Plasma is the predominant state of matter in the known universe (it is estimated that up to 99% of matter is plasma), although not on our planet, where the conditions of pressure and temperature make normal the states of matter—solid, liquid, and gas—that in global terms are exotic. If we add energy to a gas, we will partially or totally ionize it. In this way, we reach a new state of matter, plasma, made up of free electrons, atoms and molecules (electrically neutral particles), and ions. The energy needed to generate plasma can be supplied through electrical discharges in gases, in which free electrons take energy field and lose it through excitation and ionization processes of the atoms and molecules in the gas. The interaction of a plasma with a surface, either solid, liquid or belonging to a live system, is a complex process involving many different active species and reactions. The use of cold plasma for medical applications is at present in the rise as it has been proven as a powerful therapeutic tool for healing, desinfection and surface functionalization
- plasmas
- RONS
- atmospheric plasmas
- plasma material interactions
- plasma medicine
1. Introduction
In 1969, John R. Hollahan’s group experimentally demonstrated that with cold ammonia plasmas or mixtures of nitrogen and hydrogen, amino groups (–NH2) were produced, which, by adhering to the surface of different types of polymers, created materials compatible with blood [1]. Since then, the use of cold plasmas to optimize the interaction between biological systems and different types of materials has been investigated, with the ultimate goal of achieving biocompatible surfaces. Cold plasma treatment only affects the surface of the treated material. The physical, chemical, mechanical, electrical, and optical properties of the interior of the material are not altered by the cold plasma. On the other hand, the use of different acids and chemical solvents can damage the surface of many plastics and, if absorbed, affect the properties of the interior of the material.
Plasma is the predominant state of matter in the known universe (it is estimated that up to 99% of matter is plasma), although not on our planet, where the conditions of pressure and temperature make normal the states of matter—solid, liquid, and gas—that in global terms are exotic. It is enough to add energy to the solid (in the form of heat or electromagnetic radiation) for it to turn into a liquid state, from which gas is obtained through an additional supply of energy. If we continue adding energy to the gas, we will partially or totally ionize it, that is, we will remove electrons from the atoms or molecules that constitute it. In this way, we reach a new state of matter, plasma, made up of free electrons, atoms and molecules (electrically neutral particles), and ions. The energy needed to generate plasma can be supplied in several ways: through heat from a combustion process; through the interaction between laser radiation and a solid, a liquid, or a gas; or through electrical discharges in gases, in which free electrons take energy field and lose it through excitation and ionization processes of the atoms and molecules in the gas.
One of the peculiarities of plasmas is that they conduct electricity. On a macroscopic scale, plasmas are, however, electrically neutral, since the number of positive and negative charges is similar. Thus, the flame produced by burning candle wax in combination with oxygen from the air—a typical example of a plasma that is very little ionized—can conduct electricity. A graphical overview of the different kinds of plasmas according to their microscopic parameters (electron density and temperature) is displayed in Figure 1
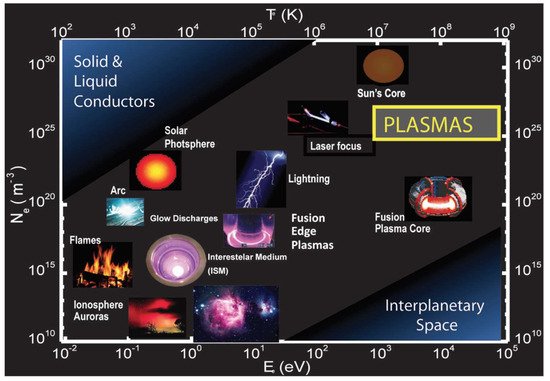
The values of density and electronic temperature, two of the main parameters that characterize plasmas, cover a wide spectrum. Thus, the electron density varies between 1 electron/cm3 and 1025 electrons/cm3; that is, it even exceeds the concentration of electrons in metals. On the other hand, the average free path of the particles in a plasma, that is, the average distance covered before a particle collides with another particle in the plasma can range from tens of millions of kilometers to just a few microns.
Classifying the diversity of types of plasmas that exist in nature or that can be generated artificially is not easy, since it is risky to choose isolated parameters that serve as criteria to establish the differences. Despite these difficulties, we can venture into a first classification of the types of plasmas, one that considers their thermal equilibrium, that is, whether or not the temperature or the average energy of the particles that make it up is the same for each type of particle. All particles have the same temperature (thermal equilibrium) for stellar interior plasma or for its terrestrial analogs, deuterium-tritium fusion plasmas and the impurities generated in experimental controlled nuclear fusion devices, like the JET and the ITER. These plasmas are also called hot or thermal plasmas since the temperature inside them reaches millions of degrees (107 °C–109 °C), the same for the electrons as for heavy species [2].
When the gas pressure is low or the electrical voltage applied in the discharge is high, the electrons in the plasma acquire, in the time between collisions with other plasma particles, kinetic energies higher than the energy associated with the random thermal movement of the neutral particles (atoms and molecules) of the plasma. We can then attribute some degree of thermal equilibrium deviation to plasmas, since electrons, ions, and neutral particles have different “temperatures” or average kinetic energies (in the case of non-Maxwellian distributions), rather common for plasmas produced at low pressure and with a small degree of ionization.
Nonthermal plasmas, also known as cold plasmas, are characterized by the fact that the temperature of heavy species (neutral particles and ions) is close to room temperature (25 °C–100 °C). Instead, the electronic temperature is much higher (between 5000 °C and 105 °C). Cold plasmas usually occur at a low pressure (p < 133 mbar) in reactors with very different geometries. Such reactors generate plasmas through direct current, radio frequency, microwave, or pulsed discharge systems.
There are special types of cold plasmas, produced in the so-called corona and dielectric barrier discharges, that are generated at atmospheric pressure by using pulses between 10–6 s and 10–9 s. In these types of discharges, referred to as CAP (cold atmospheric plasma), the highly energetic electrons are produced that, due to the shortness of the pulses used, have little time to exchange energy with their surroundings. This kind of plasmas have found many applications in the field of plasma medicine, among others, and have led to off-the-shelf devices of increasing popularity.
2. Plasma Treatment of Medical Materials
Although the very state-of-the-art of the topic is represented by the kind of research articles included in this special issue, in order to illustrate the practical applications of cold plasmas in the field of biomedicine under the light of the plasma physio-chemistry, two classical examples are addressed herein: enhancement of the wetting properties and biocompatibility of medical surfaces and generation of active radicals as bactericides, which are more commonly used for direct treatment of bacterial infections. While surface treatments are mainly (but not exclusively [11,13]) carried out under vacuum conditions, thus profiting from the high energies of the ionic species, active radicals for in situ treatments are produced at atmospheric pressure (CAP).
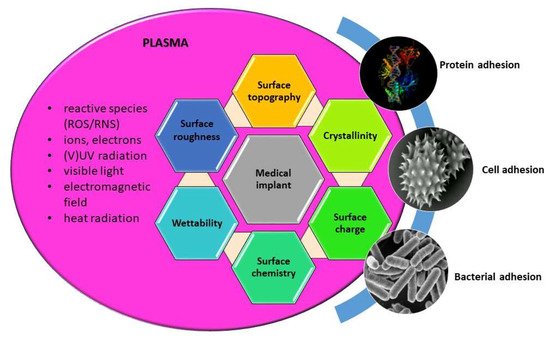
Thus the use of low pressure plasma in medicine is basically aimed at the modification of surfaces, as it is well-known that biological response is mainly governed by surface properties of materials, such as wettability, chemistry, morphology (nanotopography), crystallinity, as well as surface charge. All these surface features can be altered by plasma treatment, as already discussed in previous sections. Schematic presentation on induced surface changes and their influence on biological interactions; adhesion and conformation of proteins, adhesion and proliferation of cells, and bacterial adhesion are schematically presented in Figure 5. By appropriately adjusting the discharge and plasma parameters desired modification of material surface used for a specific medical application can be achieved.
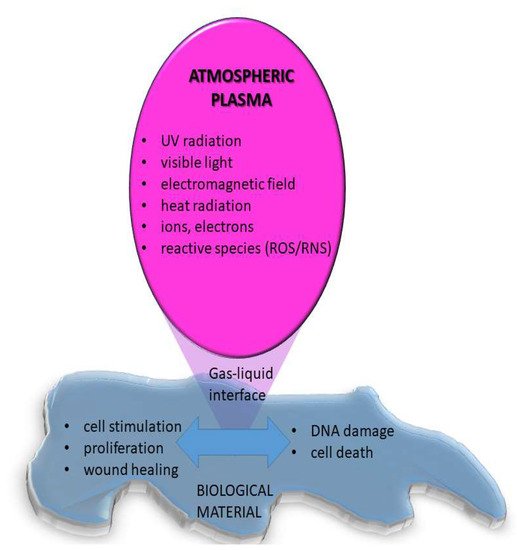
On the other hand, when atmospheric pressure plasma is used for medical applications, the interaction of plasma species with living organisms or liquids is addressed [26] as schematically displayed in Figure 6. In this case, the plasma may stimulate cell adhesion and proliferation or can cause cell death. It was shown that plasma can selectively kill cancer cells but not healthy cells, mainly due to RONs [22]. Treatment of bacterial infections, especially in case of chronic wounds was also shown to have significant success and it is already medically approved and used in praxis [21]. Results of a randomized clinical trial show that treatment of diabetic foot ulcers by CAP (kINPen Med; neoplast tools GmbH) reduced wound size, clinical infections, and microbial load compared to the initial state [27]. By optimizing the treatment conditions it is possible to achieve a desired biological response, although the exact mechanisms of interactions mainly based on redox reactions are not fully known and understood.
2.1. Enhanced Surface Hydrophilicity and Cell Adhesion
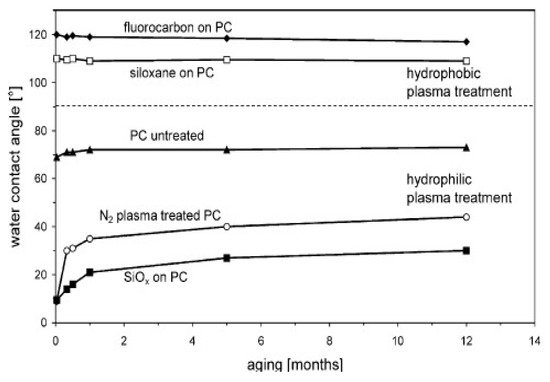
2.2. Plasma Generation of RONs for Medical Treatment
Plasma has emerged as anti-cancer therapeutic agent given its cancer-cell-apoptotic action. In particular, cancer-cell-selective killing by air or Ar plasma has been observed, although exactly how and why cancer cells are sensitive to plasma remains unknown. Although the biologically effective species generated from plasma and its cellular targets remain unknown, several lines of evidence link reactive oxygen/nitrogen species (ROS/RNS) to its biological effects, and targeting cancer cells through ROS-mediated mechanisms has become an attractive strategy for the effective and selective cancer treatment by exploiting the aberrant redox characteristics of cancer cells. It has been postulated [43] that ROS/RNS induced by air or Ar plasma effectively targets cancer cells via mitochondrial dysfunction and activation of oxidative stress signaling pathways [43,44,45]. However, this issue is highly complex as there are also other indications like immune system modulation, which may also induce cell death.
Complex chemistry is triggered when an electrical discharge is produced in air at atmospheric pressures. The initially produced molecular and atomic ions of the air components quickly undergo collisional processes releasing energy, thus heating the gas, and initiating a series of chain reactions ultimately leading to the production of the active agents. Commonly observed chemical species at significant concentrations include ozone (O3), singlet delta oxygen (O2,a1Δg), atomic nitrogen (N), hydroxyl radical (OH), various nitrogen oxides (e.g., NO, NO2, and N2O), hydrogen peroxide (H2O2) and nitric and nitrous acid (HNO3, HNO2), among others. In some cases, these species are similar or identical to known RONS of importance in biology [43].
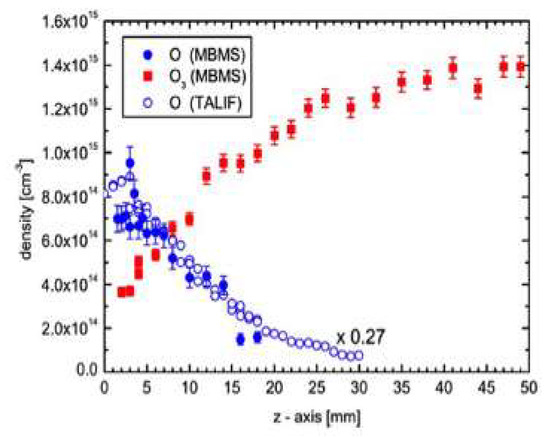
An overwhelming effort has been devoted to the simulation of these highly complex systems [6], although only through targeted experiments it is possible to disentangle the individual impact of selected candidates. As an example, Figure 9 shows the measured atomic oxygen and ozone concentration distribution for a He/O2 plasma (right) [46]. The results closely follow the predicted distribution of active O species along the plasma jet for an Ar/O2 plasma [47]. So, although a different main gas is used, very similar results are obtained, as expected from the lack of chemical effects by noble gases. Also, according to the model predictions, a very fast decay of charged particle density takes place in the jet, and metastable O2 molecules together with ozone and nitric oxide are the only species surviving one centimeter away from the nozzle [47].
In addition to electron-mediated processes, and depending on the gas flow, temperature effects may play a role in the generation of active agents. Very recently, Cejas et al. [48] developed a model of a stationary glow-type discharge in atmospheric-pressure air operated in high-gas-temperature regimes (1000 K < Tg < 6000 K), focusing on the role of associative ionization reactions involving N (2D,2P) excited atoms. The kinetic model included processes involving positive (i.e., NO+, N2+, O2+, and O+) and negative ions (O−, O2 −, and O3−), neutral species (i.e., N2 in the ground and vibrational and electronic excited states, N(2D), N(4S), N(2P), O(3P),O(1D), O(1S), O2, and NO), and electrons (e) which, together with the corresponding chemical reactions accounts for more than one hundred elemental processes in the gas phase. As a distinctive feature, the model incorporated an exoergic associative ionization reaction with the participation of N(2P) atoms and a near-threshold reaction with the participation of N(2D) atoms with a low activation barrier of 0.38 eV which can be described as:
The results of the calculations suggested a strong impact of the electronically excited states of reactants on associative ionization reactions in atomic collisions in hot air.
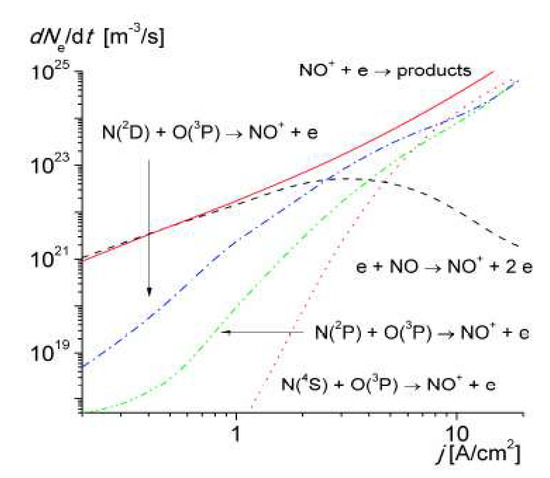
It was shown that the near-threshold associative ionization reaction involving N(2 D) atoms progressively replaced the ionization of NO molecules by electron impact at Tg > 2500 K (corresponding to a current density of > 1A/cm2), becoming the main ionization mechanism in air up to 4000–4500 K. Figure 10 shows the relative contribution of associative ionization to the electron production rate in the discharge.
Finally, it should be noted that models become exponentially expensive in terms of computer time as the number of species included in them increases. Very recently, He et al. [49] modeled the kinetics of a micro-scaled atmospheric pressure plasma jet (μAPPJ) of He/N2/O2 by using a 0-D model for the chemistry and plug flow for the gas transport (pseudo 1-D model). A total of 41 species and 516 reactions were initially considered. However, inclusion of vibrational levels of the molecular species (deemed necessary to take into account their contribution to the high energy tail of the EEDF in N2-containing plasmas [50] boosted the system to 138 species and 11733 elemental reactions. Even so, matching the experimental results on nitric oxide production by the plasma required the inclusion of metastable N(D) reactions and some tuning of the rate constants involved in its generation.
In conclusion, and is spite of the tremendous efforts made in the field, the complexity of the systems involved in the therapeutic applications of CAP research makes it difficult to predict the healing properties of these plasmas, and a rather empirical approach to the topic prevails so far. Very well-defined experiments assisted by modeling may render a deeper understanding of this highly promising subject.
This entry is adapted from the peer-reviewed paper 10.3390/molecules26071903
