Cellulose triacetate (CTA)-based hollow fiber (HF) membrane is one of the commercially successful semipermeable membranes that has had a long progress since the time the excellent semi-permeable feature of cellulose-based polymers was found in 1957. Because of the reliable and excellent performances, especially for drinking water production from seawater, CTA-HFs have been widely used as reverse osmosis (RO) membranes, especially in arid regions.
- seawater desalination
- cellulose triacetate
1. Introduction
A rapid growth of global population and urbanization especially in developing countries has resulted in a huge demand for fresh water in many regions of the world [1][2][3][4]. Fresh water shortages are becoming a crucial threat for the securing of sustainable water, human health, ecosystems, and economic development [5][6][7]. At present, about 40% of the global population faces severe water scarcity, and it is still expected to increase [7]. Because the total amount of fresh water on the earth remained almost the same despite the increasing demand, seawater desalination for reliable water securing has been sufficiently important as a major water source especially in arid regions. Seawater reverse osmosis (SWRO) has been recognized as a reliable mainstream method in the world because of its lower energy consumption compared with the conventional desalination techniques (such as evaporation) due to the absence of latent heat energy for the phase changing of water [8]. Theoretically, about 1.0 kWh of energy is needed to produce 1 m3 of fresh water with the seawater recovery ratio of 50% [9][10][11]. However, about 2.6–8.5 kWh of energy is generally required in the current RO plants [12], and minimizing the energy consumption is still needed. Furthermore, the recent environmental concerns and legal regulations require minimizing the environmental impacts of brine disposal from SWRO plants as well as wastewater treatment plants [13][14][15][16]. For example, the total global installed desalination capacity is 97.2 million m3/day while the total global cumulative contracted capacity is 114.9 million m3/day as of the middle of Feb 2020 [17], that is, a huge amount of brine (about 140 million m3/day) was discharged into the ocean (under an acceptable assumption that its water recovery ratio was 42% [16]). Therefore, brine management has been recognized as an important matter due to the rapid increase in the number of desalination plants. In fact, for example, 22 million m3/day of brine (15.5% of the total brine) is produced inland (over 50 km from the nearest coastline) [16], and it creates transportation problems for the brine discharge to say the least. Therefore, the optimization of brine management for the plants via economically viable and environmentally friendly ways will become quite important in the near future [15][18]. For this purpose, both brine volume minimization and the subsequent reuse of the brine as a valuable resource are regarded as promising options because minimizing the brine will reduce the amount of brine as well as increase the water recovery in the RO plant. In addition, the subsequent brine mining can transform waste brine into valuable products [19][20][21].
A semipermeable membrane is a key component in the SWRO plants that enables the extraction of fresh water from seawater. Moreover, it also has a huge potential for brine minimizing. Up to now, only cellulose triacetate (CTA)-based and polyamide (PA)-based membranes have been successfully used in the SWRO plants for reliable water securing since the first finding of an outstanding semipermeable membrane of cellulose-based polymers in 1957 by Reid [22]. Cellulose triacetate (CTA)-based hollow fiber (HF) membrane is therefore one of the commercially successful semipermeable membranes that has a long historical development due to its reliable excellent performances especially for drinking water production from seawater. In addition, recently, because of the versatile demands in energy consumption and environmental concerns about brine discharge as mentioned above, CTA-based HFs have caught attention for the usage of different methods, such as forward osmosis (FO) [23][24][25][26], pressure-retarded osmosis (PRO) [11][27][28][29][30][31][32][33] and brine concentration (BC) [34][35] for the emerging technologies. Because their applications require optimum characteristics individually, different strategies for designing the membrane and module are required in spite of using the same CTA material.
2. Brief Background of the CTA Membrane for Seawater Desalination
The RO process was firstly proposed by Reid of Florida University in the beginning of 1953 [23][36]. After then, the workers at Florida University announced a cellulose diacetate (CDA) film with outstanding semipermeable performance in which salt rejection was 96% or more, but its water permeability was still very small and not applicable for commercial application [37][38]. However, their pioneering work clearly showed a possibility of conducting a RO process using a semipermeable membrane for seawater desalination. After that, a remarkable progress was achieved by Loeb and Sourirajan of the University of California, Los Angeles (UCLA); they prepared an asymmetric-type CDA membrane that had quite a thinner salt rejection layer and a loose support sublayer using the same material of CDA [39][40]. After this invention, accelerated research on cellulose-acetate (CA)-based materials for practical applications was conducted to improve the membrane performance in the industry [41]. The details in the early history of RO membrane development are available elsewhere [37][41][42][43][44][45]. As CA-based polymers, cellulose triacetate (CTA) [46], cellulose acetate butylate [47], CA blends such as CDA with CTA [45][48], the cross-linked CA [49], and the composite membrane (a thin CA film on a porous support consisting of the other polymer) [50] were demonstrated in addition to the CDA. Towards its commercialization, among them, CTA membranes were well developed by some companies because CTA had a better stability in a wider range of temperatures and pH, higher resistance to chemicals and biological attack, and better membrane performance compared to the initial CDA, even though CTA was more difficult to handle in the membrane preparation than CDA [41]. Then, the preparation conditions such as dope solution composition and post-treatment condition were also further developed and optimized for the applicable industrial production. For example, the dope solution contained CA polymer, acetone, and magnesium perchlorate at −10 °C casted on a glass plate. The casted solution was set for a certain time as an evaporation procedure and then immersed in cold water [39][40]. Since the first method was difficult, an easier preparation procedure with high membrane performance was required in the industry [41]. Although various types of solvent for the dope and coagulation bath were then studied (such as acetone, acetic acid, formamide, chloroform, pyridine, dioxane and its mixtures), these solutions were difficult to handle in the industry. Therefore, polar aprotic solvents such as N-methyl pyrrolidone (NMP), dimethylacetamide (DMAc) and dimethylsulfoxide (DMSO) were preferably used as the alternative solvents because of their easy handling in the industrial production.
In addition to the membrane development, subsequent module configurations were also developed, such as tubular [42][51], plate-and-flame [52], spiral-wound [53][54], and HF [55]. Among them, the HF configuration had superior advantages to the spiral-wound in the total membrane area, the packing density, and the module performance if the original membrane performance was the same. The spiral-wound type CA membrane was commercialized by many companies and used as the industry standard through the 1960s to mid-1970s [37][56]. However, CA-based membranes were still not widely used for the large-scale desalination purpose at that time [57]. The CA-based spiral one was mainly used for pure water production for industrial processes and ultrapure water production in the semiconductor industry [57]. After that, in the case of the spiral-wound type, an alternative membrane was found via the pioneering work by Cadotte from 1972 [56][58], which was based on a situ condensation method using monomeric amine and monomeric acid halide. This innovative invention made a rapid shift from the CA-based spiral-wound to the PA-based one because of the preferable features of the PA-based membrane, such as higher flux and higher pH resistance.
With the purpose of achieving seawater desalination in mind, a significant portion of research and development was carried out using a CTA-HF membrane and module by Dow Chemical based on a research contract with the Office of Saline Water in the U.S. Department of the Interior [37]. Their development results of the RO module for brackish water desalination and for seawater desalination were published in 1970 and 1974, respectively [59][60], and the CTA-based HF RO module for low-pressure brackish water desalination was subsequently marketed in 1974 [37]. The research developments for the CA-based HF were also conducted by other companies such as Monsanto, Toyobo, and others, in addition to Dow Chemical. After that, following the early work by Dow Chemical, Toyobo successfully developed a CTA-HF RO module for high-pressure one-pass seawater desalination and subsequently marketed it in 1979 [37][45][61]. The CTA-HF RO module was often used in the fishing industry as a small-scale desalination apparatus on a shipping boat [62].
After these efforts, because of the rapid increasing demand for drinking water in the world, RO membranes have been acceptably and widely used for the large-scale seawater desalination purpose since the 1990s; the total global cumulative contracted capacities since 1965 were about 14, 25, and 60 million m3/day in 1990, 2000, and 2010, respectively [1]. In the case of the HF type, only CTA is still used for the RO membrane for seawater desalination, unlike the spiral-wound type, because of its competitive total module performance of CTA-based HF membrane module compared with the PA-based spiral-wound one [29].
3. Features of CTA-HF RO Module
3.1. CTA-HF Membrane
3.1.1. Preparation Procedure and Its Characteristics
CTA-HF RO membranes are generally prepared by spinning a solution of a CTA polymer, followed by soaking and annealing. The optimization of the preparation condition, such as using spinning technology of high concentration polymers, micropore controlling technology in manufacturing, and post-treatment by high temperature annealing, made it possible to increase the permeate water flux of the CTA-HF membrane without additional steps [37][38]. A typical illustration for HF preparation procedure for an asymmetric membrane is shown in Figure 1. Highly-controlled spinning technology provided a well-designed morphology of the HF with an outer dense layer for selective water permeation and a relatively gradient porous structure of the HF along the thickness direction from the outer to inner diameters. Figure 2 shows the optical and transmission electron microscopic images (OM and TEM) of the CTA-HF RO membranes. In the TEM image, the CTA-HF RO membrane has roughly three layers (the outermost layer in white is the dense separation layer) with gradient morphology. Water- and salt-selective permeations strongly depend on the outer dense layer, and the porous support layer provides the pressure resistance of the HF membranes. Therefore, this gradient porous morphology along the outer dense layer and the porous inner support layer provided the CTA-HF RO membranes with outstanding pressure resistance retention without additional supports. Moreover, the gradient morphology will be formed even within a dense thin layer because the dense layer in the TEM image is a lot thicker than expected from the theoretical permeability of the HF membrane estimated from its thickness in the TEM image.
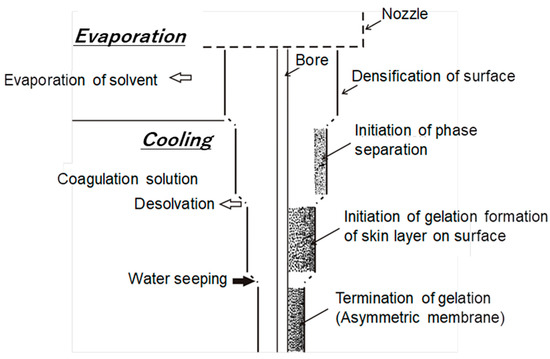
Figure 1. Typical diagram for preparation of an asymmetric membrane.
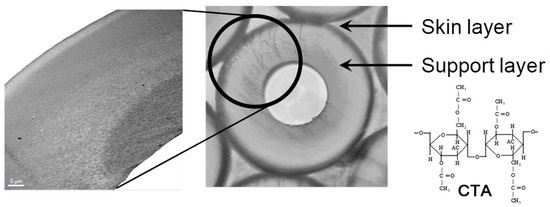
Figure 2. Image of hollow fiber (HF) membrane in cross-sectional structure and TEM image.
The HF dimensions with a fine HF structure are also important characteristics to give not only a pressure resistance of HF but also water permeation performance. The outer and inner diameters of CTA-HF RO membranes are designed to be about 140 μm and 55 μm, respectively. The suitable dimensions of the HF are controlled by the preparation condition and the nozzle dimensions in the spinning process.
3.1.2. Chlorine Resistance
A membrane clogging (fouling) caused by fouling substances (foulants) is a major problem in the daily operations in the seawater RO desalination plants because it significantly deteriorates the performance of membrane permeability and plant productivity [63]. Because the scaling is generally controlled by adjusting the pH of seawater and its recovery ratio, the most serious fouling problem in the RO plant is microorganism-based biological fouling [64]. Microorganisms adhere to the RO membrane that utilizes the foulants (such as organic substances attached onto the membrane surface), and the various microorganisms then proliferate, forming a biofilm [65]. Once a serious biofilm is formed on the RO membranes, the performance recovery would become too severe; therefore, precautions are generally taken using chemical treatments such as chlorine injection for sterilizing and suppressing the proliferation of microorganisms. For this purpose, chlorine tolerance of the membrane is an important characteristic for a stable and reliable RO plant operation without losing the salt rejection performance of the membrane. Because of CTA having less chlorine reactivity than PA [66], the CTA RO membranes have a higher chlorine resistance than that of polyamide-based (PA) RO membranes, and this enables a reliable and stable RO operation without any biological fouling problems for a long period by implementing a simply controlled intermittent chlorine injection (ICI).
3.1.3. CTA Membrane Characteristics Compared to PA-Based Membrane
In addition to the development of a CTA-based RO membrane, PA-based RO membranes also had a period of rapid development, starting from the time of Cadotte’s pioneering work in 1972 [56][58], and they were successfully used for seawater desalination purposes. Compared to the CTA-based membrane, PA-based membrane has superior performance characteristics, such as higher flux per membrane area as well as higher chemical and pH resistances. In particular, the unique preparation procedure of PA-based membranes via interfacial polymerization on the porous support layer allows for a very thin selective layer formation, which results in much higher water permeability than the CTA-based membrane. On the other hand, at the same time, the two-step membrane preparation procedure of a PA-based membrane (porous support layer preparation and subsequent interfacial polymerization) might impede the mass production of the PA-based membrane applicable for the HF configuration, especially the outer-selective layer type of the PA-based membrane [67]. Although the CTA-based membrane has lower water permeability than the PA-based membrane, the simple preparation procedure of the CTA-based membrane allows for a HF configuration and a more highly effective membrane area compared to the PA-based membrane. Consequently, the resulting module performances of CTA and PA-based membranes have been in competition with each other in the viewpoint of module performance.
The other difference between CTA and PA-based membranes is the stability of the membrane, such as chlorine resistance and membrane compaction resistance. As for the chlorine resistance, in the case of CTA-based membranes, because the CTA is an environmentally friendly polymer (biomass-based plastic and biodegradable), moderate chlorine disinfection is generally recommended to prevent the excessive degradation of the membrane during the operation, whereas chlorine disinfection is generally not recommended in the case of PA-based membranes because chlorine exposure causes performance deterioration of the PA-based membrane [68]. As for the compaction resistance, the compaction tolerance of the CTA-based membrane is less than that of PA-based membrane, which causes a flux performance reduction of the CTA-based membrane during the initial operation stage [69]. On the other hand, the PA-based membrane has higher compaction resistance than the CTA. To overcome these problems in both PA and CTA-based membranes, the recent studies have demonstrated an additional blending strategy using siloxanes [70], carbon nanotubes [71], and nanoparticles [72] for PA-based membrane with chlorine tolerance, as well as a reinforcing strategy using nonwoven fabric [73] for CTA-based membrane with compaction tolerance.
This entry is adapted from the peer-reviewed paper 10.3390/membranes11030183
References
- IDA; GWI DesalData. IDA Water Security Handbook 2019–2020; IDA and GWI DesalData: Topsfield, MA, USA, 2019; pp. 14–41.
- Pitchard, H.D. Asia’s shrinking glaciers protect large populations from drought stress. Nature 2019, 569, 649–654.
- Linares, R.V.; Li, Z.; Elimelech, M.; Amy, G.; Vrouwenvelder, H. Population Distribution and Water Scarcity. In Recent Developments in Forward Osmosis Processes; Linares, R.V., Li, Z., Elimelech, M., Amy, G., Vrouwenvelder, H., Eds.; IWA Publishing: London, UK, 2017; pp. 3–13.
- Liu, J.; Yang, H.; Gosling, S.N.; Kummu, M.; Flörke, M.; Pfister, S.; Hanasaki, N.; Wada, Y.; Zhang, X.; Zheng, C.; et al. Water scarcity assessments in the past, present, and future. Earth Future 2017, 5, 545–559.
- Caparrós-Martínez, J.L.; Rueda-Lópe, N.; Milán-García, J.; Valenciano, J.P. Public policies for sustainability and water security: The case of Almeria (Spain). Glob. Ecol. Conserv. 2020, 23, e01037.
- Al-Saidi, M.; Saliba, S. Water, Energy and Food Supply Security in the Gulf Cooperation Council (GCC) Countries: A Risk Perspective. Water 2019, 11, 455.
- Schewea, J.; Heinkea, J.; Gerten, D.; Haddeland, I.; Arnell, N.W.; Clark, D.B.; Dankers, R.; Eisner, S.; Fekete, B.M.; Colón-González, F.J.; et al. Multimodel assessment of water scarcity under climate change. Proc. Natl. Acad. Sci. USA 2014, 111, 3245–3250.
- Godart, P. Design and simulation of a heat-driven direct reverse osmosis device for seawater desalination powered by solar thermal energy. Appl. Energy 2020, 284, 116039.
- Yasukawa, M.; Suzuki, T.; Higa, M. Salinity Gradient Process: Thermodynamics, Applications, and Future Prospects. In Membrane-Based Salinity Gradient Processes for Water Treatment and Power Generation, 1st ed.; Sarp, S., Hilal, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–56.
- Mistry, K.H.; McGovern, R.K.; Thiel, G.P.; Summers, E.K.; Zubair, S.M.; Lienhard, J.H. Entropy generation analysis of desalination technologies. Entropy 2011, 13, 1829–1864.
- Tanaka, Y.; Yasukawa, M.; Goda, S.; Sakurai, H.; Shibuya, M.; Takahashi, T.; Kishimoto, M.; Higa, M.; Matsuyama, H. Experimental and simulation studies of two types of 5-inch scale hollow fiber membrane modules for pressure-retarded osmosis. Desalination 2018, 447, 133–146.
- Nassrullah, H.; Anis, S.F.; Hashaikeh, R.; Hilal, N. Energy for desalination: A state-of-the-art review. Desalination 2020, 491, 114569.
- Kurihara, M.; Sakai, H.; Tanioka, A.; Tomioka, H. Role of pressure-retarded osmosis (PRO) in the mega-ton water project. Desalin. Water Treat. 2016, 57, 26518–26528.
- Kurihara, M.; Hanakawa, M. Mega-ton Water System: Japanese national research and development project on seawater desalination and wastewater reclamation. Desalination 2013, 308, 131–137.
- Giwa, A.; Dufour, V.; Al Marzooqi, F.; Al Kaabi, M.; Hasan, S.W. Brine management methods: Recent innovations and current status. Desalination 2017, 407, 1–23.
- Jones, M.; Qadir, M.; van Vliet, M.T.; Smakhtin, V.; Kang, S.M. The state of desalination and brine production: A global outlook. Sci. Total Environ. 2019, 657, 1343–1356.
- Eke, J.; Yusuf, A.; Giwa, A.; Sodiq, A. The global status of desalination: An assessment of current desalination technologies, plants and capacity. Desalination 2020, 495, 114633.
- Missimer, T.M.; Maliva, R.G. Environmental issues in seawater reverse osmosis desalination: Intakes and Outfalls. Desalination 2018, 434, 198–215.
- Schantz, A.B.; Xiong, B.; Dees, E.; Moore, D.R.; Yang, X.; Kumar, M. Emerging investigators series: Prospects and challenges for high-pressure reverse osmosis in minimizing concentrated waste streams. Environ. Sci. Water Res. Technol. 2018, 4, 894.
- Semblante, G.U.; Lee, J.Z.; Lee, L.Y.; Ong, S.L.; Ng, H.Y. Brine pre-treatment technologies for zero liquid discharge system. Desalination 2018, 441, 96–111.
- Vanoppen, M.; Stoffels, G.; Buffel, J.; Gusseme, B.D.; Verliefde, A.R.D. A hybrid IEX-RO process with brine recycling for increased RO recovery without chemical addition: A pilot-scale study. Desalination 2016, 394, 185–194.
- Breton, E.J., Jr. Water and Ion Flow Through Imperfect Osmotic Membranes. In Research and Development Progress Report No. 16. Office of Saline Water; Washington, DC, USA, 1957; Available online: (accessed on 8 March 2021).
- Shibuya, M.; Yasukawa, M.; Takahashi, T.; Miyoshi, T.; Higa, M.; Matsuyama, H. Effect of operating conditions on osmotic-driven membrane performances of cellulose triacetate forward osmosis hollow fiber membrane. Desalination 2015, 362, 34–42.
- Shibuya, M.; Yasukawa, M.; Goda, S.; Sakurai, H.; Takahashi, T.; Higa, M.; Matsuyama, H. Experimental and theoretical study of a forward osmosis hollow fiber membrane module with a cross-wound configuration. J. Membr. Sci. 2016, 504, 10–19.
- Ahmed, M.; Kumar, R.; Garudachari, B.; Thomas, J.P. Performance evaluation of a thermoresponsive polyelectrolyte draw solution in a pilot scale forward osmosis seawater desalination system. Desalination 2019, 452, 132–140.
- Goda, S.; Sekino, M. Application of irreversible thermodynamic model to a hollow fiber forward osmosis module in sodium chloride aqueous solution system. Desalination 2020, 486, 114458.
- Saito, K.; Irie, M.; Zaitsu, S.; Sakai, H.; Hayashi, H.; Tanioka, A. Power generation with salinity gradient by pressure retarded osmosis using concentrated brine from SWRO system and treated sewage as pure water. Desalin. Water Treat. 2012, 41, 114–121.
- Kumano, A.; Marui, K.; Terashima, Y. Hollow fiber type PRO module and its characteristics. Desalination 2016, 389, 149–154.
- Higa, M.; Shigefuji, D.; Shibuya, M.; Izumikawa, S.; Ikebe, Y.; Yasukawa, M.; Endo, N.; Tanioka, A. Experimental study of a hollow fiber membrane module in pressure-retarded osmosis: Module performance comparison with volumetric-based power outputs. Desalination 2017, 420, 45–53.
- Yasukawa, M.; Shigefuji, D.; Shibuya, M.; Ikebe, Y.; Horie, R.; Higa, M. Effect of DS Concentration on the PRO Performance Using a 5-Inch Scale Cellulose Triacetate-Based Hollow Fiber Membrane Module. Membranes 2018, 8, 22.
- Kishimoto, M.; Tanaka, Y.; Yasukawa, M.; Goda, S.; Higa, M.; Matsuyama, H. Optimization of Pressure-Retarded Osmosis with Hollow-Fiber Membrane Modules by Numerical Simulation. Ind. Eng. Chem. Res. 2019, 58, 6687–6695.
- Matsuyama, K.; Makabe, R.; Ueyama, T.; Sakai, H.; Saito, K.; Okumura, T.; Hayashi, H.; Tanioka, A. Power generation system based on pressure retarded osmosis with a commercially-available hollow fiber PRO membrane module using seawater and freshwater. Desalination 2021, 499, 114805.
- Madsen, H.T.; Hansen, T.B.; Nakao, T.; Goda, S.; Søgaard, E.G. Combined geothermal heat and pressure retarded osmosis as a new green power system. Energy Convers. Manag. 2020, 226, 113504.
- Togo, N.; Nakagawa, K.; Shintani, T.; Yoshioka, T.; Takahashi, T.; Kamio, E.; Matsuyama, H. Osmotically Assisted Reverse Osmosis Utilizing Hollow Fiber Membrane Module for Concentration Process. Ind. Eng. Chem. Res. 2019, 58, 6721–6729.
- Nakagawa, K.; Togo, N.; Takagi, R.; Shintani, T.; Yoshioka, T.; Kamio, E.; Matsuyama, H. Multistage osmotically assisted reverse osmosis process for concentrating solutions using hollow fiber membrane modules. Chem. Eng. Res. Des. 2020, 162, 117–124.
- Reid, C.E.; Breton, E.J. Water and ion flow across cellulosic membranes. J. Appl. Polym. Sci. 1959, 1, 133–143.
- Kumano, A. Advances in Hollow-Fiber Reverse-Osmosis Membrane Modules in Seawater Desalination. In Advances in Water Desalination; Lior, N., Ed.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 309–375.
- Kumano, A.; Fujiwara, N. Cellulose Triacetate Membranes for Reverse Osmosis. In Advanced Membrane Technology and Applications; Li, N.N., Fane, A.G., Ho, W.S.W., Matsuura, T., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 21–46.
- Loeb, S.; Sourirajan, S. High Flow Porous Membranes for Separating Water from Saline Solutions. U.S. Patent 3,133,132, 12 May 1964.
- Loeb, S. The Loeb-Sourirajan Membrane: How It Came About. In Synthetic Membranes; Turbak, A.F., Ed.; American Chemical Society: Washington, DC, USA, 1981; Volume 153, pp. 1–9.
- Lee, K.P.; Arnot, T.C.; Mattia, D. A review of reverse osmosis membrane materials for desalination—Development to date and future potential. J. Membr. Sci. 2011, 370, 1–22.
- Glater, J. The early history of reverse osmosis membrane development. Desalination 1998, 117, 297–309.
- Belfort, G. Desalting Experience by Hyperfiltration (Reverse Osmosis) in the United States. In Synthetic Membranes Process: Fundamentals and Water Applications; Academic Press: Cambridge, MA, USA, 1984; pp. 221–280.
- Staude, E. Desalting Experience by Hyperfiltration (Reverse Osmosis) in Europe and Japan. In Synthetic Membranes Process: Fundamentals and Water Applications; Academic Press: Cambridge, MA, USA, 1984; pp. 281–341.
- Petersen, R.J. Membranes for Desalination. In Synthetic Membranes; Chenoweth, M.B., Ed.; MMI Press by Harwood Academic Publishers: New York, NY, USA, 1986; pp. 129–154.
- Mclain, E.A.; Mahon, H.I. Permselective Hollow Fibers and Method of Making. U.S. Patent 3,423,491, 2 September 1964.
- Manjikian, S. Cellulose Acetate Butyrate Semipermeable Membranes and Their Production. U.S. Patent 3,607,329, 22 April 1969.
- Hoernschemeyer, D.L. Cellulose Acetate Blend Membranes. U.S. Patent 3,878,276, 24 May 1972.
- Cannon, C.R.; Cantor, P.A. Mixed Esters of Cellulose. U.S. Patent Application No. 3,585,126, 15 June 1971.
- Del, P.J. Supported Semipermeable Membranes and Process for Preparing Same. U.S. Patent 3,762,566, 3 August 1971.
- Loeb, S. UCLA Dept. of Engineering Report 66-40; UCLA: Los Angeles, CA, USA, 1966.
- Loeb, S. UCLA Dept. of Engineering Report 62-41; UCLA: Los Angeles, CA, USA, 1961.
- Westmoreland, J.C. Spirally Wrapped Reverse Osmosis Membrane Cell. U.S. Patent 3,367,504, 6 February 1968.
- Bray, D.T. Reverse Osmosis Purification Apparatus. U.S. Patent 3,417,870, 24 December 1968.
- Mahon, H.I. Permeability Separatory Apparatus, Permeability Separatory Membrane Element, Method of Making the Same and Process Utilizing the Same. U.S. Patent 3,228,876, 19 September 1960.
- Baker, R.W. Membrane Technology and Applications, 3rd ed.; John Wiley and Sons: Hoboken, NJ, USA, 2012; pp. 207–208.
- Uemura, T.; Henmi, M. Thin-Film Composite Membranes for Reverse Osmosis. In Advanced Membrane Technology and Applications; Li, N.N., Fane, A.G., Ho, W.S.W., Matsuura, T., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 21–46.
- Cadotte, J.E. Interfacially Synthesized Reverse Osmosis Membrane. U.S. Patent 4,277,344, 22 February 1979.
- Dance, E.L.; Davis, T.E.; Mahon, E.I.; McLain, E.A.; Skiens, W.E.; Spano, J.O. Development of Cellulose Triacetate Hollow Fiber Reverse Osmosis Modules for Brackish Water Desalination; Report No. 763; U.S. Office of Saline Water Research and Development Progress: New York, NY, USA, 1971.
- Ammons, R.D.; Mahon, H.I. Development of a One-Pass Hollow Fiber Seawater Desalination Module Having a Capacity of 2500–3000 gpd; Report No. 924; U.S. Office of Saline Water Research and Development Progress: Washington, DC, USA; U.S. Government Printing Office: Washington, DC, USA, 1974; Volume 6.
- Ukai, T.; Nimura, Y.; Hamada, K.; Matsui, H. Development of one pass sea water reverse osmosis module, “HOLLOSEP”. Desalination 1980, 32, 169–178.
- Kumano, A. Recent Trends in Water Desalination Technology by Reverse Osmosis. Sen I Gakkaishi 1992, 48, 70–76.
- Badruzzaman, M.; Voutchkovn, N.; Weinrich, L.; Jacangelo, J.G. Selection of pretreatment technologies for seawater reverse osmosis plants: A review. Desalination 2019, 449, 78–91.
- Anis, S.A.; Hashaiken, R.; Hilal, N. Reverse osmosis pretreatment technologies and future trends: A comprehensive review. Desalination 2019, 452, 159–195.
- Bereschenko, L.A.; Heilig, G.H.J.; Nederlof, M.M.; van Loosdrecht, M.C.M.; Stams, A.J.M.; Euverink, G.J.W. Molecular Characterization of the Bacterial Communities in the Different Compartments of a Full-Scale Reverse-Osmosis Water Purification Plant. Appl. Environ. Microbiol. 2008, 74, 5297–5304.
- Nguyen, T.P.N.; Jun, B.-M.; Kwon, Y.-N. The chlorination mechanism of integrally asymmetric cellulose triacetate (CTA)-based and thin film composite polyamide-based forward osmosis membrane. J. Membr. Sci. 2017, 523, 111–121.
- Lim, S.; Tran, V.H.; Akther, N.; Phuntsho, S.; Shon, H.K. Defect-free outer-selective hollow fiber thin-film composite membranes for forward osmosis applications. J. Membr. Sci. 2019, 586, 281–291.
- Do, V.T.; Tang, C.Y.; Reinhard, M.; Leckie, J.O. Effects of Chlorine Exposure Conditions on Physiochemical Properties and Performance of a Polyamide Membrane-Mechanisms and Implications. Environ. Sci. Technol. 2012, 46, 13184–13192.
- Ohya, H. An expression method of compaction effects on reverse osmosis membranes at high pressure operation. Desalination 1978, 26, 163–174.
- Khairkar, S.R.; Pansare, A.V.; Shedge, A.A.; Chhatre, S.Y.; Suresh, A.K.; Chakrabarti, S.; Patil, V.R.; Nagarkar, A.A. Hydrophobic interpenetrating polyamide-PDMS membranes for desalination, pesticides removal and enhanced chlorine tolerance. Chemosphere 2020, 258, 127179.
- Ortiz-Medina, J.; Inukai, S.; Araki, T.; Morelos-Gomez, A.; Cruz-Silva, R.; Takeuchi, K.; Noguchi, T.; Kawaguchi, T.; Terrones, M.; Endo, M. Robust water desalination membranes against degradation using high loads of carbon nanotubes. Sci. Rep. 2018, 8, 2748.
- Saleem, H.; Zaidi, S.J. Nanoparticles in reverse osmosis membranes for desalination: A state of the art review. Desalination 2020, 475, 114171.
- Chen, K.; Xiao, C.; Liu, H.; Li, G.; Meng, X. Structure design on reinforced cellulose triacetate composite membrane for reverse osmosis desalination process. Desalination 2018, 441, 35–43.
