Titanium dioxide (TiO2) is widely used in various fields both in daily life and industry owing to its excellent photoelectric properties and its induced superwettability. Generally, superwettability refers to superhydrophilic, superhydrophobic, superamphiphilic, and superamphiphobic surfaces; the mechanism of the superwettability property can be explained based on the surface structure of materials, surface molecules, and external influencing factors. Over the past several decades, various methods have been reported to improve the wettability of TiO2 and plenty of practical applications have been developed. The TiO2-derived materials with different morphologies display a variety of functions including photocatalysis, self-cleaning, oil-water separation, etc.
- titanium dioxide
- controllable wettability
- ultra-fast spreading
- photoelectrode
- nanochannels
1. Introduction
TiO2 is widely used in practical applications and daily life due to its special properties [1], especially the applications of its photo-induced wettability. Many plants and animals in nature possess special wettability that can provide inspiration for research work, such as lotus leaves [2], fly eyes [3], gecko feet [4], the pitcher [5], etc. [6,7]. In 1964, Fowkes [8] provided a theory for calculating surface energy. He decomposed the surface tension into two forces: London dispersion force and polar force composed of dipole force, hydrogen bonds, and induced force. In 1969, Owens and Wendt [9] developed Fowkes theory, which considered that besides London dispersion force, there were polar effects including induced force, orientation force, and hydrogen bonds at the liquid–solid interface. In 1972, Fujishima and Honda [10] reported photolysis of water through an electrolytic cell that connects a TiO2 electrode and a platinum electrode. When the TiO2 electrode was irradiated by light, the current flow occurred and the oxygen was produced. The way oxygen is produced by oxidizing water is like the way it is produced by photosynthesis in nature. In 1997, Wang et al. [11] reported a property named “superamphiphilicity”, which denotes that the static contact angles (CAs) of both water and oil are approaching 0° on the surface of TiO2 under UV illumination. The mechanism can be explained by the formation of oxygen vacancies and the conversion of Ti4+ sites to Ti3+ sites due to UV light. These defects influence the affinity to chemisorbed water at the surrounding sites and, in turn, hydrophilic and hydrophobic nanodomains form on superamphiphilic TiO2 surfaces [12]. In addition, self-cleaning and anti-fogging characteristics are reported on TiO2 surfaces [11]. A growing number of researchers have paid attention to TiO2-derived materials with superwettability.
Generally, superwettability refers to superhydrophilic, superhydrophobic, superamphiphilic, and superamphiphobic surfaces; the mechanism of the superwettability property can be explained based on the surface structure of materials, surface molecules, and external influencing factors [13]. Surface wettability can be attributed to surface chemical composition and structure [14,15]. For TiO2, surface superwettability can be induced by photocatalysis, and with its excellent performance, TiO2 is increasingly being used in energy [16,17], textile [18,19], and catalysis [20], etc. Recently, important breakthroughs were made in the technology and basic research of TiO2, which included design of structures, modification on substrate, and multidirectional applications [21,22,23,24,25,26,27,28,29,30,31]. Some of the typical applications of TiO2 are shown in Figure 1. In this review, a number of recent works are summarized from different fields including ultra-fast spreading, controllable wettability, interfacial photocatalysis, and nanochannels.
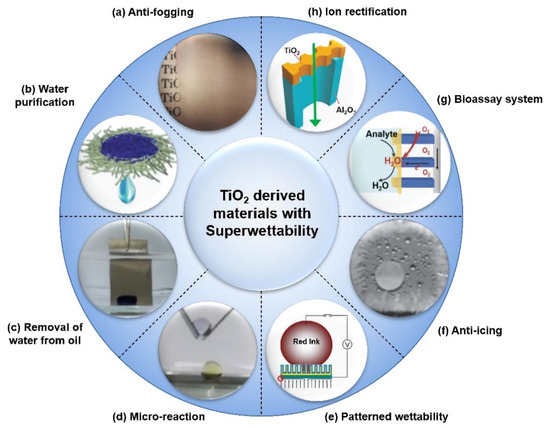
Figure 1. Typical applications of TiO2 based on photo-induced superwettability. (a) Glass substrate coated with nano-TiO2 shows anti-fogging properties. Reprint from [11]. Copyright (1997), with permission from Nature Publishing Group, Basingstoke, UK. (b) Water can be purified through a TiO2 nanomembrane by adsorption of organic pollutants. Reprint from [32]. Copyright (2013), with permission from The Royal Society of Chemistry, London, UK. (c) Removal of water from oil with UV-illumination on a superhydrophilic TiO2 nanotube array (TiO2-NTA) surface. Reprint from [33]. Copyright (2018), with permission from American Chemical Society, Washington, DC, USA. (d) Under-oil microreaction on the fabricated superhydrophilic TiO2-NTA surface. Reprint from [33]. Copyright (2018), with permission from American Chemical Society, Washington, DC, USA. (e) Patterned wettability used for printing on the TiO2 surface via the photoelectric cooperative effect. Reprint from [34]. Copyright (2009), with permission from WILEY-VCH, Weinheim, Germany. (f) Anti-icing property is shown on a 1H,1H,2H,2H-perfluorooctyltriethoxysilane-modified superhydrophobic TiO2-NTA surface. Reprint from [35]. Copyright (2014), with permission from WILEY-VCH, Weinheim, Germany. (g) The solid–liquid–air triphase bio-photoelectrode was fabricated by single-crystalline TiO2 nanowire arrays (NWs) for the bioassay system. Reprint from [36]. Copyright (2018), with permission from WILEY-VCH, Weinheim, Germany. (h) TiO2 nanochannels can be used to fabricate artificial ion channels. Reprint from [37]. Copyright (2014), with permission from WILEY-VCH, Weinheim, Germany.
2. Ultra-Fast Spreading with Superwettability
Ultra-fast spreading has long been a research topic, and TiO2-based materials are one of the widely used superspreading materials due to their unique photocatalysis properties [7,38,39]. Ultra-fast spreading is a wetting phenomenon indicating liquid permeates and diffuses into complex surface structures containing pores, taking advantage of the capillary effect [40]. Liquid shows ultra-fast spreading behaviors on TiO2-derived materials because of the aforementioned photo-induced superwettability and the structure of the surface, which can be regulated on demand [3,41,42,43].
The formation of nano-sized structures is helpful to the ultra-fast spreading behavior of droplets. Li et al. [44] reported nanostructured metal nitrides with the desirable property of superwettability. Compared to traditional TiN nanoparticle structures, the connected nanofibers and nanotubes structures have a larger surface-to-volume ratio, larger specific surface area, better mechanical stability, and independent transmission channels. A hierarchical amorphous TiO2 nanoarray reported by Li et al. [45] showed superamphiphilicity without UV-illumination. This is due to the preparation method called pulsed laser deposition, which is more cost-effective than the traditional process. Meanwhile, many TiO2 surfaces have special structures that show the property of superwettability. Zorba et al. [46] reported a self-similar porous TiO2 surface with superhydrophilic properties without UV-irradiation. The water CAs release to less than 5° quickly, in 160 ms. With the introduction of TiO2 onto the glass substrate, the coating showed obvious anti-fogging performance. Denison et al. [47] reported ultra-fast spreading behavior of water droplets on a transparent mesoporous superhydrophilic TiO2 film prepared by reverse micelle, sol–gel, and spin coating technology. The photo-induced stick-slip behavior on mesoporous TiO2 can be observed by investigating the spreading of water droplets on the semiconductor surface irradiated by ultraviolet light. By means of electrospinning, Ganesh et al. [48] reported that a rice-shaped TiO2 nanostructure showed superhydrophilicity. The superhydrophilicity of TiO2 coatings depends on their thickness. Electrospinning TiO2 shows better photocatalytic performance than commercial Degussa P25. Funakoshi et al. [49] prepared a superhydrophilic anatase TiO2 film by a titanium alkoxide hydrolysis operation. The water CAs and the transmittance of visible light to its substrate vary with the duration of tetraethyl orthotitanate (TEOT) hydrolysis operation. In the hydrolysis process of TEOT, the water CAs on the film shows about 35°, and when the process is longer than 60 min, the CAs drops below 1°. With the prolongation of the hydrolysis time of TEOT, the transmittance of light through its substrate is low.
In addition to the formation of nano-sized structures, fabrication of microstructures also influences surface superwettability. Kobayashi et al. [50] observed ultra-fast spreading on a TiO2 micropillar arrays with overhanging roofs. When the geometrical angle between the overhanging roof and the vertical micropillar shaft decreased from 51°to 9°, the wettability transition time when storing in dark and under UV illumination decreased from 180 min to 6 min.
In contrast to nanostructures and microstructures, the micro-nano composite structure can make the surface ultra-fast spreading more obvious. Joung et al. [51] fabricated a superhydrophilic micro-nano composite porous TiO2 structure by electrophoretic deposition and breakdown anodization. The thin film prepared by the electrophoretic deposition method shows a nanostructure and a basis for producing a superhydrophilic porous structure by the breakdown anodization method. On the basis of the superwettability property of TiO2, the spreading ability of TiO2 can be greatly enhanced by adjusting the structure, such as a to a mesh structure. Wang et al. [52] reported a superhydrophilic TiO2 fibrous mesh with an ultra-fast spreading property (Figure 2a). The mesh is made by tetrabutyl titanate and poly (vinyl pyrrolidone) composed of polycrystalline anatase TiO2, and it has three-dimensional micropores surrounded by nanofibers, and the nanochannel structure of a single fiber promotes the superhydrophilicity of water (Figure 2b). The water droplet spreads more quickly on the surface of a fiber mesh composed of the superhydrophilic fibers (Figure 2c). The capillary effect in three dimensions induces the wetting of water in the vertical direction (Figure 2d). The final wetting state shows that CAs is 0°. Similar to TiO2 fiber mesh, Wen et al. [32] reported superhydrophilic SiO2-TiO2 porous nanofibrous membranes through electrospinning and calcination. This material shows the characteristics of ultra-fast water wettability (Figure 2f) and the graded porous structure of the nanofiber membrane is beneficial for improving adsorption capacity and permeability. The membrane was useful for water purification (Figure 2g). Based on the micro-nano composite structure, the fabrication of the internal structure of the fiber depends on electrospray technology and helps to form a superhydrophilic surface. In 2008, Chen et al. [53] proposed a simple fluid electrospray technology to produce multi-component microcapsules. A novel multicompartment accomplished one-step multi-component encapsulation. This simple and effective composite fluid electrospray technique is crucial for separating and encapsulating active components. In 2010, Chen et al. [54] proposed a multifluidic coaxial electrospinning method for nanowire-in-microtube structured preparation. Compared with the multifluidic electrospinning method, the new one method adds an extra middle fluid between the polyacrylonitrile core and the polystyrene shell fibers. The advantage is the introduction of the additional intermediate fluid is that it can effectively reduce the interaction between the other two fluids. Further, in 2011, Chen et al. [55] reported a hierarchically porous inorganic nanofiber structure using microemulsion electrospinning. With the increase of precursor concentration, the diameter of nanopores increases. Meanwhile, the internal structure of the fiber can be controlled by changing the composition of micro-emulsion and the conditions of cations.
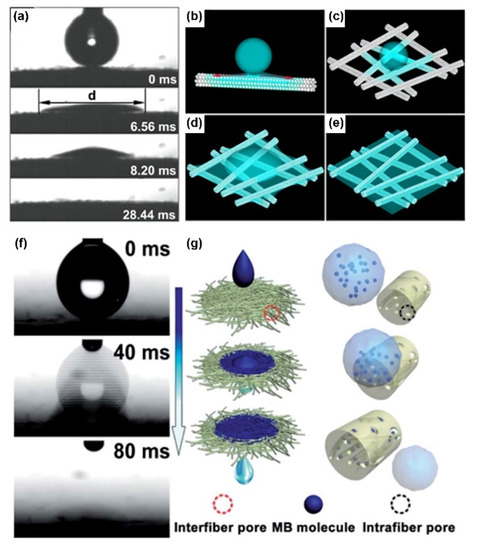
Figure 2. Ultra-fast spreading behaviors on the TiO2 surface for water purification. (a) A water droplet spreads on a TiO2 superhydrophilic mesh in 28.44 ms. (b)The one-dimensional nanochannels of a single nanofiber, which generate a capillary effect to pull water along with the precursor water film. (c) The capillary effect of several nanofibers promotes the diffusion of water on the top surface of the fiber mesh. (d) The capillary effect caused by 3D micropores further promotes the diffusion of water droplets after inducing the invasion of water in the vertical direction. (e) The final state of the mesh shows contact angles (CAs) around 0°. Reprint from [52]. Copyright (2009), with permission from Elsevier, Amsterdam, The Netherlands. (f) A water droplet spreads on a flexible SiO2-TiO2 composite porous nanomembrane in 80 ms. (g) The methylene blue solution spreads rapidly on the SiO2-TiO2 composite porous nanofibrous membrane and permeates through the interspace between nanofibers in the membrane. Meanwhile, methylene blue molecules are captured by intra-fiber pores, and then a water droplet is obtained. Reprint from [32]. Copyright (2013), with permission from The Royal Society of Chemistry, London, UK.
In addition to superspreading properties, controllable superwettability, which denotes the effective conversion between superhydrophilicity and superhydrophobicity, is widely studied and used in daily life because of its applications in smart devices such as printing, microfluidics, and water collection [56,57,58,59,60].
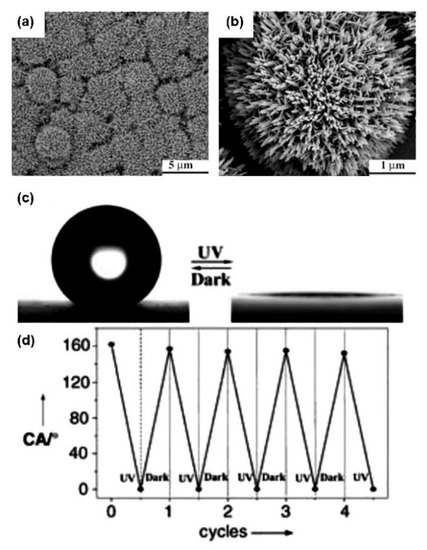
UV-induced superwettability was first reported by Fujishima et al. [11]. With UV-light illuminated on TiO2 film, the water CA is 0°. After storing the film in the dark, a high CA of about 72° of the film is recovered. After that, much related research was further carried out. Kang et al. [33] reported on the under-oil switchable wettability of TiO2 nanotube arrays (TiO2-NTAs). Comparison between TiO2-NTAs and TiO2-films showed that they have the same superhydrophilicity in air but different wettability under-oil. With UV irradiation, TiO2-NTAs reveal CAs of about 0° under oil. After heating at 150 °C in air, the superhydrophobicity appears again. Zeng et al. [61] reported a new heterostructure with the TiO2-NTAs composited with Ni(OH)2, which shows a photo-induced color change by oxidative energy storage. The oxidative energy is generated by TiO2 nanotubular arrays under UV light, and the oxidative energy can be reserved in the Ni(OH)2 layer. The color change phenomenon can be shown with reducing agents’ bleaching such as with alcohol, hydrogen peroxide, and ketone solutions. This storage-releasing oxidative energy-induced color change switch shows good reversibility. Gao et al. [62] reported a switchable superwettability membrane called a biomimetic TiO2-titanium mesh. A controllable underwater superwettability was shown by UV-illumination and heating. In terms of nanotube array structure, Chagas et al. [63] reported fabrication of TiO2 nanoparticle@trimethoxypropyl silane on a polypropylene substrate. The water CAs showed a change between superhydrophobic and superhydrophilic with UV light and heating. Additionally, UV light can increase the adhesion of the TiO2 nanoparticle@trimethoxypropyl silane coating. Caputo et al. [64] reported controllable wettability on a colloidal TiO2 nanocrystal film under UV irradiation. A low water CA was shown after UV illumination. Meanwhile, a vacuum environment can accelerate the change of wettability. Wang et al. [65] reported a nanotube-structured TiO2 film with switchable water adhesion. The hydrophilic region is generated by ultraviolet irradiation, and the alternating hydrophilic/superhydrophobic alignment is realized by using a mask. This phenomenon was applied to potential in-water drop manipulation. Feng et al. [66] prepared a TiO2 nanorod film with a special micro-nano composite structure on glass substrate, which shows superhydrophobic behavior. After UV irradiation, water CAs of the sample change from superhydrophobic to superhydrophilic. The TiO2 nanorod is shown in Figure 3a,b. As a photosensitive material, with UV illumination, the photogenerated hole combines with lattice oxygen to form surface oxygen vacancies, then, the film revealed water CAs of about 0°. After storage in the dark, finally, the surface wettability converted from superhydrophilic to superhydrophobic again (Figure 3c). Repeated reversible superhydrophilic-superhydrophobic transition is shown in Figure 3d.
In addition to ultraviolet irradiation, other external stimuli can also be applied to achieve controllable wettability of TiO2. Lian et al. [67] reported a controllable wettability on TiO2 rough surfaces with ethanol immersion and dark storage. The experiment phenomenon shows the changing CAs via the change of hydroxyl. A special nanostructure was reported by Lai et al. [68] who designed three controllable adhesion and superhydrophobic nanostructures, which include nanopores, nanotubes, and a nanovesuvianite array. The adhesion of water can be controlled by changing the diameter or length of nanotubes. This design guides the new functional nanomaterials with custom-tailored surface hydrophobicity and adhesion. Hu et al. [35] reported superhydrophobic TiO2-NTAs with controllable water adhesion. These TiO2-NTAs were modified by 1H,1H,2H,2H-perflfluorooctyl-triethoxysilane (PTES). The result shows that when a nanotube’s diameter or length increased, the adhesion of water was reduced. Following this feature, a water droplet can be transferred from having low adhesive superhydrophobic TiO2-NTAs to high adhesive superhydrophobic TiO2-NTAs, and then to a smooth Ti film. The high adhesive TiO2-NTAs were used as “mechanical hands”, and an anti-icing property was revealed on the low adhesion TiO2-NTA surface. Moreover, a switch of the electric field can control the dynamic wettability. Li et al. [69] reported a strategy for achieving controllable liquid manipulation and transportation on a micro-nano structured elastomer film via an electric field. Wettability is controlled according to the stretching/releasing of the elastomer TiO2 surface. After coating with carbon grease and applying an electric field, when the voltage was applied, the shortening of the distance of the micro-nano composite structure clusters could be triggered. Liu et al. [70] reported a TiO2/reduced graphene oxide composite aerogel that was used for selectively degrading pollutants in water and depended on its photocatalysis properties. TiO2 nanostructures have been grown on three-dimensional reduced graphene oxide porous structures and play an important role. Pan et al. [71] reported a TiO2 nanoparticle (NP) coating modified with 1H,1H,2H,2H-perfluorooctyl(trimethoxy)silane. The CAs could rapidly change from 165° to 0° in 10 min after heating. Meanwhile, the membrane can be used to separate oil and water.
Controllable wettability can be applied to oil-water separation. Shi et al. [72] fabricated a TiO2@copper wire mesh. The TiO2@copper wire mesh covered with a TiO2 micro-nano composite structure has a transforming wettability of less than 30 min in addition to its property of switchable wettability applied for oil-water separation. Yan et al. [73] designed a carbon nanotubes/TiO2 composite membrane, which was used to separate oil-water systems. The membrane exhibited high separation efficiency in oil-water separation. With the corrosive test and after several cycles, the material also showed a stable separation effect. Ren et al. [74] reported a superhydrophobic TiO2@CA/AS coating that was fabricated with chitosan (CS) and stearic acid (SA). The wettability can be controlled with ammonic and heat treatment and is used for separating oil and water.
Moreover, patterned printing based on controllable wettability has also become attractive. Tian et al. [34] reported a superhydrophobic nanorod array surface whose patterned wettability transition could be controlled by the photoelectric cooperative. Under the condition of only applying voltage, the wettability of the nanorod surface cannot be regulated. However, under a certain voltage, by controlling illumination, a controllable wettability interface with photoelectric cooperation can be formed (Figure 4a,b). With this property, a desired pattern ”H” is obtained (Figure 4c). Guo et al. [75] reported an approach for patterned liquid permeation with a TiO2-NTAs coated Ti mesh. The wettability of this coated material can be controlled by the photoelectric response. The mechanism can be explained as follows. At first, the surface of the superhydrophobic Ti mesh with TiO2-NTAs becomes hydrophilic by introducing appropriate voltage and light. However, if the voltage is too high or too low, the hydrophilic phenomenon of photoelectric synergy cannot be obtained. In the same year, Guo et al. [76] reported a ZnO mesh surface with the micro-nano composite structure for liquid patterned printing. By adding voltage and illumination, a great difference can be created between patterned and un-patterned areas due to the micro-nano composite structure of the ZnO mesh. Patterned liquid printing is realized in this way. With different mechanisms on patterned printing, Zheng et al. [57] reported a TiO2-SiO2 composite coating used for electrostatic patterning. Temporary conductivity of the insulation surface was realized by electrostatic powder spraying. The water droplets sprayed on the superhydrophilic surface can diffuse rapidly and spread into a water film, and the first pass transfer efficiency is promoted to more than 80%. After spraying the powder particles on the water film and heating it, an epoxy-polyester film on a porous composite coating was accomplished (Figure 4d,e). Surface patterning was completed by electrostatic attraction of negative charge of epoxy polyester powder and positive charge of water (Figure 4f,g). The micro-grade lines with different sizes are shown in Figure 4h.
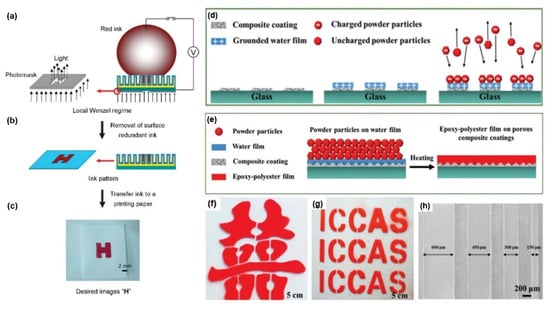
Figure 4. Liquid-patterned printing by TiO2 and its superwettability. (a) Red ink was printed on the composite TiO2 nanorod-array surface with illumination through the photomask. (b) After the illumination and voltage were turned off, an ink-patterned “H” was shown with the removal of the ink. (c) When the pattern is transferred onto the paper, the desired image “H” is obtained. Reprint from [34]. Copyright (2009), with permission from WILEY-VCH, Weinheim, Germany. (d) Process of the electrostatic attraction-induced patterning on the glass by spraying droplets on the glass patterned with a superhydrophilic nanoparticle (NP) coating. Electrostatic powder was adsorbed on the water film. (e) After heating for 20 min, a patterned film formed on the glass. (f) The pattern of the “Red Double Happiness”. (g) The pattern of “ICCAS”. (h) SEM image shows the patterned glass with different sizes of micro-grade lines. Reprint from [57]. Copyright (2016), with permission from WILEY-VCH, Weinheim, Germany.
This entry is adapted from the peer-reviewed paper 10.3390/catal11040425
