Extracellular vesicles (EVs) play major roles in intracellular communication and participate in several biological functions in both normal and pathological conditions. Surface modification of EVs via various ligands, such as proteins, peptides, or aptamers, offers great potential as a means to achieve targeted delivery of therapeutic cargo, i.e., in drug delivery systems (DDS). This study summarizes recent studies pertaining to the development of EV-based DDS and its advantages compared to conventional nano drug delivery systems (NDDS).
- extracellular vesicles
- exosomes
- drug delivery systems
- targeted delivery
1. Introduction
A drug delivery system (DDS) consists of various formulation which enable therapeutic substance to reach the desired site of action specifically without going to non-target sites [1]. In nano drug delivery systems (NDDS), different biodegradable and biocompatible materials with size approximately 10-100 nm are utilized as nanocarriers [2][3]. These nanocarriers can be either natural or synthetic polymers, lipids, and metals such as nanoparticles [1][2][3][4]. Although NDDS have been used with several drugs including anti-cancer drugs [5][6][7], very few have been approved for use in humans by the Food and Drug Administration [8]. Cytotoxicity and rapid clearance of most of the synthetic NDDS via the mononuclear phagocyte system or reticuloendothelial system have been major bottlenecks in their transition from bench to bedside in clinical setting [9][10]. Several approaches have been employed to modify the nanoparticles (NPs). One example is coating the NPs with polyethylene glycol (PEG); this enhanced circulation time but impeded interaction between the target cells or tissues and the NDDS, thereby interfering with their biodistribution [11][12][13]. Another approach is to look for natural DDS, which could be expected to yield higher therapeutic value owing to their better in vivo biocompatibility as compared to the synthetic NDDS [14][15][16]. Extracellular vesicles (EVs) are natural nanovesicles released from most cells and biofluids; they carry various cargo including nucleic acids, proteins, and lipids [17]. EVs have attracted tremendous attention in the context of NDDS due to their ability to facilitate intracellular communication and the transportation of cargo to the target recipient cells [18][19]. Based on their size range and biogenesis, EVs are categorized into three major types, namely exosomes, microvesicles (MVs), and apoptotic bodies (ABs) [20]. Exosomes are of endocytic origin. They have sizes in the range of 30–100 nm; structurally, exosomes are composed of a lipid bilayer carrying cargoes of different composition including functional proteins, DNA, mRNA, miRNA, and lncRNA (Figure 1) [21][22][23].

Figure 1. Representative structure of an exosome and its composition.
The biogenesis of exosomes takes place by inward budding of the plasma membrane that forms the endosome vesicle, and the multivesicular bodies (MVBs). MVBs fuse with lysosomes and degrade or fuse with the plasma membrane and form exosomes which are released from cell into extracellular space (Figure 2A) [24][25]. They are released from various cell types like red blood cells, platelets, lymphocytes, dendritic cells (DCs), epithelial cells, adipocytes, fibroblasts, neural cells, stem cells, and cancer cells [26]. They have also been found in various biofluids such as blood, plasma, urine, cerebrospinal fluid (CSF), milk, amniotic fluid, malignant ascites, saliva, and synovial fluid [27][28][29]. They play a major role in cell-to-cell communication in the signalling pathways of both physiological and pathological processes [30], and transferring molecules such as proteins and RNA from donor cells to recipient cells [27][31]. Various specific proteins are present on the surface of exosomes, such as tetraspanin proteins (CD9, CD63, and CD81) [31], lysosomal protein (Hsp70), tumor sensitive gene 101 (Tsg101), and fusion proteins (annexin, and flotillin) [32]. These proteins are associated with the endosomal pathway, and are characteristic of exosomes, distinguishing them from MVs and ABs. MVs are another set of EVs with size in the range of 100–1000 nm; they are formed and released by budding off cell membrane [33]. In contrast, ABs are in the size range of 50–5000 nm, and are released from cells undergoing apoptosis [34] as shown in Figure 2B, C. Some scientists have subdivided the category of EV into subtypes, partially based on size, marker and biogenesis [35][36]; in this review the term EV refers to the general category, unless we specifically refer to a subgroup.
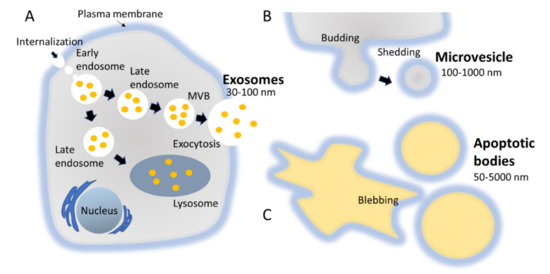
Figure 2. Biogenesis pathways followed by different types of EVs, namely exosomes, MVs, and ABs. (A) MVBs fuse with lysosomes and degrade or fuse with the plasma membrane and form exosomes which are released from cell into extracellular space. (B) MVs are a set of EVs with size in the range of 100–1000 nm; they are formed and released by budding off cell membrane. (C) ABs are in the size range of 50–5000 nm, and are released from cells undergoing apoptosis.
A diverse range of studies have been carried out exploring the application of EVs in drug delivery. It has been found that miRNA and protein can be loaded on EVs and delivered to t tumor cells [37][38]. EVs also show the capability to inhibit tumor growth by delivering chemical drugs [39]. Interestingly, EVs can avoid phagocytosis by macrophages and prolong the half-life of chemical drugs in comparison to artificial NDDS, and are considered as the natural carrier of chemical drugs to improve the efficiency of biological drug delivery [40][41][42]. Previous review articles have covered various aspects of EVs in NDDS. This review updates those summaries with an in-depth discussion of the latest methods for surface modification of EVs, the importance of cell origin, and the importance of loading efficiency in EV-based targeted drug delivery systems. Here we shed light on the structural similarity between liposome and EVs, and their different roles in targeted delivery.
2. How Crucial Is the Origin of Exosomes for Drug Delivery?
There are various sources of EVs which can be employed for developing DDS (Figure 3). To achieve the desired effect and to avoid any potential harmful effects, it is necessary to understand the pros and cons of selecting any particular source. For example, the proportion of lipid to surface protein in an EV differs, depending on the source [43]. This proportion can affect certain properties that are crucial for effective delivery, and thus should be considered when selecting a source. It has also been found that the proportion of certain lipids is enhanced in exosomes as compared to the amount of lipids in their donor cells, such as sphingolipid, phosphatidylserine, phosphatidylinositol, and cholesterol. These lipids facilitate in enhancing the rigidity of exosomal membrane [44]. Another factor to consider is the relationship between surface proteins and target cells. Some sources produce EVs with surface proteins that are detrimental to the recipient target cells. A third factor is biocompatibility; and finally yield should also be taken into consideration while making selection of donor source cells for isolation of EVs for developing into EVs-based DDS.

Figure 3. Different sources of EVs for developing EV-based DDS, their advantages, and disadvantages.
2.1. Choice between Autologous and Heterologous EVs
The choice between autologous and heterologous (also referred as allogenic) EVs for developing into DDS is one of the crucial factors for effective delivery. It has been found that the uptake of autologous EVs and the uptake of heterologous EVs by target recipient cells differ distinctly. As the compositions of EVs have been reported to mimic their parent cells, the selection of heterologous EVs may induce an immune response in the target recipient cells. Therefore, theoretically, autologous EVs may be more suitable for therapeutic purposes [45]. In practice, however, heterologous EVs from MSCs have been found to be safe and reliable for therapeutic purposes. Still, we shouldn’t forget about autologous EVs, for example, pathological tissues are generally considered waste; however, if the EVs from these tissues could be isolated and their disease-causing cargo removed, these EVs could be valuable DDs [46]. Lessi et al. demonstrated that human primary macrophage-derived EVs could deliver drugs efficiently, [47], suggesting that autologous EVs derived from peripheral blood-derived primary monocytes could be suitable as theranostic agents. The safety profile of these EVs needs to be assessed before developing them into DDS; however, some evidence indicates they are safer than EVs derived from plasma [48].
2.2. Tumor-Derived EVs
Tumor-derived EVs (TEVs) have been employed by many research groups for drug delivery [49][50]. TEVs have several advantages compared to other delivery carriers. For example, tumor cells release significantly high numbers of EVs [51], suggesting their suitability for studies requiring large amounts of EVs. In addition, TEVs carry MHC class-I molecules and antigens specific to the originating tumor cells. Moreover, TEVs can induce immune response against cancer cells by delivering antigens to DCs [52]. Interestingly, the tetraspanin proteins, common markers for exosomes, have been found to bind with various ligands in a diverse range of tissues, suggesting their suitability for targeted delivery [53]. TEVs from melanoma patients have been found to increase the release of myeloid-derived suppressor cells (MDSCs), crucial for avoiding immune recognition [54][55]. It is striking that although TEVs have been shown to have potential for targeted delivery, there is also a chance that they can initiate tumor progression due to various of their constituents, such as urokinase plasminogen activator, which can promote cancer cell invasion, and adhesion modulators like vimentin, and annexin A1 [56]. Therefore, again, selection of appropriate source for isolating EVs is a crucial factor for developing successful and effective EV-based targeted DDS.
2.3. Immune Cell-Derived EVs
Another important source from which EVs can be isolated are immune cells. For example cells like macrophages and monocytes have gained attention for EV-based immunotherapy [57][58]. Immune cell derived EVs (IEVs) can evade phagocytosis, a clearance mechanism, which is a major limitation for most of the other types of EVs. Therefore, IEVs possess longer circulation time and better efficacy [59]. Importantly, the DC-derived EVs (DCEVs) seem to have a great potential as various clinical studies have demonstrated their effectiveness on different cancers. DCEVs play a major role as intercellular communicators in adaptive immunity for modulation of immune responses. Therefore, most of the researches related to DCEVs are about immunotherapy of cancer leading to clinical advantage [60][61]. Notably, in a Phase-I clinical trial, Escudier et al. reported the feasibility and safety of administering DCEVs pulsed with MAGE 3 peptides for immunization in melanoma patients under stage- III/IV [58]. DCEVs have also been found to promote tumor rejection via transporting peptide-MHC complexes from DCs (exposed to an antigen) to other DCs (not exposed to same antigen) [57][62][63].
2.4. Biofluid-Derived EVs
EVs derived from biofluids such as plasma [64], and ascites [65], have shown potential as delivery carriers. Biofluid-derived EVs have several advantages as delivery carriers. For example, unlike cell culture-derived EVs, plasma-derived EVs are enriched with lyso-phospholipids and do not contain phosphatidylserine (PS). The absence of PS on the surface of plasma-derived EVs prevent their removal from circulation [64][66]. In addition, plasma-derived EVs can cross the blood-brain barrier (BBB), which suggests their applicability for brain delivery [64]. Ascites-derived EVs along with granulocyte–macrophage colony-stimulating factor have been found to be safe and effective for immunotherapy of colorectal cancer [65]. It has also been reported that human peripheral blood-derived EVs loaded with miRNA have potential for treating cardiac diseases [67]. Another study showed that EVs in peripheral blood can be important mediators of lung injury via exosomal shuttling of miR-155 [68]. Blood EVs have been shown to be crucial for targeting brain disease. For example, dopamine-loaded blood EVs can be used as delivery platform in treating Parkinson’s disease and other central nervous system-related disorders [69]. Urine- and saliva-derived EVs have not been much explored for their therapeutic potential as delivery carriers; however, they have been extensively studied for developing biomarker of different diseases including cancer [70][71]. Conclusively, biofluids such as blood and ascites are great sources of EVs for developing novel DDS.
2.5. Plant and Bovine Milk-Derived EVs
Due to safety concerns related to TEVs and IEVs, scientists have explored the applicability of plant-derived EVs (PEVs) as DDS, such as grape-derived EVs [72] or bovine milk-derived EVs (BMEVs) [73]. There are several advantages of using PEVs as DDS, including better safety, consistency of source, scalability for large production, and relative cost effectiveness [72]. Several research groups have isolated EVs from different plants or food sources and showed a diverse range of applications [74]. For example Ju et al. demonstrated that grape- derived EVs are useful in protecting intestinal damage in mice via facilitating growth and differentiation of intestinal stem cells [72]. Subsequently, Wang et al. demonstrated that modification of grapefruit derived EVs improved their ability to target tumors and loaded those EVs with doxorubicin and curcumin. Interestingly, those EVs were found to be effective against inflammation in vivo [75]. Bovine milk is another important source of EVs for DDS. Mungala et al. demonstrated the enhanced activity of various therapeutic cargo loaded BMEVs against lung cancer in vitro and in vivo. They further showed that modification of BMEVs with folate could enhance tumor-targeting ability as compared to the free drug [73]. Recently, because many clinical trials for the treatment of Alzheimer’s disease (AD) using synthetic drugs have failed [76][77][78], scientists are trying to develop targeted delivery using EVs and developing precision medicine-loaded EVs for the treatment of AD [79][80]. Plant-derived traditional medicine have been studied in preclinical models of AD [81][82][83]. Targeted delivery of plant-derived bioactive components using EVs could be more effective for the treatment of AD.
3. How Does Loading Efficiency Play a Crucial Role in EV-Based DDS?
Loading therapeutic cargo into EVs is one of the crucial parts in the process of developing EV-based DDS. The high loading efficiency of EVs ensures better bioavailability of the cargo when it reaches the target site. The major factors that need consideration while loading any cargo onto EVs are: how better encapsulation or loading efficiency can be achieved, how the structural integrity of EVs can be maintained, and how the functional properties of the therapeutic cargo can be maintained.
Therapeutic cargo such as proteins, drugs, or small nucleic acids such as miRNA can be loaded in two different ways. First, the therapeutic cargo can be incorporated into donor cells, followed by isolation of EVs; this is referred to as in vitro loading [84]. Second, the therapeutic cargo can be loaded after isolation of EVs via various methods including incubation, sonication, electroporation, extrusion, permeabilization, or the freeze-thaw method; this is referred to as ex vivo loading (Figure 4) [59]. In the simple incubation method, EVs are incubated with drugs, and the drugs enter EVs via diffusion due to the concentration gradient. Incubation is found to be suitable for loading hydrophobic drugs as they interact with lipid layers of EVs’ membranes [59][85]. One disadvantage of simple incubation is low loading efficiency. Another method of loading is incubation of drugs with donor cells, followed by isolation of EVs [84]. Table 1 summarizes different loading methods with their advantages and disadvantages. The physicochemical properties of the therapeutic cargo partially determine what method is employed for their encapsulation in EVs. For example hydrophobic drugs such as curcumin can be loaded within the inner layers of fatty acid via incubation, whereas hydrophilic molecules including siRNA, miRNA can be loaded by forming transient pores on the membrane of EVs via methods like electroporation [16][86][87].
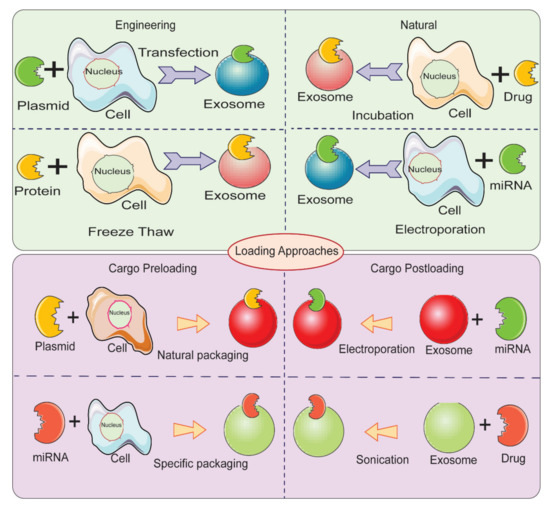
Figure 4. Different methods for loading therapeutic cargos in EVs for developing EV-based delivery systems.
Table 1. Different techniques for loading cargos in EVs with advantages and disadvantages.
| Loading Methods | Steps Involved | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|
| Electroporation | Phospholipid bilayer of EVs are disorganized by an electric field, creating pores in the membrane which allow the passage of drug to vesicle. | Loading with large molecules is possible | Disrupts integrity of EVs; Low loading efficiency | [86][88][89][90] |
| Sonication | Exosomes derived from donor cells are mixed with drug and sonicated through probe sonicator which permits the drug to flow into exosome | Increased loading efficiency; applicable for small RNAs | Potential deformation of membrane of EVs; Not efficient for hydrophobic drugs. |
[91][92] |
| Extrusion | Exosomes are mixed with drug and loaded into syringe-based lipid extruder and extruded through membrane with 100–400 nm pore size at controlled temperature. | High drug loading efficiency | Potential deformation of membrane. |
[91] |
| Freeze/Thaw Method | Exosome are mixed with drug and incubated, subsequently frozen at −80 °C or in liquid nitrogen and are thawed at room temperature. | Medium loading;Fusion of membranespossible | Exosomes may aggregate; Low loading efficiency |
[91] |
| Saponin-Assisted Loading | Saponin is incubated with exosomes to generate pores in their membrane by interacting with cholesterol which leads to increased membrane permeability | High drug loading compared to the other methods used in early reports | Generates pores in exosomes; Saponin can cause haemolysis; Toxicity concerns; Saponin concentration control & washing required |
[59][93][94] |
| Dialysis | Exosomes mixed with drug are dialyzed by stirring to obtain drug loaded exosome. | Promotes loading efficiency | Poor cellular uptake; No substantial impact on photodynamic effect |
[95] |
Recently, membrane permeabilization of EVs has been found to be a promising method for enhancing the loading efficiency of EVs. Saponin has been shown to be particularly effective in enhancing the loading of different cargos in EVs from various sources. Being a surfactant, saponin is able for form a complex with cholesterol in the membranes of cells and create pores, thereby facilitating permeabilization [93]. Haney et al. demonstrated that loading efficiency of catalase into exosomes can be enhanced via incubation with saponin, as compared with simple incubation technique. Interestingly, the activity of catalase was not affected by the saponin [59]. Another recent research showed that passing saponin through the microfluidic channels enhances loading of doxorubicin in glioma stem cell-derived exosomes as compared with other conventional methods. The authors reported two different microfluidic channels; one linear. and another sigmoid, which suggests that designing advanced microfluidic channels along with using permeabilizing agent may have a synergistic effect to achieve augmented efficiency of loading cargoes in EVs [16][96]. Fuhrmann et al. showed that incubation of a small hydrophilic molecule, porphyrin, with saponin could enhance the loading efficiency as compared with a passive loading technique excluding saponin [94]. Nevertheless, there are some concerns associated with the use of saponin for in vivo purposes because of its hemolytic activity [93].
This entry is adapted from the peer-reviewed paper 10.3390/molecules26061544
References
- Rojo, J.; Sousa-Herves, A.; Mascaraque, A. Perspectives of Carbohydrates in Drug Discovery. In Comprehensive Medicinal Chemistry III; Elsevier: Amsterdam, The Netherlands, 2017; pp. 577–610.
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351.
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71.
- Chowdhury, A.; Kunjiappan, S.; Panneerselvam, T.; Somasundaram, B.; Bhattacharjee, C. Nanotechnology and nanocarri-er-based approaches on treatment of degenerative diseases. Int. Nano Lett. 2017, 7, 91–122.
- Hinde, E.; Thammasiraphop, K.; Duong, H.T.T.; Yeow, J.; Karagoz, B.; Boyer, C.; Gooding, J.J.; Gaus, K. Pair correlation microscopy reveals the role of nanoparticle shape in intracellular transport and site of drug release. Nat. Nanotechnol. 2017, 12, 81–89.
- Liu, D.; Yang, F.; Xiong, F.; Gu, N. The Smart Drug Delivery System and Its Clinical Potential. Theranostics 2016, 6, 1306–1323.
- Sharma, A.K.; Gothwal, A.; Kesharwani, P.; Alsaab, H.; Iyer, A.K.; Gupta, U. Dendrimer nanoarchitectures for cancer diagnosis and anticancer drug delivery. Drug Discov. Today 2017, 22, 314–326.
- Matsumura, Y. The Drug Discovery by NanoMedicine and its Clinical Experience. Jpn. J. Clin. Oncol. 2014, 44, 515–525.
- Haque, S.; Whittaker, M.R.; McIntosh, M.P.; Pouton, C.W.; Kaminskas, L.M. Disposition and safety of inhaled biodegradable nanomedicines: Opportunities and challenges. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1703–1724.
- Luan, X.; Sansanaphongpricha, K.; Myers, I.; Chen, H.; Yuan, H.; Sun, D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin. 2017, 38, 754–763.
- Sun, L.; Wu, Q.; Peng, F.; Liu, L.; Gong, C. Strategies of polymeric nanoparticles for enhanced internalization in cancer therapy. Colloids Surf. B Biointerfaces 2015, 135, 56–72.
- Chow, T.-H.; Lin, Y.-Y.; Hwang, J.-J.; Wang, H.-E.; Tseng, Y.-L.; Wang, S.-J.; Liu, R.-S.; Lin, W.-J.; Yang, C.-S.; Ting, G. Improve-ment of biodistribution and therapeutic index via increase of polyethylene glycol on drug-carrying liposomes in an HT-29/luc xenografted mouse model. Anticancer Res. 2009, 29, 2111–2120.
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51.
- Vader, P.; Mol, E.A.; Pasterkamp, G.; Schiffelers, R.M. Extracellular vesicles for drug delivery. Adv. Drug Deliv. Rev. 2016, 106, 148–156.
- Batrakova, E.V.; Kim, M.S. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J. Control. Release 2015, 219, 396–405.
- Thakur, A.; Sidu, R.K.; Zou, H.; Alam, K.; Yang, M.; Lee, Y. Inhibition of Glioma Cells’ Proliferation by Doxorubicin-Loaded Exosomes via Microfluidics. Int. J. Nanomed. 2020, 15, 8331–8343.
- Thakur, A.; Qiu, G.; Ng, S.-P.; Wu, C.-M.L.; Lee, Y. Detection of membrane antigens of extracellular vesicles by surface plasmon resonance. J. Lab. Precis. Med. 2017, 2, 98.
- Milane, L.; Singh, A.; Mattheolabakis, G.; Suresh, M.; Amiji, M.M. Exosome mediated communication within the tumor microenvironment. J. Control. Release 2015, 219, 278–294.
- Thakur, A.; Qiu, G.; Xu, C.; Han, X.; Yang, T.; Ng, S.P.; Chan, K.W.Y.; Wu, C.M.L.; Lee, Y. Label-free sensing of exosomal MCT1 and CD147 for tracking metabolic reprogramming and malignant progression in glioma. Sci. Adv. 2020, 6, eaaz6119.
- Thakur, A.; Qiu, G.; Ng, S.-P.; Guan, J.; Yue, J.; Lee, Y.; Wu, C.-M.L. Direct detection of two different tumor-derived extracellular vesicles by SAM-AuNIs LSPR biosensor. Biosens. Bioelectron. 2017, 94, 400–407.
- Mashouri, L.; Yousefi, H.; Aref, A.R.; Ahadi, A.M.; Molaei, F.; Alahari, S.K. Exosomes: Composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol. Cancer 2019, 18, 75.
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19.
- Li, D.; Liu, J.; Guo, B.; Liang, C.; Dang, L.; Lu, C.; He, X.; Cheung, H.Y.-S.; Xu, L.; Lu, C.; et al. Osteoclast-derived exosomal miR-214-3p inhibits osteoblastic bone formation. Nat. Commun. 2016, 7, 10872.
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289.
- György, B.; Szabó, T.G.; Pásztói, M.; Pál, Z.; Misják, P.; Aradi, B.; László, V.; Pállinger, É.; Pap, E.; Kittel, Á.; et al. Membrane vesicles, current state-of-the-art: Emerging role of extracellular vesicles. Cell. Mol. Life Sci. 2011, 68, 2667–2688.
- Isola, A.L.; Chen, S. Exosomes: The Messengers of Health and Disease. Curr. Neuropharmacol. 2016, 15, 157–165.
- Urbanelli, L.; Magini, A.; Buratta, S.; Brozzi, A.; Sagini, K.; Polchi, A.; Tancini, B.; Emiliani, C. Signaling Pathways in Exosomes Biogenesis, Secretion and Fate. Genes 2013, 4, 152–170.
- Wall, N.R.; Aspe, J.R. Survivin-T34A: Molecular mechanism and therapeutic potential. OncoTargets Ther. 2010, 3, 247–254.
- Keller, S.; Ridinger, J.; Rupp, A.-K.; Janssen, J.W.G.; Altevogt, P. Body fluid derived exosomes as a novel template for clinical diagnostics. J. Transl. Med. 2011, 9, 86.
- Rodriguez-Dorantes, M.; Romero-Cordoba, S.; Peralta-Zaragoza, O.; Salido-Guadarrama, I.; Hidalgo-Miranda, A. MicroRNAs transported by exosomes in body fluids as mediators of intercellular communication in cancer. OncoTargets Ther. 2014, 7, 1327–1338.
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579.
- Kahlert, C.; Melo, S.A.; Protopopov, A.; Tang, J.; Seth, S.; Koch, M.; Zhang, J.; Weitz, J.; Chin, L.; Futreal, A.; et al. Identification of Double-stranded Genomic DNA Spanning All Chromosomes with Mutated KRAS and p53 DNA in the Serum Exosomes of Patients with Pancreatic Cancer. J. Biol. Chem. 2014, 289, 3869–3875.
- Battistelli, M.; Falcieri, E. Apoptotic Bodies: Particular Extracellular Vesicles Involved in Intercellular Communication. Biology 2020, 9, 21.
- Kakarla, R.; Hur, J.; Kim, Y.J.; Kim, J.; Chwae, Y.-J. Apoptotic cell-derived exosomes: Messages from dying cells. Exp. Mol. Med. 2020, 52, 1–6.
- Willms, E.; Cabañas, C.; Mäger, I.; Wood, M.J.A.; Vader, P. Extracellular Vesicle Heterogeneity: Subpopulations, Isolation Techniques, and Diverse Functions in Cancer Progression. Front. Immunol. 2018, 9, 738.
- Roefs, M.T.; Sluijter, J.P.; Vader, P. Extracellular Vesicle-Associated Proteins in Tissue Repair. Trends Cell Biol. 2020, 30, 990–1013.
- Vlassov, A.V.; Magdaleno, S.; Setterquist, R.; Conrad, R. Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta (BBA) Gen. Subj. 2012, 1820, 940–948.
- Tkach, M.; Théry, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232.
- Schiffelers, R.M.; Kooijmans, S.A.; Vader, P.; Van Dommelen, S.M.; Van Solinge, W.W. Exosome mimetics: A novel class of drug delivery systems. Int. J. Nanomed. 2012, 7, 1525–1541.
- Ohno, S.-I.; Takanashi, M.; Sudo, K.; Ueda, S.; Ishikawa, A.; Matsuyama, N.; Fujita, K.; Mizutani, T.; Ohgi, T.; Ochiya, T.; et al. Systemically Injected Exosomes Targeted to EGFR Deliver Antitumor MicroRNA to Breast Cancer Cells. Mol. Ther. 2013, 21, 185–191.
- Rani, S.; Ryan, A.E.; Griffin, M.D.; Ritter, T. Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Mol. Ther. 2015, 23, 812–823.
- Van Dommelen, S.M.; Vader, P.; Lakhal, S.; Kooijmans, S.; Van Solinge, W.W.; Wood, M.J.; Schiffelers, R.M. Microvesicles and exosomes: Opportunities for cell-derived membrane vesicles in drug delivery. J. Control. Release 2012, 161, 635–644.
- Osteikoetxea, X.; Balogh, A.; Szabó-Taylor, K.; Németh, A.; Szabó, T.G.; Pálóczi, K.; Sódar, B.; Kittel, Á.; György, B.; Pállinger, É.; et al. Improved Characterization of EV Preparations Based on Protein to Lipid Ratio and Lipid Properties. PLoS ONE 2015, 10, e0121184.
- Frydrychowicz, M.; Kolecka-Bednarczyk, A.; Madejczyk, M.; Yasar, S.; Dworacki, G. Exosomes—Structure, Biogenesis and Biological Role in Non-Small-Cell Lung Cancer. Scand. J. Immunol. 2015, 81, 2–10.
- Ren, J.; He, W.; Zheng, L.; Duan, H. From structures to functions: Insights into exosomes as promising drug delivery vehicles. Biomater. Sci. 2016, 4, 910–921.
- Federici, C.; Petrucci, F.; Caimi, S.; Cesolini, A.; Logozzi, M.; Borghi, M.; D’Ilio, S.; Lugini, L.; Violante, N.; Azzarito, T.; et al. Exosome release and low pH belong to a framework of resistance of human melanoma cells to cisplatin. PLoS ONE 2014, 9, e88193.
- Iessi, E.; Logozzi, M.; Lugini, L.; Azzarito, T.; Federici, C.; Spugnini, E.P.; Mizzoni, D.; Di Raimo, R.; Angelini, D.F.; Battistini, L.; et al. Acridine Orange/exosomes increase the delivery and the effectiveness of Acridine Orange in human melanoma cells: A new prototype for theranostics of tumors. J. Enzym. Inhib. Med. Chem. 2017, 32, 648–657.
- Campanella, C.; Bavisotto, C.C.; Logozzi, M.; Gammazza, A.M.; Mizzoni, D.; Cappello, F.; Fais, S. On the Choice of the Extracellular Vesicles for Therapeutic Purposes. Int. J. Mol. Sci. 2019, 20, 236.
- Mahaweni, N.M.; Kaijen-Lambers, M.E.; Dekkers, J.; Aerts, J.G.; Hegmans, J.P. Tumour-derived exosomes as antigen delivery carriers in dendritic cell-based immunotherapy for malignant mesothelioma. J. Extracell. Vesicles 2013, 2, 22492.
- Vázquez-Ríos, A.J.; Molina-Crespo, Á.; Bouzo, B.L.; López-López, R.; Moreno-Bueno, G.; De La Fuente, M. Exosome-mimetic nanoplatforms for targeted cancer drug delivery. J. Nanobiotechnol. 2019, 17, 85.
- Spugnini, E.P.; Logozzi, M.; Di Raimo, R.; Mizzoni, D.; Fais, S. A Role of Tumor-Released Exosomes in Paracrine Dissemination and Metastasis. Int. J. Mol. Sci. 2018, 19, 3968.
- Wolfers, J.; Lozier, A.; Raposo, G.; Regnault, A.; Théry, C.; Masurier, C.; Flament, C.; Pouzieux, S.; Faure, F.; Tursz, T.; et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat. Med. 2001, 7, 297–303.
- Rana, S.; Yue, S.; Stadel, D.; Zöller, M. Toward tailored exosomes: The exosomal tetraspanin web contributes to target cell selection. Int. J. Biochem. Cell Biol. 2012, 44, 1574–1584.
- Frey, A.B. Myeloid suppressor cells regulate the adaptive immune response to cancer. J. Clin. Investig. 2006, 116, 2587–2590.
- Taylor, D.D.; Gercel-Taylor, C. Exosomes/microvesicles: Mediators of cancer-associated immunosuppressive microenvi-ronments. Semin. Immunopathol. 2011, 33, 441–454.
- Harris, D.A.; Patel, S.H.; Gucek, M.; Hendrix, A.; Westbroek, W.; Taraska, J.W. Exosomes Released from Breast Cancer Carcinomas Stimulate Cell Movement. PLoS ONE 2015, 10, e0117495.
- Shenoda, B.B.; Ajit, S.K. Modulation of Immune Responses by Exosomes Derived from Antigen-Presenting Cells. Clin. Med. Insights Pathol. 2016, 9.
- Escudier, B.; Dorval, T.; Chaput, N.; André, F.; Caby, M.-P.; Novault, S.; Flament, C.; Leboulaire, C.; Borg, C.; Amigorena, S.; et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: Results of thefirst phase I clinical trial. J. Transl. Med. 2005, 3, 10.
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release 2015, 207, 18–30.
- Shi, S.; Rao, Q.; Zhang, C.; Zhang, X.; Qin, Y.; Niu, Z. Dendritic Cells Pulsed with Exosomes in Combination with PD-1 Antibody Increase the Efficacy of Sorafenib in Hepatocellular Carcinoma Model. Transl. Oncol. 2018, 11, 250–258.
- Pitt, J.M.; Charrier, M.; Viaud, S.; André, F.; Besse, B.; Chaput, N.; Zitvogel, L. Dendritic Cell–Derived Exosomes as Immunotherapies in the Fight against Cancer. J. Immunol. 2014, 193, 1006–1011.
- Théry, C.; Duban, L.; Segura, E.; Véron, P.; Lantz, O.; Amigorena, S. Indirect activation of naïve CD4+ T cells by dendritic cell–derived exosomes. Nat. Immunol. 2002, 3, 1156–1162.
- Viaud, S.; Terme, M.; Flament, C.; Taieb, J.; André, F.; Novault, S.; Escudier, B.; Robert, C.; Caillat-Zucman, S.; Tursz, T.; et al. Dendritic Cell-Derived Exosomes Promote Natural Killer Cell Activation and Proliferation: A Role for NKG2D Ligands and IL-15Rα. PLoS ONE 2009, 4, e4942.
- Jakubec, M.; Maple-Grødem, J.; Akbari, S.; Nesse, S.; Halskau, Ø.; Mork-Jansson, A.E. Plasma-derived exosome-like vesicles are enriched in lyso-phospholipids and pass the blood-brain barrier. PLoS ONE 2020, 15, e0232442.
- Dai, S.; Wei, D.; Wu, Z.; Zhou, X.; Wei, X.; Huang, H.; Li, G. Phase I Clinical Trial of Autologous Ascites-derived Exosomes Combined with GM-CSF for Colorectal Cancer. Mol. Ther. 2008, 16, 782–790.
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228.
- Kang, J.; Park, H.; Kim, H.; Mun, D.; Park, H.; Yun, N.; Joung, B. Human peripheral blood-derived exosomes for microRNA delivery. Int. J. Mol. Med. 2019, 43, 2319–2328.
- Jiang, K.; Yang, J.; Guo, S.; Zhao, G.; Wu, H.; Deng, G. Peripheral Circulating Exosome-Mediated Delivery of miR-155 as a Novel Mechanism for Acute Lung Inflammation. Mol. Ther. 2019, 27, 1758–1771.
- Qu, M.; Lin, Q.; Huang, L.; Fu, Y.; Wang, L.; He, S.; Fu, Y.; Yang, S.; Zhang, Z.; Zhang, L.; et al. Dopamine-loaded blood exosomes targeted to brain for better treatment of Parkinson’s disease. J. Control. Release 2018, 287, 156–166.
- Nair, S.; Tang, K.D.; Kenny, L.; Punyadeera, C. Salivary exosomes as potential biomarkers in cancer. Oral Oncol. 2018, 84, 31–40.
- Huebner, A.R.; Somparn, P.; Benjachat, T.; Leelahavanichkul, A.; Avihingsanon, Y.; Fenton, R.A.; Pisitkun, T. Exosomes in Urine Biomarker Discovery. In Urine Proteomics in Kidney Disease Biomarker Discovery; Springer: Dordrecht, The Netherlands, 2015; pp. 43–58.
- Ju, S.; Mu, J.; Dokland, T.; Zhuang, X.; Wang, Q.; Jiang, H.; Xiang, X.; Deng, Z.-B.; Wang, B.; Zhang, L.; et al. Grape Exosome-like Nanoparticles Induce Intestinal Stem Cells and Protect Mice From DSS-Induced Colitis. Mol. Ther. 2013, 21, 1345–1357.
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Gupta, R.C. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016, 371, 48–61.
- Dad, H.A.; Gu, T.-W.; Zhu, A.-Q.; Huang, L.-Q.; Peng, L.-H. Plant Exosome-like Nanovesicles: Emerging Therapeutics and Drug Delivery Nanoplatforms. Mol. Ther. 2021, 29, 13–31.
- Wang, Q.; Ren, Y.; Mu, J.; Egilmez, N.K.; Zhuang, X.; Deng, Z.; Zhang, L.; Yan, J.; Miller, D.; Zhang, H.-G. Grapefruit-Derived Nanovectors Use an Activated Leukocyte Trafficking Pathway to Deliver Therapeutic Agents to Inflammatory Tumor Sites. Cancer Res. 2015, 75, 2520–2529.
- Huang, L.-K.; Chao, S.-P.; Hu, C.-J. Clinical trials of new drugs for Alzheimer disease. J. Biomed. Sci. 2020, 27, 1–13.
- Oxford, A.E.; Stewart, E.S.; Rohn, T.T. Clinical Trials in Alzheimer’s Disease: A Hurdle in the Path of Remedy. Int. J. Alzheimer’s Dis. 2020, 2020, 5380346.
- Aisen, P.S. Editorial: Failure After Failure. What Next in AD Drug Development? J. Prev. Alzheimer’s Dis. 2019, 6, 150.
- Yin, Q.; Ji, X.; Lv, R.; Pei, J.-J.; Du, Y.; Shen, C.; Hou, X. Targetting Exosomes as a New Biomarker and Therapeutic Approach for Alzheimer’s Disease. Clin. Interv. Aging 2020, 15, 195–205.
- Losurdo, M.; Pedrazzoli, M.; D’Agostino, C.; Elia, C.A.; Massenzio, F.; Lonati, E.; Mauri, M.; Rizzi, L.; Molteni, L.; Bresciani, E.; et al. Intranasal delivery of mesenchymal stem cell-derived extracellular vesicles exerts immunomodulatory and neuroprotective effects in a 3xTg model of Alzheimer’s disease. Stem Cells Transl. Med. 2020, 9, 1068–1084.
- Iyaswamy, A.; Krishnamoorthi, S.K.; Liu, Y.W.; Song, J.X.; Kammala, A.K.; Sreenivasmurthy, S.G.; Malampati, S.; Tong, B.C.K.; Selvarasu, K.; Cheung, K.H.; et al. Yuan-Hu Zhi Tong Prescription Mitigates Tau Pathology and Alleviates Memory Deficiency in the Preclinical Models of Alzheimer’s Disease. Front. Pharmacol. 2020, 11, 584770.
- Iyaswamy, A.; Krishnamoorthi, S.K.; Song, J.-X.; Yang, C.-B.; Kaliyamoorthy, V.; Zhang, H.; Sreenivasmurthy, S.G.; Malampati, S.; Wang, Z.-Y.; Zhu, Z.; et al. NeuroDefend, a novel Chinese medicine, attenuates amyloid-β and tau pathology in experimental Alzheimer’s disease models. J. Food Drug Anal. 2020, 28, 132–146.
- Durairajan, S.S.K.; Iyaswamy, A.; Shetty, S.G.; Kammella, A.K.; Malampati, S.; Shang, W.; Yang, C.; Song, J.; Chung, S.; Huang, J.; et al. A modified formulation of Huanglian-Jie-Du-Tang reduces memory impairments and β-amyloid plaques in a triple transgenic mouse model of Alzheimer’s disease. Sci. Rep. 2017, 7, 6238.
- Pascucci, L.; Coccè, V.; Bonomi, A.; Ami, D.; Ceccarelli, P.; Ciusani, E.; Viganò, L.; Locatelli, A.; Sisto, F.; Doglia, S.M.; et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: A new approach for drug delivery. J. Control. Release 2014, 192, 262–270.
- Sun, D.; Zhuang, X.; Xiang, X.; Liu, Y.; Zhang, S.; Liu, C.; Barnes, S.; Grizzle, W.; Miller, D.; Zhang, H.-G. A Novel Nanoparticle Drug Delivery System: The Anti-inflammatory Activity of Curcumin Is Enhanced When Encapsulated in Exosomes. Mol. Ther. 2010, 18, 1606–1614.
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345.
- Zhuang, X.; Xiang, X.; Grizzle, W.; Sun, D.; Zhang, S.; Axtell, R.C.; Ju, S.; Mu, J.; Zhang, L.; Steinman, L.; et al. Treatment of Brain Inflammatory Diseases by Delivering Exosome Encapsulated Anti-inflammatory Drugs From the Nasal Region to the Brain. Mol. Ther. 2011, 19, 1769–1779.
- Wahlgren, J.; Karlson, T.D.L.; Brisslert, M.; Sani, F.V.; Telemo, E.; Sunnerhagen, P.; Valadi, H. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res. 2012, 40, e130.
- Shtam, T.A.; Kovalev, R.A.; Varfolomeeva, E.; Makarov, E.M.; Kil, Y.V.; Filatov, M.V. Exosomes are natural carriers of exoge-nous siRNA to human cells in vitro. Cell Commun. Signal. 2013, 11, 88.
- Kooijmans, S.A.; Stremersch, S.; Braeckmans, K.; de Smedt, S.C.; Hendrix, A.; Wood, M.J.; Schiffelers, R.M.; Raemdonck, K.; Vader, P. Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. J. Control. Release 2013, 172, 229–238.
- Antimisiaris, S.G.; Mourtas, S.; Marazioti, A. Exosomes and Exosome-Inspired Vesicles for Targeted Drug Delivery. Pharmaceutics 2018, 10, 218.
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 655–664.
- Podolak, I.; Galanty, A.; Sobolewska, D. Saponins as cytotoxic agents: A review. Phytochem. Rev. 2010, 9, 425–474.
- Fuhrmann, G.; Serio, A.; Mazo, M.; Nair, R.; Stevens, M.M. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J. Control. Release 2015, 205, 35–44.
- Fu, S.; Wang, Y.; Xia, X.; Zheng, J.C. Exosome engineering: Current progress in cargo loading and targeted delivery. NanoImpact 2020, 20, 100261.
- Thakur, A.; Zou, H.; Yang, M.; Lee, Y. Abstract 3720: Augmented loading efficiency of doxorubicin into glioma-derived exo-somes by an integrated microfluidic device. In Cancer Research; American Association for Cancer Research: Philadelphia, PA, USA, 2018; p. 3720.
