The modern boron applications have adsorbed the mineral processors’ attention to improve typical boron mineral’s (BM) beneficiation methods. In this regard, dry treatment and pretreatment processes—such as magnetic separation and calcination as environmentally friendly methods, due to their minimal or zero adverse effect on the environment—need more consideration. Over the years, anionic flotation has become the main technique for beneficiation of friable BMs; however, there is a gap in the investigation of cationic flotation separation since BMs’ surface negatively charges in a wide pH range. At present, enriching BMs’ flotation via surface modification is taking center stage, which can also be considered for reprocessing long-forgotten BM tailings.
- borax
- ulexite
- colemanite
- flotation
- magnetic separation
- flocculation
- calcination
1. Introduction
Over 150 Boron “B” minerals (BM) have been recorded, and the most frequent ones are borax or tyncal (Na2B4O5 (OH)4·10H2O), ulexite (NaCaB5O6 (OH)6·5H2O), colemanite (Ca2B6O115H2O), and kernite (Na2B4O7·4H2O) [1][2][3]. It was reported that boron compounds are generally produced from brines rich in this element [4][5]. Typically, BMs are naturally found as hydrated oxides of alkali metals (especially sodium and calcium), which lose crystallization water through the heating [6]. Worldwide, the estimated BM reserves exceed a billion metric tons, against a yearly production of about four million tons. Turkey, where the largest borate reserve globally is found, is followed by Russia, South America, and the United States. These four countries/regions account for an estimated 95% of the world’s Boron reserves (data from Eti Maden AS). Simultaneously, countries such as China, Italy, and Germany meet only the local demand due to their smaller deposits [7].
BMs have been used in various applications dating from the eighth century when applied in the refining and assaying of gold and silver [8]. They have been used in several other applications such as medicines, food preservatives, ceramic glazes, and metal fluxes [9]. The modern applications of BMs and boron derivatives include microbicides, insecticides, wood treatment agents, washing products (e.g., detergents and soaps), special alloys, fertilizers, fire retardants, fiberglass, and heat resistant glass (e.g., Pyrex) [10][11][12][13]. BMs also can be used in the metallurgical industries [2][14]. Boron compounds have a considerable potential to be used in the energy sector where “B” as a hydrogen carrier in the form of BH4, has a higher hydrogen storage capacity than other hydrides and compounds; hence, it can be used as a hydrogen carrier. However, it was reported that the glass, ceramics, and agricultural sectors consume about 80% of refined borates and boric acid produced in the world (Figure 1) [15].
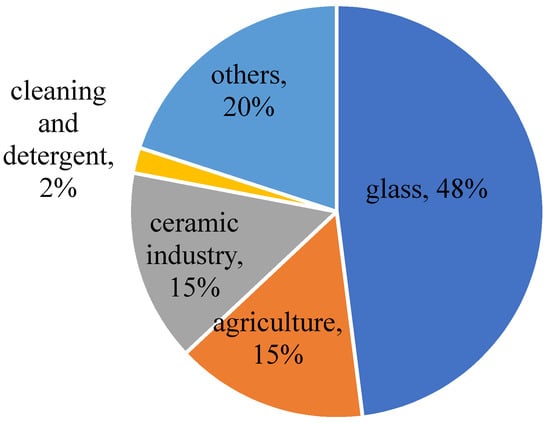
Figure 1. General applications of boron.
Although significant attention has now been drawn to BMs, their Processing poses severe challenges. BMs and their associated gangues are usually salt-type minerals, naturally hydrophilic, soluble, and exhibit similar surface characteristics. For instance, colemanite and realgar (its associated valuable mineral) show similar water contact angles [16]. Ulexite, borax, and associated clay gangue minerals exhibit negative zeta potentials at all practical pH [1][2][11][17]. Moreover, BMs are relatively friable; thus, their grinding generates fines, which enhance slime coating and loss of a sufficient amount of valued minerals to the tailings [18][19]. Furthermore, the conventional wet mechanical attrition and washing processes used for pretreatment of BMs for further processing promote slime coating and are environmentally unfriendly [6][20]. All of these obstacles limit the efficiencies of beneficiation methods employed in the processing of BMs.
Therefore, removing gangue minerals from BMs utilizes several preprocessing techniques such as ultrasonic pretreatment [21], attrition-scrubbing/classification [22][23], and thermal decomposition/decrepitation [24]. These pretreatment techniques are followed by various beneficiation methods such as electrostatic and magnetic separations [25], and flotation [4][23][26][27]. These processes exhibit grave limitations and may lead to loss of BMs in the tailings and cause various environmental problems. Hence, they need to be controlled and optimized by size reduction, surface property modification, zeta potential and pH regulation, and the application of appropriate reagent types. However, there are gaps in the scientific literature concerning BMs’ Processing as accentuated by the limited publications before 2010 on BMs referenced in this work.
2. Particle Size
Calcination at 450 °C successfully processes BMs, especially borax, which causes the minerals to expand in size to a maximum degree. This is possible at up to 50 mm particle size. Fragmentation then occurs once the calcined BMs reach their full growth in size due to heat, with air classification utilized after that to separate the fine gangue minerals from the BMs. [6]. However, an upper threshold of around 9.5 mm was reported as the largest particle size for the efficient BM processes. Decreasing the BM particle sizes increases their solubility [28]. This increase is detrimental to selecting an efficient upgrading process. Traditional methods for concentrating BMs are scrubbing, washing, and classification, followed by the size reduction steps [9][29][30]. Nevertheless, these non-chemical processes are generally successful only at a high grade and coarse particle size fractions, and their performances diminish at fine particle ranges [30]. As BMs are friable, the mining and processing steps continuously magnify the fine particle production [18]. These fine particles are the roots of severe predicaments in storage and environmental pollution [18][19], discharging in tailings and losing a substantial amount of valuable minerals. Typically, for the physical upgrading processes, size fractions below 0.2–3 mm are designated as fines and challenge these types of processes [19][29]. It was reported if particle sizes finer than 150–200 μm is delivered to the tailings, it would lead to loss of around 20–26% of B2O3 [19][23][30]. Hence, significant attention has been turned to developing innovative methods for processing BM fines. For other processes such as magnetic separation, the smaller size limit is 75 μm [31][32]. It was documented that particle size of about 38 μm is generally used as the lower threshold limit to prevent slimes’ adverse effects for beneficiation by froth flotation [20][33].
3. Magnetic Separation
Calcium and magnesium borates pose a severe challenge to wet method processing. Thus, dry methods—such as calcination, magnetic separation, and ultrasound techniques—could have a high potential for their processing. Calcination through decrepitation concentrates borates in the coarse fractions [7]. However, calcination cannot remove iron impurities. Several investigations have suggested that magnetic separation has a high potential of being used for processing diamagnetic BMs from their typical associated clay gangue phase [27][31][34][35][36]. This separation is possible because minerals of as low as 5 × 10−6 m3/kg are still capable of being separated at field gradients of about 50–500 T/m with a considerable high magnetic field around 0.8 T [31]. Generally, the magnetic susceptibility of clay gangues depends on their iron content. Therefore, they may respond to a high magnetic field (when they have adequately high susceptibilities). It was reported that a magnetic separator operating at 0.45 T using a steel ball matrix could reduce BM’s concentrate iron component to as low as 0.6%, which is within the market limit of 0.7%. Nevertheless, its recovery of 80% is not encouraging; hence, it can be considered as a pre-concentration technique [7].
The paramagnetic properties of the colemanite ore are contingent on magnetite [7]. These properties also depend on silicate minerals in their structure, including the smectite group, in which iron is distributed (iron readily experiences oxidation). It was well understood that the whole sample’s magnetic susceptibility is increased by the high mass fraction of iron-containing silicates [37]. Thus, samples with high colemanite content may turn out to have low susceptibilities. Reference [35] indicated that samples with ≥63 wt % colemanite content exhibited low susceptibilities. This lower attraction to magnet may lead to a selective separation of diamagnetic colemanite from its paramagnetic iron-bearing silicate gangue in a high magnetic field. Dry magnetic separation as a simple, environmentally acceptable beneficiating process is preferable to flotation and decrepitation for processing colemanite ores [2][26]. However, as mentioned, it has a size limitation, and for processing fine BMs, flotation has to be used.
4. Flotation
Flotation has the highest potential for processing of the BM fine particles [11][12]. However, as ultrafine particles (i.e., slimes) have an unfavorable impact on the BMs’ flotation recovery and grade [30][38], various pretreatment methods can be implemented before flotation separation. It should be noted that these modification processes could stand alone in some instances.
4.1. Pretreatment
4.1.1. Ultrasonic Treatment
Ultrasound is a cost-effective and environmentally friendly pretreatment method that can disperse clay minerals before removing them and decreasing their BM coating rate [20][21][39]. The attachment of clay minerals on the surface of colemanite particles is a significant factor for decreasing their flotation efficiency. Hence, a series of flotation experiments have been conducted to determine ultrasonic treatment’s ability in dispersing clays from the colemanite surface [11]. Celik et al. reported that flotation could recover 5% of colemanite without ultrasonic pretreatment, while over 90% of the ultrasonically treated sample could be recovered by flotation separation [11]. In-situ ultrasound treatment detaches clay particles from the colemanite surfaces and increases anionic collector adsorption rate on their surfaces [6]. In other words, ultrasonic pretreatment improves reagents’ effectiveness due to the improvement of their uniform distributions and activities in the suspension (Table 1) [40][41][42][43]. It was also reported that conditioning through in-situ sonication requires a lower collector addition (several times lower than the prepared sample by conventional conditioning); hence, it serves as a cheaper separation method [11] (Figure 2). The shearing forces of ultrasound waves are therefore responsible for the reduction in the quantity of collectors/reagents used.
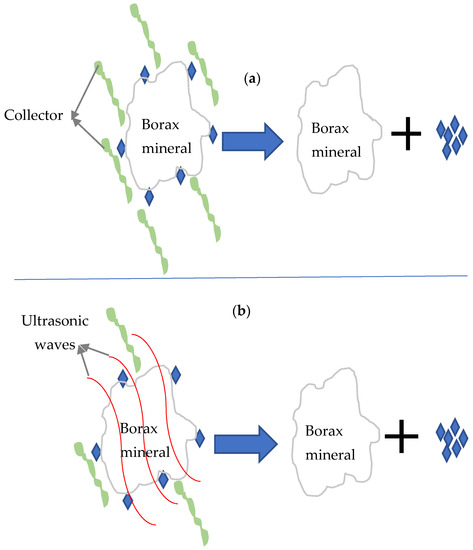
Figure 2. Impact of ultrasonic waves on the flotation of borax mineral: (a) clay minerals coated the surface of borax and more collector is needed for beneficiation; (b) ultrasonic waves cleaned the surface of borax and lower quantity of collector is required.
Table 1. Influence of ultrasonic waves on the flotation of borax minerals
| Reference | Condition | Results | ||||||
|---|---|---|---|---|---|---|---|---|
| Grade (%) | Recovery (%) | |||||||
| Size | Collector | Ultrasound Frequency (kHz) | Power (W) | With Ultrasound | Without Ultrasound | With Ultrasound | Without Ultrasound | |
| [39] | −250 + 38 (µm) | Cytec R825 2000 mg/g |
35 | 120 | 49 | 45 | 90 | 80 |
| [21] | −6 (mm) | 35 | 250 | 35 | 25 | 69 | 67 | |
| [11] | −150 + 74 (µm) | Sodium dodecyl sulfate | 90 | 5 | ||||
Hence, the ultrasound pretreatment technique is superior to mechanical attrition and washing, as its application enhances reagent absorption on the surfaces of BMs, improving flotation (Table 2) [20]. The utilization of ultrasonic waves, followed by the application of reagents, significantly increased the percentage grade of concentrate. This increase in grade is proportional to the reduction of calcined material, with the dispersion of clay particles being improved as particle size increased. The rise in the dispersion can be attributed to better penetration of the waves among coarser particles, resulting in cleaner concentrates [20]. The results showed that ultrasound could be used to obtain high-grade concentrates. However, this technique faced two significant challenges: the need for higher capacity tanks because of long residence time and the considerable initial capital cost. Nevertheless, these difficulties could be offset by low energy requirements and operating costs. Besides, high power ultrasonic generators could occasion superior results, and environmental degradation could be reduced [20].
Table 2. Comparison between ultrasonic beneficiation and mechanical attrition and washing methods [20].
| Pretreatment Method | Particle Size (mm) | Weight (%) | B2O3 (%) | Distribution (%) |
|---|---|---|---|---|
| Ultrasonic beneficiations | −3.000 + 0.038 | 56.25 | 41.69 | 97.01 |
| −0.038 + dissolved | 43.75 | 1.65 | 2.99 | |
| Mechanical attrition and washing | −3.000 + 0.038 | 62.16 | 33.01 | 84.93 |
| −0.038 + dissolved | 37.84 | 9.62 | 15.07 |
4.1.2. Calcination
The effect of heat on BMs and their gangues has been extensively investigated from beneficiation results of such minerals [6][44]. It was documented that calcination can be used as a single processing method or a pretreatment technique for processing BMs. Moderate temperatures cause shrinkage of ulexite size [44][45] and decrepitation in colemanite [24][25]. Calcination successfully has been used to process borax when the unaltered gangue resides in the coarse fraction [7]. Unlike the reactions of ulexite and colemanite to heat, borax experienced expansion under heating [6][25][44]. The borax processing via heat pretreatment resulted in a concentrate grade of 51.05% B2O3 with 93.73% recovery [6]. Piskin demonstrated that borax starts to expand above 100 °C and reaches 300% expansion at 450 °C (before shrinking to the original size, borax acquires a flat shape and has greyish color). Temperature, heating time, and particle size can affect the growth of pure borax [44].
There is a positive correlation between the optimum expansion of borax and its optimum upgrading states; as a function of particle size, both growth and upgrading reach their optimum conditions over the 375–475 °C [44]. It was revealed that particle size controls heat access to the particles, and at a given time and temperature, coarse particles would be less affected than finer ones [6]. The maximum concentrate grade (>52%) was obtained at a large particle size fraction (−12.6 + 4.76 mm) at 475 °C, but it was possible to arrive at a grade of above 50% with >85–90% recovery at a smaller particle size fraction at 375–425 °C (Table 3). The gangue, mainly clay minerals, undergo changes as an increase in heat application gradually hardens the clay minerals, making them separate material within the borax matrix [6]. This phenomenon could lead to a selective separation of BMs from clay minerals [44]. Concerning grades, 15 min was reported as the optimum heating time for most fractions (Table 3). However, an upper limiting particle size of 9.51 mm was required for obtaining over 80% recoveries. Therefore, investigators suggested this narrow particle size range enhances both grade and recovery in real plant operations [6].
Table 3. Effect of particle size, temperature, and heating time on borax recovery and grade [6].
| Particle Size (mm) | Feed | Concentrate | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Weight (%) | % B2O3 Grade |
Optimum % B2O3 Grade |
Temp. (°C) |
Optimum % B2O3 Recovery | Temp. (°C) |
Optimum % B2O3 Grade |
Heating Time (min) |
Optimum % B2O3 Recovery |
Heating Time (min) |
|
| +0.50–1.00 | 9.00 | 29.91 | 43.8 | 325 | 85.0 | 375 | 46.0 | 15 | 90.2 | 10 |
| +1.00–3.00 | 17.51 | 54.15 | 49.6 | 375 | 90.0 | 375 | 55.0 | 15 | 86.1 | 10 |
| +3.00–4.76 | 10.81 | 25.17 | 53.0 | 375 | 87.5 | 375 | 58.1 | 15 | 96.0 | 10 |
| +4.76–9.51 | 14.93 | 22.71 | 53.5 | 425 | 88.0 | 375 | 57.3 | 15 | 90.3 | 10 |
| +9.51–12.50 | 8.63 | 23.54 | 48.5 | 475 | 81.0 | 475 | 61.0 | 25 | 79.8 | 15 |
| +12.50–25.00 | 21.58 | 23.92 | 52.4 | 475 | 90.0 | 475 | 60.1 | 15 | 70.3 | 15 |
| +25.00–50.00 | 5.92 | 18.02 | 54.0 | 575 | 75.0 | 575 | 60.2 | 25 | 50.1 | 10 |
Calcination is environmentally friendly in comparison to wet methods. Calcination only utilizes the application of heat, wherein the minerals expand to such extend that the gangue material becomes easily separable from the desired BMs, without the use of reagents. The wet process encompasses the use of reagents, which, when not properly supervised, pose an environmental problem. Comparing the outcomes obtained from the currently operating industrial-scale wet process showed 33% grade and 83% recovery, while a pilot plant scale dry process resulted in 55% grade and 90% recovery of B2O3 [6]. Another significant advantage of calcination (a dry scheme) over the wet methods is the product’s pelletization or briquette [46]. However, there is a need to conduct more studies into calcination as a beneficiation method for BMs, especially to ascertain the correlation between growth and optimum upgrading [6].
4.2. Surface Properties
4.2.1. Zeta Potential
Zeta potential (ζ) and isoelectric points (IEPs) for a series of BMs in water and when conditioned with reagents have been reported. For instance, colemanite exhibits IEP at a pH of around 10.5 in distilled water (Figure 3) [2][19][26]. Inderite (magnesium borate) and tunellite (strontium borate) show positive ζ potentials with no clear IEP throughout the pH range 7 to 11 [25]. Similarly, ulexite [2][25] and borax [17] exhibit no IEP down to about pH 7 but possess negative ζ potentials.
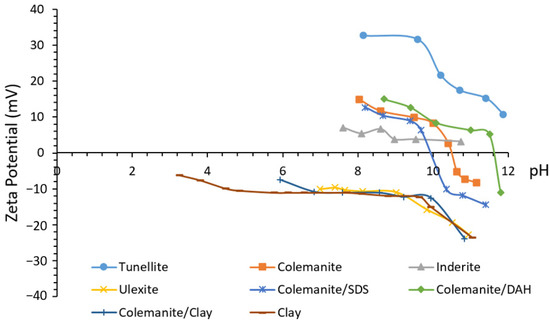
Figure 3. Zeta potential of boron minerals in the presence of clay and some flotation reagents [11][17][47].
Divalent ion (Ca2+) (for colemanite), the anion B4O2−7, and the H+ and OH− ions, which control the HCO3/CO32− ratio, are the constituent lattice members for BMs and determinants for the ζ potentials [25]. It has been proven that ionic strength affects the ζ potentials of colemanite and ulexite [2]. In detail, monovalent ions decrease the charge of colemanite due to the electrical double layer compression; however, they oppositely affect ulexite because of the decrease in calcium [2]. The release of Ca2+ ions in solution depends on the amount of solid percentage, suggesting that the ζ potential of colemanite is directly proportional to changes in the solid concentration [25]. Therefore, solid concentrations of more than 1% or prolonged conditioning time to release all Ca2+ ions produce reliable zeta potential results [29]. For instance, ζ potential studies have shown that clay content as low as 1% can reverse the colemanite surface charge from positive to negative. This reversal indicated that the strong affinity between the negatively charged clay minerals and positively charged colemanite surface through electrostatic attraction is responsible for slime coating during flotation [11].
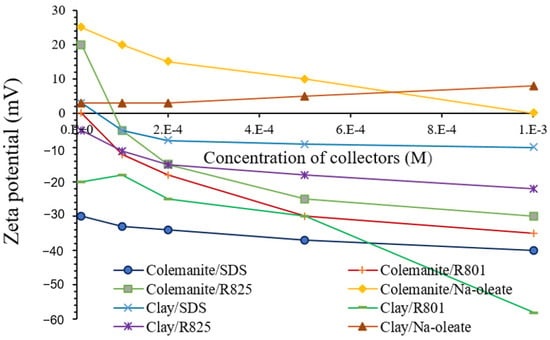
Dodecylamine hydrochloride (DAH) can increase the colemanite IEP to around 11.6, while sodium dodecyl sulfate (SDS) negligibly decreases it to around 10 (Figure 3). The SDS and Na-oleate adsorption ensues with the formation of Ca(DS)2 and Ca(Oleate)2 on colemanite surfaces, making its zeta potential mostly negative at pH values higher than 10.2 [13]. The electrokinetic experiments results reveal that both sodium oleate and SDS engendered only small changes to the zeta potential of colemanite and its clayey gangues, as concentration increases even though there were charge reversals in some instances (Figure 4). Furthermore, the carboxylate seems to adsorb more significantly on the clays than on colemanite. However, there were substantial affinities for sulfate adsorption on both minerals. These adsorptions indicate that these two reagents are not reliable for selective separation. On the other hand, sulfonates (R801 and R825) exhibited extensive changes in the zeta potential of colemanite with large negative values after charge reversals and signifying effective adsorption onto the mineral surface. The R801 adsorption seems more prominent than R825. The difference in electrical charge on the surface of colemanite and clay in the presence of sulfonates implies that they could enhance selective separation [19].
4.2.2. Contact Angle
Colemanite should be considered as a highly hydrophilic mineral, primarily due to its chemical structure and relatively high solubility. Realgar also exhibits low contact angles at its interface with water. Increasing pH can cause a negligible change in the contact angle of colemanite (34–32°) and realgar surface (26–27°) [16]. Anionic and cationic collectors affect the colemanite-liquid interfaces’ contact angles (Table 4) [16][19]. Measurements indicated that R801, as an anionic collector, occasioned the greatest hydrophobicity on the surface of colemanite at a low concentration range than any other examined collectors, with a contact angle of up to 73°. This observation suggested that R801 (sulfonates) can generate the required hydrophobicity for colemanite flotation [19]. Moreover, the BMs’ contact angle can increase with a rise in sodium oleate dosage (a carboxylate) at pH 9 (beyond this point, the contact angle slightly decreases). Armac-T (amine), as a cationic collector, in proportions up to 0.10% generated a rise in colemanite contact angle from 32 to 47° at pH 9 [16].
| [19] | [16] | |||||
|---|---|---|---|---|---|---|
| Colemanite | Reagent | Conc. (M) | Colemanite | Realgar | ||
| Reagent | Conc. (M) | Contact Angle (°) | Contact Angle (°) | |||
| R801 | E−5 to E−4 M | 67.0–73.0 | KAX | E−4 to E−2 M | 32.0–34.0 | 25.0–62.0 |
| R825 | E−4 to E−3 M | 57.0–58.0 | KEX | E−4 to E−2 M | 32.0–36.0 | 30.0–63.0 |
| SDS | E−4 to E−3 M | 53.0–56.0 | R825 | 0.01–0.50% | 32.0–35.0 | 32.0–47.0 |
| Na-oleate | E−4 to E−3 M | 50.0–51.0 | R840 | 0.01–0.05% | 36.0–43.0 | 25.0–39.0 |
| Armac-T | 0.01–0.10% | 44.0–48.0 | 32.0–43.0 | |||
| Na-silicate | E−4 to E−2 M | 27.0–34.0 | 26.0–42.0 | |||
Contact angle measurement on the surface of realgar indicated an increase with increasing the concentrations of collectors. Measurements showed high contact angle values (62°), at a concentration of 10−2 M of KAX and KEX at pH 9 (Table 4). These collectors engendered the highest contact angles for realgar and generated the widest difference in hydrophobicities between colemanite and realgar. Whereas colemanite generally exhibited higher contact angles with the sulfonates than the xanthate counterparts, the latter exceedingly surpassed the former as per increasing the contact angle of realgar. These observations indicated that these particular xanthates did not chemically adsorb onto the colemanite surface even at relatively high concentrations. This suggestion agreed with the presented electrokinetic potential measurements. The addition of the sodium silicate as a depressant to the flotation separation changed the contact angles of colemanite and realgar to 27° and 42°, respectively [16].
4.2.3. Effect of Ions
BMs contain monovalent salts in their lattice structure (for instance, sodium being the prominent one in borax). Monovalent ions compress the double layers of minerals and change the surface activity for collector adsorption by modifying the bulk water structure and micellization of collectors. Some investigations showed that monovalent cations and anions change the bulk structure of water and transport the same operation to the surface, depending on their water-breaking and water-making structures. It was also stressed that monovalent ions are adsorbed at the solid surfaces and interact with water [5]. The mechanism of BM flotation was investigated using the chlorides of monovalent ions Na+, K+, and Cs+ in the presence of SDS and DAH surfactants. NaCl and KCl can be structure makers or breakers, while CsCl is a potent structure breaker [28][48]. High dosages of these monovalent salts (≥0.1 M) make electrostatic interactions ineffective (Figure 5) [2][6][49].
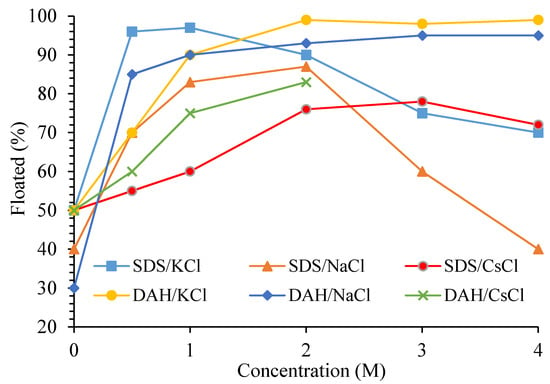
Figure 5. Effect of monovalent salts on colemanite flotation with 4×10−5 M of anionic (SDS) and cationic (DAH) collectors, pH 9.3 ± 0.1. Colemanite was conditioned in the reagents for 10 min and floated for 1 min [11].
Multivalent ions, especially Ca2+, can play different roles in BMs’ flotation separation [26][29]. In the presence of SDS, three areas characterized the reaction of colemanite with cations: (1) the adsorption of divalent ions onto the surface of colemanite; (2) the adsorption of an anionic surfactant on the surface; and (3) the bulk precipitation of metal dodecyl sulfate onto the surface of colemanite (surface precipitation occurs before bulk precipitation) [50]. Relative to their solubility, multivalent ions react with collectors; for instance, with dodecyl sulfate (DS), the product strengths of collector salts show the following order Ba(DS)2 < Ca(DS)2 < Mg(DS)2 [26]. As Figure 6 showed, the least soluble Ba(DS)2 activates colemanite most; the most soluble Mg(DS)2 activates it the least. It was also reported that Ca(DS)2 unavoidably precipitates at a high salt concentration [6][51]. Flotation experiments on colemanite samples from Emet mines in Turkey indicated that increasing Ca2+ and Mg2+ ion concentrations from 0 to 300 ppm reduced B2O3% recovery values from 99–65% for combined usage of Ca and Mg ions. The addition of these ions increased the tailings grade values by 18–25% [52].
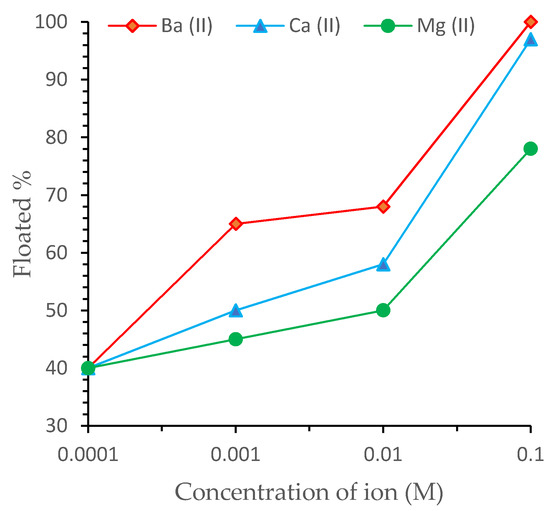
Figure 6. Effect of multivalent ion concentration on the activation of colemanite at pH 9.3 ± 0.1 [11].
The role of multivalent ions on the surface of these BMs is dependent on pH. At pH 9.3, multivalent ions depressed the floatability of ulexite, while at higher pHs, the same multivalent ions enhance its floatability [6][29][50]. At the pH range of 4.5–8.5, precipitation of Al(OH)3 on the ulexite surface increases its positive charge, whereas the electrical charge is reversed above this [53]. It was suggested that particles become hydrophobic where metal ions in the solution react with the collectors and precipitate them [6][11].
4.3. Flocculation
Flocculation of BMs, especially colemanite, effectively enhances the efficiency of flotation separation. Colemanite particles in the suspension agglomerate due to the hydrocarbon chain association of the surfactant. The surfactant adsorbs on the particle layers and contacts with each other. The hydrocarbon chain association depends on the carbon number of surfactants (long-chain surfactants are more effective than shorter ones). Shear flocculation closely correlates with the particle’s hydrophobicity in suspension [54][55][56]. Increasing the surfactant concentration can lead to an increase in colemanite’s shear flocculation, thereby improving the particles’ surface hydrophobicity. The energy barrier should increase as negative zeta potential increases, and hindering particle flocculation. However, an increase in surface charge due to the surfactant’s adsorption on the colemanite surfaces did not decrease the colemanite flocculation [13].
Sodium oleate (C17H33COONa) and sodium dodecyl sulfate (C12H25SO4Na) in the role of flocculants can positively influence the aggregation of colemanite. Sodium oleate proved to be more efficient than sodium dodecyl sulfate in the shear flocculation of colemanite [54][57]. It was also documented that Aero 801 performs better than SDS in flocculating colemanite [18][19]. Ucbeyiay and Ozkan (2014) showed two-stage flocculation could provide higher concentrate grades for shear flocculation and column flotation methods. They indicated the use of sodium pyrophosphate (SPP) and sodium hexametaphosphate (SHMP) as dispersants enhanced the shear flocculation of colemanite in the presence of Aero 801 and SDS [18].
Stirring speed and flocculation time are critical parameters in the flocculation of colemanite. An optimized stirring speed ruptures the flocs while stirring rates below their optimum value may reduce particles’ collision efficiency in the suspension and reduce the suspension’s BMs’ flocculation. Studies showed that a stirring speed of 500 rpm and conditioning for 3 min affects the highest degree of flocculation with sodium oleate and SDS (Figure 7). Shear flocculation, a slow process [54], has shown high flocculation degrees at low flocculation times for some minerals. There was no significant decrease resulting from a floc rupturing process at flocculation times above the 3 min when colemanite is aggregated in a suspension treated with oleate and SDS. Therefore, 3 min proved to be sufficient time for colemanite flocculation. Flocculation time depends on the surface charge, the particle size, the particle concentration, and electric charges on the surface generate kinetic energy barrier [52][55]. Overcoming this barrier requires appropriate stirring strength. Decreasing the surface charge decreases the critical shear rate needed for shear flocculation of minerals. A high surface charge requires prolonged flocculation time, while a low charge may cause a rapid agglomeration. In general, coarser particles require lower rates than finer particles [52][54][55].
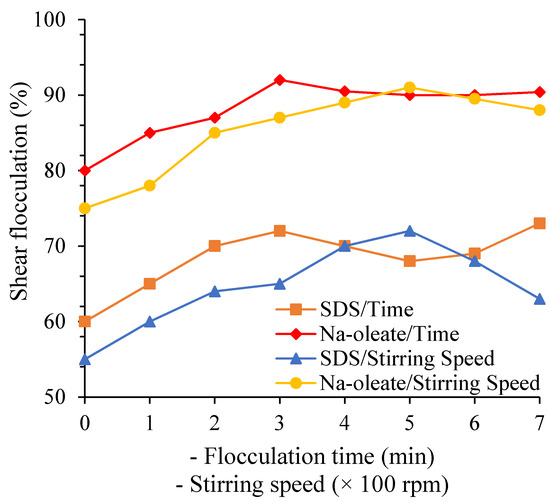
Figure 7. Effect of flocculation time and stirring speed on the degree of shear flocculation [54].
Multivalent ions have been studied for their effects on colemanite shear flocculation (Figure 8). Multivalent ions such as Mg2+, Ba2+, and Fe3+ enhance BMs’ flocculation as a function of pH [13]. They reduce the BMs’ negative surface charge and enhance their coagulation in the suspension [39][58][59]. Celik et al. [11] indicated that barium ion is more effective in colemanite flocculation than magnesium ion. The difference in their product solubility with SDS was suggested as the main reason [11]. However, significant decreases in colemanite flocculation were observed at concentrations higher than these ranges [13]. In the presence of multivalent ions, the maximum degrees of colemanite flocculation was reached at pH between 9–11.5 [11][13].
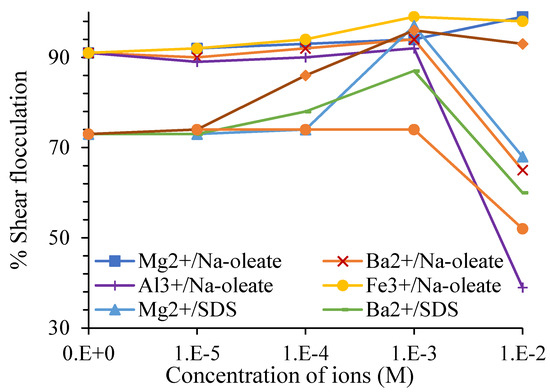
Figure 8. Effect of multivalent ions on the shear flocculation of colemanite with 60 mg/L of collector at pH 11.5 [13].
4.4. Direct Flotation
Nevertheless, the most crucial reagent in the flotation process is the collector. Both cationic and anionic collectors can be considered to recover BMs through direct flotation [11][29]; however, anionic reagents are generally the collector of choice for BMs. The most prominent are the sulfonates, xanthates, carboxylates (sodium oleate or oleic acid), and sulfate (SDS) (Table 5). It is essential to restate here that although KAX and KEX are capable of floating realgar, they were not effective in floating colemanite [16]. In general, sulfonates, especially petroleum sulfonates, have proven to be potent collectors for BMs’ flotation. For instance, experiments at pH 10 with a combination of anionic collectors (R-801, R-825, and Hoechst F-698) through the colemanite flotation resulted in the B2O3 recovery and grade of 86.1% and 44.5%, respectively [23]. Other investigations have also shown that petroleum sulfonates performed better in floating colemanite than SDS, olefin sulfonates, sodium oleate, and even cationic collectors [16][19][26]. Their higher efficiencies could be due to their higher molecular weight and the corresponding hydrocarbon chain length.
Table 5. Conditions used for the direct flotation separation of boron minerals
| Collector | Collector Type | BM | Gangue | Depressant | pH | Recovery (% B2O3) |
Grade (% B2O3) |
Reference |
|---|---|---|---|---|---|---|---|---|
| R-801, R-825 and Hoechst F-698 | Sulfonate, anionic | Colemanite | Clays and Carbonates | Ke-1365, dextrine, Na2SiO3, Cataflot P-40 | 10 | 86.1 | 44.5 | [23] |
| R801 | Sulfate, anionic | Colemanite | Clay | 7 | 98.47 | 45.42 | [30] | |
| Dodiflood V3622; MW, 383 | Petroleum sulfonate, anionic | Colemanite | Clay | 7 | 96% | [26] | ||
| Flotigam T | Amine, cationic | Colemanite | Clay | 10 | 95% | |||
| SDS | Sulfate, anionic | Colemanite | Clay | 8.3 | 59 | [2] | ||
| R801 | sulfonate, anionic | Colemanite | Clay, quartz, calcite | Corn starch | 9.3 | 99 | 46 | [19] |
| R825 | sulfonate, anionic | Colemanite | Clay, quartz, calcite | Corn starch | 9.3 | 70 | 40 | |
| SDS | Sulfate, anionic | Colemanite | Clay, quartz, calcite | Corn starch | 9.3 | 85 | 38 | |
| Sodium oleate | Carboxylate, anionic | Colemanite | Clay, quartz, calcite | Corn starch | 9.3 | 98 | 38 | |
| SDS | Sulfate, anionic | Ulexite | Clay | 8 | 45 | [2] | ||
| DAH | Amine, cationic | Ulexite | Clay | 9.3 | 97 |
Anionic collectors showed different adsorption mechanisms on the surface of BMs. A study showed that R825 was not chemically adsorbed on the colemanite surface, as were sodium oleate and R840 [16]. R801 has an electrostatic and chemical in nature interaction by the surface of BMs because of the existence of Ca2+ in the solution [60][61], which may involve micellization and precipitation of surface complexes such as Ca(OH)RSO3 [51][62]. The flotation recovery of colemanite by anionic collectors decreased by increasing pH and was very low at the IEP. These phenomena indicated a coulombic attraction between the anionic collectors and the positively charged colemanite surface. Celik et al. [11] indicated that the recovery of colemanite, floated with SDS, decreased by increasing pH; though its recovery was regained at the high collector concentrations. These observations could be ascribed to the better collector performance in its competition with clay particles for the colemanite surface at higher collector concentrations [11].
From the perspective of impact on shear flocculation, it was earlier stated that sodium oleate was better than SDS for colemanite. Like petroleum sulfonate, this performance can be attributed to the longer chain of the oleate radical. This assertion agrees with the previous conclusion that longer chains are more effective because alkyl chain interaction is contingent on the carbon number of adsorbed surfactant chains [54][57]. Although sodium oleate is a common collector for floating many salt-type minerals [63][64], its selectivity is low, and it is highly sensitive to water hardness and flotation temperature [65].
Due to the relatively low selectivity of anionic fatty acids and their derivatives, cationic collectors are also used in the flotation of these minerals with modification of parameters and the use of modifiers/depressants [64]. They include Flotigam T, DAH, Armac-T, and DTAC. Generally, they are capable of floating colemanite and ulexite. For example, Armac-T and DAH can float both colemanite and realgar in a similar manner [2][12]. Since ulexite and the clay minerals associated with colemanite carry the same surface charge and properties, cationic collectors may not selectively separate colemanite from them. The most efficient colemanite recovery with an amine collector could be achieved at pH around 10, where the highest amounts of ion-molecular complexes form [5][26]. The absence of difference in adsorption bands observed with colemanite and cationic collector indicates the only adsorption would be physical (electrostatic) [16]. Celik et al. revealed that primary amines could float colemanite at lower concentrations than quaternary amines and that the interaction with the former could involve hydrogen bonding. The better performance of primary amines can be ascribed to the structural arrangement of their functional groups [11].
4.5. Reverse Flotation
BMs are generally recovered by direct flotation; however, some conditions render direct flotation inadequate for their selective separations. Borax and its associated montmorillonite clay can both be floated equally by collectors because of their similar surface properties [40]. In light of this, reverse flotation (the direct flotation of the less valued mineral) is applied. Reverse flotation is also recommended to recover fine BMs from tailing dams because it can be more selective than direct flotation (based on the mineralogy of gangue phases). Fortunately, a combination of various reagents can improve the efficiency of the process (Table 6).
Table 6. Flotation conditions for the reverse flotation of BMs.
| Gangue Mineral | Collector | Collector Type | Borate Mineral | Depressant | pH | Recovery (% B2O3) |
Grade (% B2O3) |
Reference |
|---|---|---|---|---|---|---|---|---|
| Realgar | KAX | Xanthate, anionic | Colemanite | Sodium silicate | 9 | 96.99 | 33.93% | [16] |
| Calcite, dolomite, and clay | Sodium oleate | Carboxylate, anionic | Borax | Sodium silicate | 8 | 81.78 | 23.47 | [40] |
| Clay | R801 | Xanthate, anionic | Colemanite | Tannic acid | 9.3 | 2.5 (floated) | [26] |
Calcite serves as a hindrance to the selective flotation of borax. Therefore, sodium silicate was used to depress borax. The sodium oleate’s maximum chemisorbed on the calcite surface can occur at pH ranges 10–11 [66][67]. Though, Çilek and Üresin showed that sodium oleate dosage is the essential variable affecting both the grade and recovery of BMs in the concentrate (Figure 9) [40].
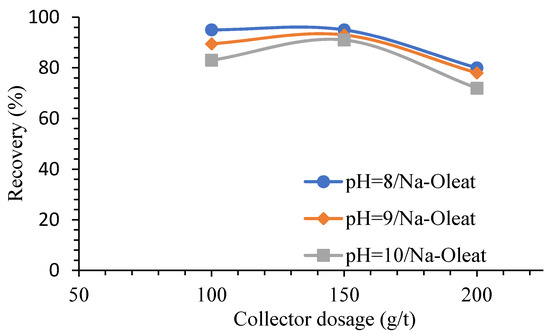
Figure 9. Influence of different Na-Oleat dosages on boron mineral recovery in various pHs (reverse flotation) [40].
Koca et al. (2003) investigated the effects of potassium amyl xanthate (KAX) on flotation separation of realgar from colemanite since the xanthates do not efficiently float colemanite [12]. However, they are one of the best collectors for realgar. KAX was employed for the reverse flotation recovery of colemanite from its ore-containing realgar. After successful trials with two artificial samples, a concentrate recovery of 96.99% B2O3 and a grade of 33.93% B2O3 was achieved. This result represented a reduction in arsenic content by 31.20% [12][16].
In the context of separating BMs from each other using the flotation process, the method can be termed direct flotation or reverse flotation of the BMs, depending on the BM being floated or depressed. Celik and Bulut (1996) indicated that DAH is not selective for separating colemanite from ulexite in equal amounts of the collector [12]. However, ulexite is only marginal floated by anionic collectors such as SDS. Therefore, an anionic reagent would be more appropriate for selective colemanite and ulexite [2].
4.6. Depressants
Various gangue minerals, in varying amounts, are usually associated with BMs. The clays, carbonates, and arsenic minerals are prominent [12][16][19][23]. Montmorillonite-type clay minerals are transported into the concentrate by mechanical entrainment. The carbonate mineral surface becomes hydrophobic using the Na-oleate collector in the alkaline condition [40]. The arsenic minerals are floated in equal proportions as the BMs with the conventional collectors [12]. Hence, separating gangues from the valued minerals requires different additives (modifiers) in the flotation systems [63][68][69].
Depressants play a prominent role in the selective flotation process. They are generally used to suppress clay minerals as the main gangue phases. The ones applied in depressing minerals in the recovery of BMs include Ke-1365, dextrin, Cataflot P-40 [23], sodium silicate [12][16][23][40], sodium pyrophosphate, and sodium hexametaphosphate, and corn starch. However, starch and sodium silicate are typical depressants used for the direct and reverse flotations of BMs to decrease gangue minerals’ presence in the concentrate (Table 5 and Table 6) [16][19]. Sodium silicates’ effects on boron minerals recovery depend on three essential parameters: pH, collector dosage, and depressant dosage (Figure 10) [40].
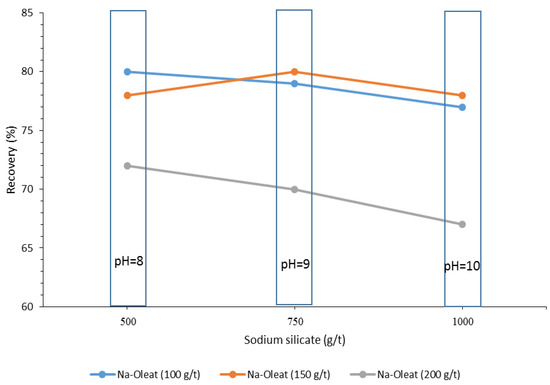
Figure 10. Effect of sodium silicate dosage on the recovery of the boron minerals [40].
This entry is adapted from the peer-reviewed paper 10.3390/min11030318
References
- Hançer, M. Doygun Çözeltilerde Boraksın Flotasyon Kimyasının Incelenmesi. In Proceedings of the Türkiye XIII. Madencilik Kongresi, Istanbul, Turkey, 10–14 May 1993.
- Celik, M.; Bulut, R. Mechanism of selective flotation of sodium-calcium borates with anionic and cationic collectors. Sep. Sci. Technol. 1996, 31, 1817–1829.
- Çolak, M.M.; Helvaci, C.; Maggetti, M. Saponite from the Emet colemanite mines, Kutahya, Turkey. Clays Clay Miner. 2000, 48, 409–423.
- Alp, I. Application of magnetic separation technology for the Processing of a colemanite ore. J. S. Afr. Inst. Min. Metall. 2009, 109, 139–145.
- Hancer, M.; Celik, M.; Miller, J.D. The significance of interfacial water structure in soluble salt flotation systems. J. Colloid Interface Sci. 2001, 235, 150–161.
- Batar, T.; Kahraman, B.; Cirit, E.; Celik, M.S. Dry Processing of borax by calcination as an alternative to wet methods. Int. J. Miner. Process. 1998, 54, 99–110.
- Flores, H.; Villagran, P. Borates in the Argentine puna: A new application of magnetic separation. Phys. Sep. Sci. Eng. 1992, 3, 155–169.
- Travis, N.; Cocks, E. The Tincal Trail: A History of Borax; Harrap: London, UK, 1984; Volume 311, pp. 115–124.
- Kistler, R.B.; Helvaci, C. Boron and borates. Ind. Miner. Rocks 1994, 6, 171–186.
- Woods, W.G. An introduction to boron: History, sources, uses, and chemistry. Environ. Health Perspect. 1994, 102 (Suppl. 7), 5–11.
- Celik, M.; Hancer, M.; Miller, J. Flotation chemistry of boron minerals. J. Colloid Interface Sci. 2002, 256, 121–131.
- Koca, S.; Savas, M. Contact angle measurements at the colemanite and realgar surfaces. Appl. Surf. Sci. 2004, 225, 347–355.
- Sahinkaya, H.U.; Ozkan, A. Investigation of shear flocculation behaviors of colemanite with some anionic surfactants and inorganic salts. Sep. Purif. Technol. 2011, 80, 131–139.
- Ozkan, S.G. Flotation Studies of Colemanite Ores from the Emet Deposits of Turkiye; University of Birmingham: Birmingham, UK, 1994.
- Çelikkan, H.; Şahin, M.; Aksu, M.L.; Veziroğlu, T.N. The investigation of the electrooxidation of sodium borohydride on various metal electrodes in aqueous basic solutions. Int. J. Hydrogen Energy 2007, 32, 588–593.
- Koca, S.; Savas, M.; Koca, H. Flotation of colemanite from realgar. Miner. Eng. 2003, 16, 479–482.
- Muduroglu, M.; Ozbayoglu, G.; Arol, A.I. Mineral processing on the verge of the 21st century. In Proceedings of the 8th International Mineral Processing Symposium, Antalya, Turkey, 16–18 October 2000.
- Ucbeyiay, H.; Ozkan, A. Two-stage shear flocculation for enrichment of fine boron ore containing colemanite. Sep. Purif. Technol. 2014, 132, 302–308.
- Ucar, A.; Yargan, M. Selective separation of boron values from the tailing of a colemanite processing plant. Sep. Purif. Technol. 2009, 68, 1–8.
- Sonmez, E.; Koca, S.A.; Ozdag, H.; Ipek, H.A. Beneficiation of colemanite concentrates from fine wastes by using ultrasound waves. Miner. Eng. 2004, 17, 359–361.
- Alp, I.; Özdağ, H. Investigation of the Processing of colemanite tailings by ultrasonic sound waves. In Mineral Processing on the Verge of the 21st Century; Özbayoğlu, G., Atalay, M.U., Arol, A.I., Hosten, C., Hicyilmaz, C., Eds.; Balkema: Rotterdam, The Netherlands, 2000; pp. 693–696.
- Özdag, H.; Bozkurt, R.; Uçar, A. Benefication of boron from tailings of Kestelek concentrator. In Proceedings of the 2nd International Mineral Processing Symposium, Izmir, Turkey; 1988.
- Gül, A.; Kaytaz, Y.; Önal, G. Beneficiation of colemanite tailings by attrition and flotation. Miner. Eng. 2006, 19, 368–369.
- Celik, M.; Uzunoglu, H.; Arslan, F. Decrepitation properties of some boron minerals. Powder Technol. 1994, 79, 167–172.
- Celik, M.; Yasar, E. Effect of temperature and impurities on electrostatic separation of boron minerals. Miner. Eng. 1995, 8, 829–833.
- Hancer, M.; Celik, M. Flotation mechanisms of boron minerals. Sep. Sci. Technol. 1993, 28, 1703–1714.
- Watson, J.; Beharrell, P. Extracting values from mine dumps and tailings. Miner. Eng. 2006, 19, 1580–1587.
- Celik, M.S.; Somasundaran, P. Wettability of reservoir minerals by flotation and correlation with surfactant adsorption. In Proceedings of the SPE Oilfield and Geothermal Chemistry Symposium, Stanford, CA, USA, 28–30 May 1980; Society of Petroleum Engineers.
- Ozdemir, O.; Çelik, M.S. Surface properties and flotation characteristics of boron minerals. Open Miner. Process. J. 2010, 3.
- Ucar, A.; Sahbaz, O.; Kerenciler, S.; Oteyaka, B. Recycling of colemanite tailings using the Jameson flotation technology. Physicochem. Probl. Miner. Process. 2014, 50, 645–655.
- Svoboda, J. Magnetic Techniques for the Treatment of Materials; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2004.
- Alp, I. Application of magnetic separation technology for the recovery of colemanite from plant tailings. Waste Manag. Res. 2008, 26, 431–438.
- Acarkan, N.; Bulut, G.; Kangal, O.; Önal, G. A new process for upgrading boron content and recovery of borax concentrate. Miner. Eng. 2005, 18, 739–741.
- Elder, J.; Domenico, J. The role of physical Processing enhancing the quality of industrial minerals. In Proceedings of the 14th Industrial Minerals International Congress, Belgorod, Russia, 7 June 2000.
- Flores, H.; Mattenella, L.; Valdez, S. Physical and physicochemical properties of borates from Argentinean Puna. Inf. Tecnol. 2002, 13, 103–108.
- Ata, O.N.; Colak, S.; Copur, M.; Celik, C. Determination of the optimum conditions for boric acid extraction with carbon dioxide gas in aqueous media from colemanite containing arsenic. Ind. Eng. Chem. Res. 2000, 39, 488–493.
- Schuette, R.; Goodman, B.A.; Stucki, J.W. Magnetic properties of oxidized and reduced smectites. Phys. Chem. Miner. 2000, 27, 251–257.
- Trahar, W.; Warren, L. The flotability of very fine particles—A review. Int. J. Miner. Process. 1976, 3, 103–131.
- Ozkan, S.G.; Gungoren, C. Investigation of ultrasonics use for colemanite flotation. Int. Multidiscip. Sci. Geoconf. SGEM 2010, 1, 661.
- Çilek, E.C.; Üresin, H. Beneficiation of borax by reverse flotation in boron saturated brine. J. Colloid Interface Sci. 2005, 290, 426–430.
- Cilek, E.C.; Ozgen, S. Improvement of the flotation selectivity in a mechanical flotation cell by ultrasound. Sep. Sci. Technol. 2010, 45, 572–579.
- Mitome, H. Action of ultrasound on particles and cavitation bubbles. In Proceedings of the World Congress on Ultrasonics, Paris, France, 7–10 September 2003.
- Ozkan, S.G.; Gungoren, C. Enhancement of colemanite flotation by ultrasonic pretreatment. Physicochem. Probl. Miner. Process 2012, 48, 455–462.
- Piskin, S. Thermal Properties of Hydratet Boron Minerals. Ph.D. Thesis, Istanbul Technical University, İstanbulc, Turkey, 1983.
- Şener, S.; Özbayoǧlu, G. Separation of ulexite from colemanite by calcination. Miner. Eng. 1995, 8, 697–704.
- Kocakuşak, S.; Akcay, K.; Ayok, T.; Koöroǧlu, H.J.; Koral, M.; Savaşçi, Ö.T.; Tolun, R. Production of anhydrous, crystalline boron oxide in fluidized bed reactor. Chem. Eng. Process. Process Intensif. 1996, 35, 311–317.
- Emrullahoglu, O.F.; Kara, M.; Tolun, R.; Celik, M.S. Beneficiation of calcined colemanite tailings. Powder Technol. 1993, 77, 215–217.
- Somasundaran, P.; Hanna, H. Adsorption of sulfonates on reservoir rocks. Soc. Pet. Eng. J. 1979, 19, 221–232.
- Israelachvili, J. Forces between surfaces in liquids. Adv. Colloid Interface Sci. 1982, 16, 31–47.
- Morgan, L.J.; Ananthapadmanabhan, K.; Somasundaran, P. Oleate adsorption on hematite: Problems and methods. Int. J. Miner. Process. 1986, 18, 139–152.
- Fuerstenau, M.C.; Miller, D.J.; Kuhn, M.C. Chemistry of Flotation; Society for Mining Metallurgy: Englewood, CO, USA, 1985.
- Ozkan, S.; Acar, A. Investigation of impact of water type on borate ore flotation. Water Res. 2004, 38, 1773–1778.
- Li, C.; Somasundaran, P. Reversal of bubble charge in multivalent inorganic salt solutions—effect of magnesium. J. Colloid Interface Sci. 1991, 146, 215–218.
- Warren, L. Shear flocculation. In Colloid Chemistry in Mineral Processing; Elsevier: Amsterdam, The Netherlands, 1992; pp. 309–329.
- Song, S.; Lu, S. Theory and applications of hydrophobic flocculation technology. In Developments in Mineral Processing; Elsevier: Amsterdam, The Netherlands, 2000; pp. C5-31–C5-38.
- Song, S.X.; Lopez-Valdivieso, A.; Reyes-Bahena, J.L.; Lara-Valenzuela, C. Floc flotation of galena and sphalerite fines. Miner. Eng. 2001, 14, 87–98.
- Zollars, R.L.; Ali, S.I. Shear coagulation in the presence of repulsive interparticle forces. J. Colloid Interface Sci. 1986, 114, 149–166.
- Pascoe, R.; Doherty, E. Shear flocculation and flotation of hematite using sodium oleate. Int. J. Miner. Process. 1997, 51, 269–282.
- Shibata, J.; Fuerstenau, D. Flocculation and flotation characteristics of fine hematite with sodium oleate. Int. J. Miner. Process. 2003, 72, 25–32.
- Aplan, F.; Fuerstenau, D. Principles of nonmetallic mineral flotation. Froth Flotat. 1962, 50, 170.
- Nalaskowski, J.; Veeramasuneni, S.; Miller, J. Interaction forces in the flotation of colemanite as measured by atomic force microscopy. In Proceedings of the Innovations Mineral Coal Processing: Proceedings 7th International Mineral Processing Symposium, Istanbul, Turkey, 15–17 September 1998.
- Zhang, R.; Somasundaran, P. Advances in adsorption of surfactants and their mixtures at solid/solution interfaces. Adv. Colloid Interface Sci. 2006, 123, 213–229.
- Gibson, B.; Wonyen, D.; Chelgani, S.C. A review of pretreatment of diasporic bauxite ores by flotation separation. Miner. Eng. 2017, 114, 64–73.
- Wonyen, D.G.; Kromah, V.; Gibson, B.; Nah, S.; Chelgani, S.C. A review of flotation separation of Mg carbonates (dolomite and magnesite). Minerals 2018, 8, 354.
- Xu, L.; Hu, Y.; Tian, J.; Wu, H.; Yang, Y.; Zeng, X.; Wang, Z.; Wang, J. Selective flotation separation of spodumene from feldspar using new mixed anionic/cationic collectors. Miner. Eng. 2016, 89, 84–92.
- Young, C.; Miller, J. Effect of temperature on oleate adsorption at a calcite surface: An FT-NIR/IRS study and review. Int. J. Miner. Process. 2000, 58, 331–350.
- Cebeci, Y.; Sönmez, İ. A study on the relationship between critical surface tension of wetting and oil agglomeration recovery of calcite. J. Colloid Interface Sci. 2004, 273, 300–305.
- Jafari, M.; Abdollahi, H.; Shafaei, S.Z.; Gharabaghi, M.; Jafari, H.; Akcil, A.; Panda, S. Acidophilic bioleaching: A review on the process and effect of organic–inorganic reagents and materials on its efficiency. Miner. Process. Extr. Metall. Rev. 2019, 40, 87–107.
- Jafari, M.; Shafaei, S.Z.; Abdollahi, H.; Gharabaghi, M.; Chelgani, S.C. Effect of flotation reagents on the activity of L. ferrooxidans. Miner. Process. Extr. Metall. Rev. 2018, 39, 34–43.
