miRNAs in body fluids, protecting them against degradation even under nonphysiological conditions. ExomiRs are reportedly resistant to multiple freeze-thaw cycles, are stable during long-term storage at room temperature, and remain stable for up to five years when stored at −20°C [7][8][9][10][11]. Their enhanced stability compared to proteins and other nucleic acids, both in the circulation and in fixed tissues, makes exomiRs well-suited to sampling and analysis [12][13]. Moreover, exosome secretion from malignant tissue is much higher than that from the corresponding normal tissue, and higher concentrations of exomiRs are typically detected in tumor liquid biopsy samples such as plasma, urine, and ascites [14][15][16][17]. Coupled with the stability of miRNAs, this increased load of circulating exosomes in the malignant state has enabled the identification of several potential exomiR biomarkers. Another attractive RNA molecule exists in exosome is lncRNA, which is larger in size and has less been studied than exomiRs—specifically, 534 versus 52 reports regarding miRNA and lncRNA, respectively, were searched in PubMed until January 2020 [18]. Nevertheless, granting exosomal lncRNA seems to be a promising biomarker for cancer; its application has some limitations, including an analysis only on the known lncRNAs, the false positives derived from nonspecific hybridizations, high variability for little expressed genes, and the sealed lncRNAs sequence variants. On the basis of these findings, the obvious superiority for the exomiRs as a noninvasive biomarker in liquid biopsies has been confirmed.
MiRNAs are taken up by nearby or distal target recipient cells as a cargo of exosomes, reflecting a cell-to-cell communication method that can influence the pathogenesis of cancer [19][20]. Therefore, miRNAs carried by exosomes can provide information about dominant cells from which they are derived, as well as the target and cellular state, including potential therapy resistance. Recent evidence suggests that the transmission of onco-miRNAs via tumor cell-derived exosomes can promote tumor cell proliferation and invasion, as well as angiogenesis, distant metastasis, and remodeling of the tumor microenvironment [21].
MiRNAs are key regulators of gene expression in cancer, functioning as either tumor-suppressors or oncogenes depending on the target mRNA, and play a constructive role in tumorigenesis, metastasis, and resistance to diverse treatments [22][23]. Cancer cell-released exomiR-21, exomiR-23, exomiR-29, exomiR-103, and exomiR-210 promote tumor proliferation, angiogenesis, and migration [21][24][25][26][27][28][29][30]. In particular, exo-miR-21 may be a promising biomarker for many types of cancer. The roles of exomiRs in cancer are depicted in Figure 2.
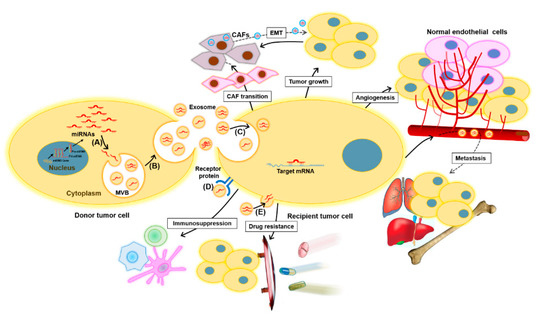
Figure 2. Functions of exomiRs in cancer. ExomiRs play multiple roles in tumor initiation and development. Tumor cells may transfer exomiRs to the surrounding normal or tumor cells as follows: (A) the sorting of miRNAs into exosomes during MVB formation, (B) the release of exosomes into the extracellular space, (C) the delivery of exomiRs to recipient cells by endocytosis, (D) the binding of exosomes to receptors to activate specific signaling pathways, and (E) the fusion of exosomes with the plasma membrane of recipient cells. Subsequently, the transmitted exomiRs can regulate tumor cell growth, angiogenesis, and metastasis by binding to their downstream targets. Drug-resistant tumor cells can transmit their resistant phenotype to drug-sensitive tumor cells through the transfer of exomiRs that confer chemoresistance. Moreover, tumor-derived exomiRs play a key role in exchanging information between tumor cells and immune cells, such as macrophages, T cells, and dendritic cells, to suppress the immune response and promote tumor progression. Tumor-derived exomiRs are also instrumental modulators of the tumor microenvironment, inducing CAF transition, thus facilitating tumorigenesis and tumor development. On the other hand, fibroblast-derived exomiRs are also transferred to tumor cells and can contribute to EMT. CAF, cancer-associated fibroblast; EMT, epithelial–mesenchymal transition; ExomiR, exosomal miRNA; and MVB, multivesicular body.
3. Exosomal miRNAs as Potential Biomarkers for Bladder Cancer
As exomiRs are one of the major components of exosomes and play a functional role in cell-to-cell communication, many studies of urinary exosomal biomarkers have focused on exomiRs. A study examining miRNAs in matched tumor tissue, plasma, urine exosomes, and white blood cells from patients with bladder cancer found that miR-4454, miR-205-5p, miR-200c-3p, miR-200b-3p, miR-21-5p, miR-29b-3p, and miR-720 /3007a were common to NMIBC tissues and urinary exosomes [31]. Although these results were from a small cohort (n = 16), they support the hypothesis that exomiRs reflect the molecular signals of parental tumor cells, thereby serving as desirable biomarkers for tumor characterization. The potential use of exosomes and exomiRs as diagnostic tools in BCa requires further investigation; however, increasing evidence suggests they have great potential. Previous clinical studies exploring the use of exomiRs as potential BCa biomarkers are summarized in Table 1.
Table 1. List of exomiRs identified in BCa.
| Markers | Biological Source | Regulation | Clinical Significance | Reference |
|---|---|---|---|---|
| miR-1285-3p, miR-142-3p, miR-16-1-3p, miR-195-3p, miR-196b-5p, miR-23b-3p, miR-28-5p, miR-299-3p, miR-3155a, miR-3162-5p, miR-3678-3p, miR-4283, miR-4295, miR-4311, miR-4531, miR-492, miR-5096, miR-513b-5p, miR-5187-5p, miR-601, miR-619-5p, miR-92a-2-5p | Urine | Up | Diagnosis | [32] |
| miR-375, miR-146a | Urine | Up | Diagnosis | [33] |
| miR-155-5p, miR-15a-5p, miR-21-5p, miR-132-3p, miR-31-5p (especially miR-21-5p) |
Urine | Up | Diagnosis | [34] |
| Four-miRNA panel: mir-21, miR-93, miR-200c, and miR-940 | Urine | Up | Diagnosis | [35] |
| Three-miRNA panel: miR-30a-5p, let-7c-5p and miR-486-5p | Urine | Up | Diagnosis | [36] |
| Six-miRNA panel: miR-152, miR-148b-3p, miR-3187-3p, miR-15b-5p, miR-27a-3p and miR-30a-5p | Serum | Up | Diagnosis | [37] |
| Seven-miRNA panel: miR-7-5p, miR-22-3p, miR-29a-3p, miR-126-5p, miR-200a-3p, miR-375 and miR-423-5p | Urine | Up | Diagnosis | [38] |
| Twenty-five-miRNA panel: miR-652, miR-199a-3p, miR-140-5p, miR-93, miR-142-5p, miR-1305, miR-30a, miR-224, miR-96, miR-766, miR-223, miR-99b, miR-140-3p, let-7b, miR-141, miR-191, miR-146b-5p, miR-491-5p, miR-339-3p, miR-200c, miR-106b, miR-143, miR-429, miR-222 and miR-200a | Urine | Up | Diagnosis | [39] |
| Ratio of miR-6124/miR-4511 | Urine | Up | Diagnosis | [40] |
| miR-30a-3p, miR-99a-5p, miR-137-3p | Cells | Up | Discrimination of MIBC from NMIBC | [41] |
| miR-141-3p, miR-205-5p | Cells | Down | Discrimination of MIBC from NMIBC | [41] |
| miR-99a, miR-125b | Urine | Down | Diagnosis | [42] |
| miR-152 | Serum | Up | Prediction of NMIBC recurrence | [37] |
| miR-22-3p, miR-200a-3p | Urine | Up | Prediction of NMIBC recurrence | [38] |
| Six-miRNA panel: miR16, miR200c, miR205, miR21, miR221 and miR34a | Urine | Up | Prediction of NMIBC recurrence | [43] |
| Four-miRNA panel: miR-422a-3p, miR-486-3p, miR-103a-3p and miR-27a-3p | Serum | Up | Prediction of MIBC survival | [44] |
| miR-214 | Urine | Down | Prediction of NMIBC recurrence | [45] |
| miR-21-5p | Cells | Up | Prediction of response to chemotherapy | [46] |
| miR-Let-7i-3p | Cells | Down | Prediction of response to chemotherapy | [46] |
BCa, bladder cancer; MIBC, muscle invasive bladder cancer; NMIBC, non-muscle invasive bladder cancer; and exomiRs: exosomal miRNAs.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22041713
References
- Di Meo, A.; Bartlett, J.; Cheng, Y.; Pasic, M.D.; Yousef, G.M. Liquid biopsy: A step forward towards precision medicine in urologic malignancies. Mol. Cancer 2017, 16, 80.
- Siravegna, G.; Marsoni, S.; Siena, S.; Bardelli, A. Integrating liquid biopsies into the management of cancer. Nat. Rev. Clin. Oncol. 2017, 14, 531–548.
- Mandel, P. Les acides nucleiques du plasma sanguin chez 1 homme. CR Seances Soc. Biol. Fil. 1948, 142, 241–243.
- Iyer, M.; Niknafs, Y.; Malik, R.; Malik, R.; Singhal, U.; Sahu, A.; Hosono, Y.; Barrette, T.R.; Prensner, J.R.; Evans, J.R.; et al. The landscape of long noncoding RNAs in the human transcriptome. Nat. Genet. 2015, 47, 199–208.
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of exosome composition. Cell 2019, 177, 428–445.e18.
- Yokoi, A.; Villar-Prados, A.; Oliphint, P.A.; Zhang, J.; Song, X.; De Hoff, P.; Morey, R.; Liu, J.; Roszik, J.; Clise-Dwyer, K.; et al. Mechanisms of nuclear content loading to exosomes. Sci. Adv. 2019, 5, eaax8849.
- Thind, A.; Wilson, C. Exosomal miRNAs as cancer biomarkers and therapeutic targets. J. Extracell. Vesicles 2016, 5, 31292.
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; How Huang, K.; Jen Lee, M.; Galas, D.J.; Wang, K. The microRNA spectrum in 12 body fluids. Clin. Chem. 2010, 56, 1733–1741.
- Yun, S.J.; Jeong, P.; Kim, W.-T.; Kim, T.H.; Lee, Y.-S.; Song, P.H.; Choi, Y.-H.; Kim, I.Y.; Moon, S.-K.; Kim, W.-J. Cell-free microRNAs in urine as diagnostic and prognostic biomarkers of bladder cancer. Int. J. Oncol. 2012, 41, 1871–1878.
- Minciacchi, V.R.; Freeman, M.R.; Di Vizio, D. Extracellular vesicles in cancer: Exosomes, microvesicles and the emerging role of large oncosomes. Semi. Cell Dev. Biol. 2015, 40, 41–51.
- Tomasetti, M.; Lee, W.; Santarelli, L.; Neuzil, J. Exosome-derived microRNAs in cancer metabolism: Possible implications in cancer diagnostics and therapy. Exp. Mol. Med. 2017, 49, e285.
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518.
- Xi, Y.; Nakajima, G.; Gavin, E.; Morris, C.G.; Kudo, K.; Hayashi, K.; Ju, J. Systematic analysis of microRNA expression of RNA extracted from fresh frozen and formalin-fixed paraffin-embedded samples. RNA 2007, 13, 1668–1674.
- Rabinowits, G.; Gerçel-Taylor, C.; Day, J.M.; Taylor, D.D.; Kloecker, G.H. Exosomal microRNA: A diagnostic marker for lung cancer. Clin. Lung Cancer 2009, 10, 42–46.
- Taylor, D.D.; Gercel-Taylor, C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008, 110, 13–21.
- Andre, F.; Schartz, N.E.; Movassagh, M.; Flament, C.; Pautier, P.; Morice, P.; Pomel, C.; Lhomme, C.; Escudier, B.; Le Chevalier, T. Malignant effusions and immunogenic tumour-derived exosomes. Lancet 2002, 360, 295–305.
- Valenti, R.; Huber, V.; Filipazzi, P.; Pilla, L.; Sovena, G.; Villa, A.; Corbelli, A.; Fais, S.; Parmiani, G.; Rivoltini, L. Human tumor-released microvesicles promote the differentiation of myeloid cells with transforming growth factor-β–mediated suppressive activity on T lymphocytes. Cancer Res. 2006, 66, 9290–9298.
- Zhou, B.; Xu, K.; Zheng, X.; Chen, T.; Wang, J.; Song, Y.; Shao, Y.; Zheng, S. Application of exosomes as liquid biopsy in clinical diagnosis. Signal. Transduct. Target. Ther. 2020, 5, 144.
- Magy-Bertrand, N. Actualités sur les amyloses. La Rev. De Médecine Interne 2016, 37, 529–535.
- Wang, M.; Yu, F.; Ding, H.; Wang, Y.; Li, P.; Wang, K. Emerging function and clinical values of exosomal MicroRNAs in cancer. Mol. Ther. Nucleic Acids 2019, 16, 791–804.
- Rahbarghazi, R.; Jabbari, N.; Sani, N.A.; Asghari, R.; Salimi, L.; Kalashani, S.A.; Feghhi, M.; Etemadi, T.; Akbariazar, E.; Mahmoudi, M. Tumor-derived extracellular vesicles: Reliable tools for Cancer diagnosis and clinical applications. Cell Commun. Signal. 2019, 17, 73.
- Liu, J.; Sun, H.; Wang, X.; Yu, Q.; Li, S.; Yu, X.; Gong, W. Increased exosomal microRNA-21 and microRNA-146a levels in the cervicovaginal lavage specimens of patients with cervical cancer. Int. J. Mol. Sci. 2014, 15, 758–773.
- Cui, S.; Cheng, Z.; Qin, W.; Jiang, L. Exosomes as a liquid biopsy for lung cancer. Lung Cancer 2018, 116, 46–54.
- Hsu, Y.; Hung, J.; Chang, W.; Lin, Y.; Pan, Y.; Tsai, P.; Wu, C.; Kuo, P. Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1. Oncogene 2017, 36, 4929–4942.
- Bao, L.; You, B.; Shi, S.; Shan, Y.; Zhang, Q.; Yue, H.; Zhang, J.; Zhang, W.; Shi, Y.; Liu, Y. Metastasis-associated miR-23a from nasopharyngeal carcinoma-derived exosomes mediates angiogenesis by repressing a novel target gene TSGA10. Oncogene 2018, 37, 2873–2889.
- Sruthi, T.; Edatt, L.; Raji, G.R.; Kunhiraman, H.; Shankar, S.S.; Shankar, V.; Ramachandran, V.; Poyyakkara, A.; Kumar, S.V. Horizontal transfer of miR-23a from hypoxic tumor cell colonies can induce angiogenesis. J. Cell Physiol. 2018, 233, 3498–3514.
- Lin, X.-J.; Fang, J.-H.; Yang, X.-J.; Zhang, C.; Yuan, Y.; Zheng, L.; Zhuang, S.-M. Hepatocellular carcinoma cell-secreted exosomal microRNA-210 promotes angiogenesis in vitro and in vivo. Mol. Ther. Nucleic Acids 2018, 11, 243–252.
- Fang, J.H.; Zhang, Z.J.; Shang, L.R.; Luo, Y.W.; Lin, Y.F.; Yuan, Y.; Zhuang, S.M. Hepatoma cell-secreted exosomal microRNA-103 increases vascular permeability and promotes metastasis by targeting junction proteins. Hepatology 2018, 68, 1459–1475.
- Liu, Y.; Luo, F.; Wang, B.; Li, H.; Xu, Y.; Liu, X.; Shi, L.; Lu, X.; Xu, W.; Lu, L. STAT3-regulated exosomal miR-21 promotes angiogenesis and is involved in neoplastic processes of transformed human bronchial epithelial cells. Cancer Lett. 2016, 370, 125–135.
- Wang, X.-F.; Zhang, X.-W.; Hua, R.-X.; Du, Y.-Q.; Huang, M.-Z.; Liu, Y.; Cheng, Y.F.; Guo, W.-J. Mel-18 negatively regulates stem cell-like properties through downregulation of miR-21 in gastric cancer. Oncotarget 2016, 7, 63352–63361.
- Armstrong, D.A.; Green, B.B.; Seigne, J.D.; Schned, A.R.; Marsit, C.J. MicroRNA molecular profiling from matched tumor and bio-fluids in bladder cancer. Mol. Cancer 2015, 14, 194.
- Yasui, T.; Yanagida, T.; Ito, S.; Konakade, Y.; Takeshita, D.; Naganawa, T.; Nagashima, K.; Shimada, T.; Kaji, N.; Nakamura, Y.; et al. Unveiling massive numbers of cancer-related urinary-microRNA candidates via nanowires. Sci. Adv. 2017, 3, e1701133.
- Andreu, Z.; Oshiro, R.O.; Redruello, A.; López-Martín, S.; Gutiérrez-Vázquez, C.; Morato, E.; Marina, A.I.; Gómez, C.O.; Yáñez-Mó, M. Extracellular vesicles as a source for non-invasive biomarkers in bladder cancer progression. Eur. J. Pharm. Sci. 2017, 98, 70–79.
- Matsuzaki, K.; Fujita, K.; Jingushi, K.; Kawashima, A.; Ujike, T.; Nagahara, A.; Ueda, Y.; Tanigawa, G.; Yoshioka, I.; Ueda, K.; et al. MiR-21-5p in urinary extracellular vesicles is a novel biomarker of urothelial carcinoma. Oncotarget 2017, 8, 24668–24678.
- Long, J.D.; Sullivan, T.B.; Humphrey, J.; Logvinenko, T.; Summerhayes, K.A.; Kozinn, S.; Harty, N.; Summerhayes, I.C.; Libertino, J.A.; Holway, A.H.; et al. A non-invasive miRNA based assay to detect bladder cancer in cell-free urine. Am. J. Transl. Res. 2015, 7, 2500–2509.
- Pardini, B.; Cordero, F.; Naccarati, A.; Viberti, C.; Birolo, G.; Oderda, M.; Di Gaetano, C.; Arigoni, M.; Martina, F.; Calogero, R.A.; et al. microRNA profiles in urine by next-generation sequencing can stratify bladder cancer subtypes. Oncotarget 2018, 9, 20658–20669.
- Jiang, X.; Du, L.; Wang, L.; Li, J.; Liu, Y.; Zheng, G.; Qu, A.; Zhang, X.; Pan, H.; Yang, Y.; et al. Serum microRNA expression signatures identified from genome-wide microRNA profiling serve as novel noninvasive biomarkers for diagnosis and recurrence of bladder cancer. Int. J. Cancer 2015, 136, 854–862.
- Du, L.; Jiang, X.; Duan, W.; Wang, R.; Wang, L.; Zheng, G.; Yan, K.; Wang, L.; Li, J.; Zhang, X.; et al. Cell-free microRNA expression signatures in urine serve as novel noninvasive biomarkers for diagnosis and recurrence prediction of bladder cancer. Oncotarget 2017, 8, 40832–40842.
- Urquidi, V.; Netherton, M.; Gomes-Giacoia, E.; Serie, D.J.; Eckel-Passow, J.; Rosser, C.J.; Goodison, S. A microRNA biomarker panel for the non-invasive detection of bladder cancer. Oncotarget 2016, 7, 86290–86299.
- Piao, X.M.; Jeong, P.; Kim, Y.H.; Byun, Y.J.; Xu, Y.; Kang, H.W.; Ha, Y.S.; Kim, W.T.; Lee, J.Y.; Woo, S.H. Urinary cell-free microRNA biomarker could discriminate bladder cancer from benign hematuria. Int. J. Cancer 2019, 144, 380–388.
- Baumgart, S.; Meschkat, P.; Edelmann, P.; Hartmann, A.; Bohle, R.; Pryalukhin, A.; Heinzelmann, J.; Stöckle, M.; Junker, K. Invasion-associated miRNAs as possible diagnostic biomarkers of muscle invasive bladder cancer in tumor tissues and urinary exosomes. J. Urol. 2018, 199, e1038.
- Zhang, D.Z.; Lau, K.M.; Chan, E.S.; Wang, G.; Szeto, C.C.; Wong, K.; Choy, R.K.; Ng, C.F. Cell-free urinary microRNA-99a and microRNA-125b are diagnostic markers for the non-invasive screening of bladder cancer. PLoS ONE 2014, 9, e100793.
- Sapre, N.; Macintyre, G.; Clarkson, M.; Naeem, H.; Cmero, M.; Kowalczyk, A.; Anderson, P.D.; Costello, A.J.; Corcoran, N.M.; Hovens, C.M. A urinary microRNA signature can predict the presence of bladder urothelial carcinoma in patients undergoing surveillance. Br. J. Cancer 2016, 114, 454–462.
- Jiang, X.; Du, L.; Duan, W.; Wang, R.; Yan, K.; Wang, L.; Li, J.; Zheng, G.; Zhang, X.; Yang, Y.; et al. Serum microRNA expression signatures as novel noninvasive biomarkers for prediction and prognosis of muscle-invasive bladder cancer. Oncotarget 2016, 7, 36733–36742.
- Kim, S.M.; Kang, H.W.; Kim, W.T.; Kim, Y.J.; Yun, S.J.; Lee, S.C.; Kim, W.J. Cell-Free microRNA-214 From Urine as a Biomarker for Non-Muscle-Invasive Bladder Cancer. Korean J. Urol. 2013, 54, 791–796.
- Fanous, H.; Sullivan, T.; Rieger-Christ, K. Distinct exosomal miRNA profiles in chemoresistant bladder carcinoma cell lines. J. Urol. 2017, 197, e1179–e1180.
