TiO2 is the most widely used photocatalyst in many energy and environmental applications. This entry describes the basic structure and properties of TiO2 as a nanomaterials. It also enlists the special properties of TiO2 which make it a best candidate for photocatalysis reaction. It also explains the drawbacks of TiO2 nanomaterials along with the strategies to overcome those.
- Photocatalysts
- Nanocomposites
- Green Chemistry
- Renewable Energy
Introduction
TiO2 has received wide attention ever since 1972 when Fujishima and Honda discovered photocatalytic splitting of water on a TiO2 electrode under ultraviolet (UV) light [1]. Over the past decades, TiO2 has served as a “benchmark photocatalyst” for the degradation of a wide class of organic compounds and microorganisms in the UV range [2] [3]. TiO2 has been the most widely studied and used in many applications because of its strong oxidizing abilities, superhydrophilicity, chemical stability, long durability, nontoxicity, biocompatibility, photocorrosion-free, low cost, and transparency to visible light [4]. In addition, it has the following advantages.
- TiO2 can be fabricated with interesting morphologies such as spheres, nanorods, fibers, tubes and sheets. This enables them to attain unique chemical, physical, and electronic properties increasing its photocatalytic efficiency [5] [6].
- TiO2 nanomaterials can be prepared in large scale at mild temperatures and conditions. [7] [8]
- TiO2 can be supported on various substrates such as glass, fibers, stainless steel, inorganic materials, sand, and activated carbon which allows its continuous reuse [9].
Therefore, tremendous interest has been shown in studies of TiO2 nanomaterial structures and their photocatalytic applications.
2.1. Structure of TiO2
TiO2 exists in three mineral forms, viz., anatase, rutile and brookite. These polymorphic phases of TiO2 are shown in figure 3. In all the three forms, titanium (Ti4+) atoms are coordinated to six oxygen (O2-) atoms, forming TiO6 octahedra. Anatase is made up of face (vertice) sharing arrangements of octahedra at (001) planes (Fig. 1 a) resulting in a tetragonal structure. In rutile, the octahedra share edges at (001) planes to give a tetragonal structure (Fig. 1 b), and in brookite both edges and corners are shared to give an orthorhombic structure (Fig. 1 c)[10]
Amongst the various phases, TiO2 in the brookite phase has been scarcely used as photocatalysts. Between TiO2 in the anatase and rutile phases, the anatase TiO2 is more active for photocatalysis applications, even though the rutile TiO2 possesses a smaller band gap. The reasons anatase TiO2 is more actively used for photocatalysis are as follows [11]:
- The conduction band position of anatase TiO2 is more negative compared to rutile TiO2. This is responsible for the higher reducing power of anatase TiO2 towards the adsorbed reactants and thus results in the higher photocatalytic activity of anatase TiO2 in comparison to rutile TiO2
- Recombination reactions adversely affect photocatalytic activity and the degree of recombination is found to be higher for rutile TiO2 than for anatase TiO2. The recombination reactions limit rutile TiO2’s activity.
The photocatalytic activity of TiO2 increases with an increase in the density of physically adsorbed OH- groups on its surface. The surface hydroxyl groups accept holes generated by UV and solar irradiation to form hydroxyl radicals and prevent electron-hole recombination thus increasing the photocatalytic activity of TiO2. Generally, the surface of the anatase phase of TiO2 is very hydroxylated; whereas, rutile TiO2, being obtained at higher temperatures, has a very low density of physically adsorbed hydroxyl groups. This leads to lower photoactivity of rutile TiO2 in comparison to anatase TiO2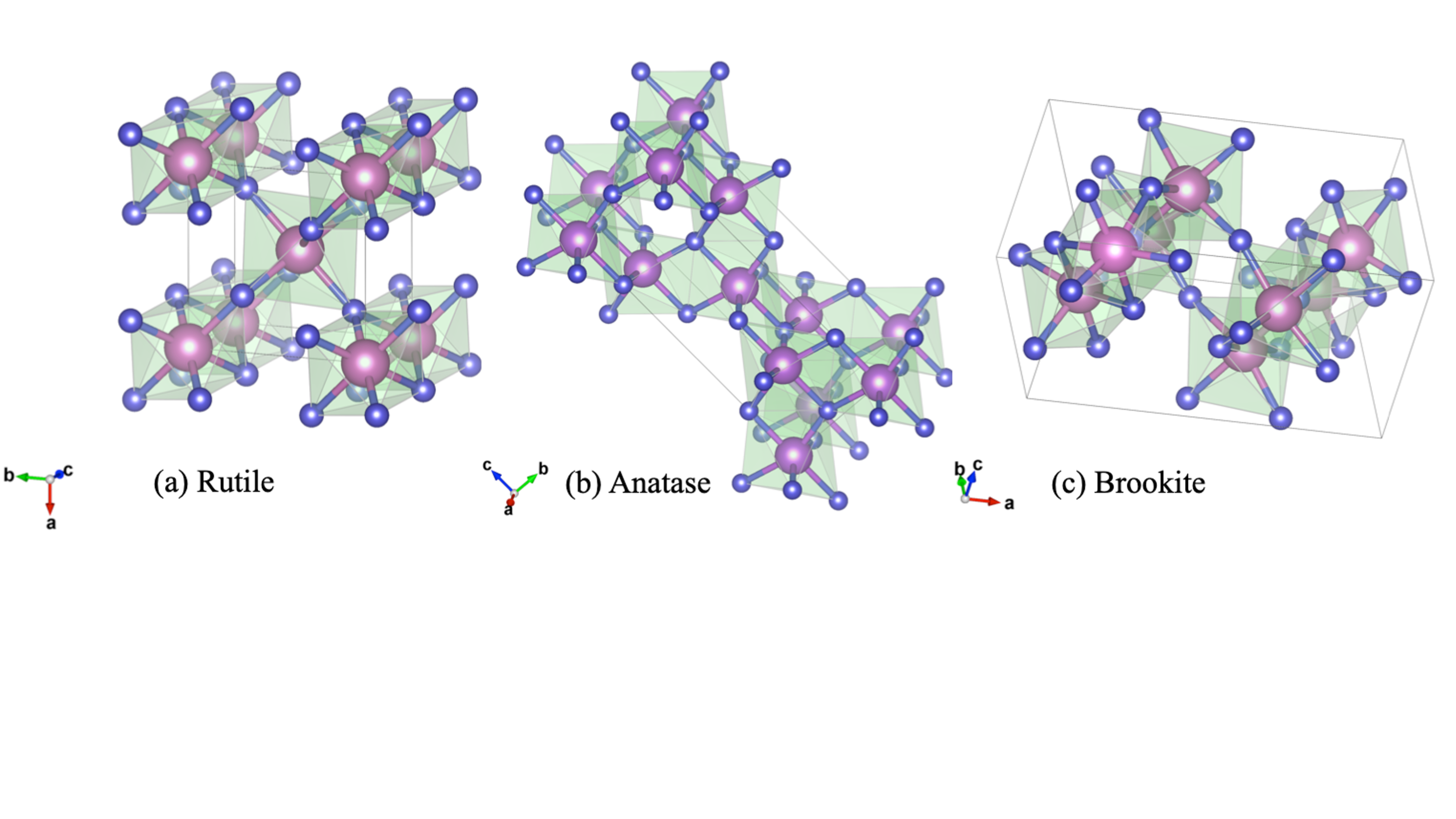
Figure 1. Polymorphic phases of TiO2
2.2. Drawbacks of TiO2
Besides the aforementioned inherent advantages of TiO2, it has following drawbacks
-
- The large band gap of TiO2 (∼ 3.2 eV for anatase and brookite, ∼3.0 eV for rutile) requires an excitation wavelength that falls in the UV region. Given that less than ∼5% of the solar flux incident at the earth’s surface lies in this spectral regime (solar light consists of ∼5% UV, ∼43% visible, and ∼52% harvesting infrared), only a very small portion of the solar light could be used by pure TiO2 photocatalysts.
- Massive recombination of photogenerated charge carriers limits its overall photocatalytic efficiency
2.3 Strategies to modify TiO2 nanomaterials
Various strategies have been adopted for improving the photocatalytic efficiency of TiO2. Some of them are listed in Fig. 2.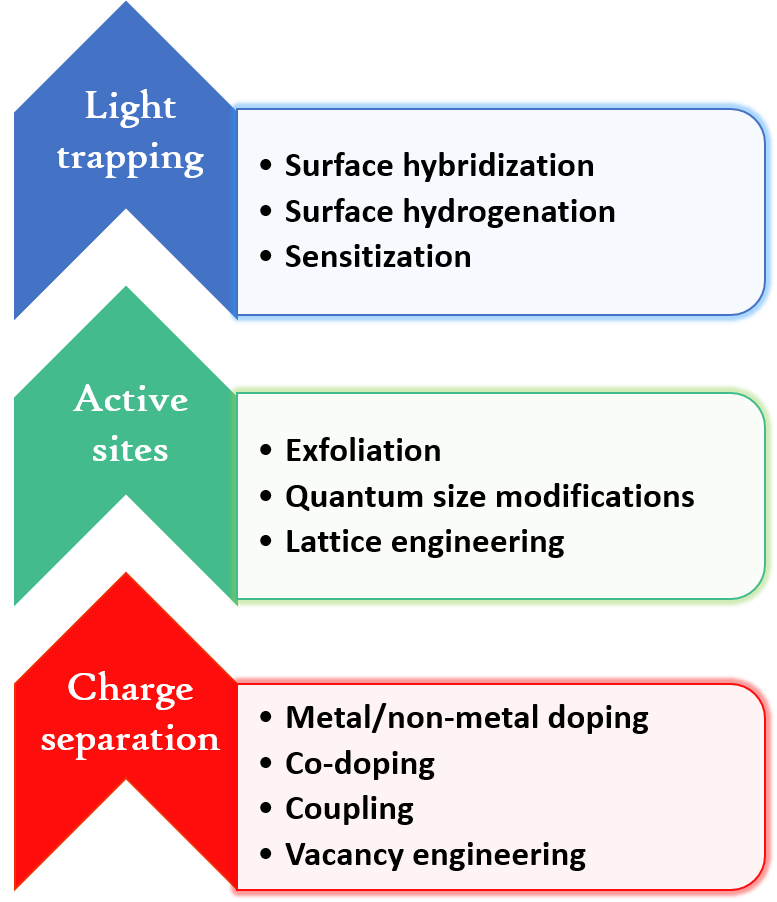
Figure 2. Strategies to modify TiO2
