Formic acid is a liquid organic hydrogen carrier giving hydrogen on demand using catalysts. Metal complexes are known to be used as efficient catalysts for the hydrogen production from formic acid decomposition. Their performance could be better than those of supported catalysts with metal nanoparticles. However, difficulties to separate metal complexes from the reaction mixture limit their industrial applications. This problem can be resolved by supporting metal complexes on the surface of different supports, which may additionally provide some surface sites for the formic acid activation.
- formic acid decomposition
- hydrogen
- biomass
- metal complex
- heterogeneous catalyst
- ruthenium
- iridium
- iron
1. Introduction
Hydrogen is mainly used for ammonia synthesis and the petrochemical industry. Its traditional production involves non-renewable sources and processes giving a significant emission of carbon dioxide leading to global warming. Among these processes are steam reforming of natural gas and gasification of coal performed at very high temperatures (>900 K). Recently, the International Energy Agency reported that the hydrogen production reached 75 mln of tons and that it was accompanied by emission of 830 mln tons of CO2 [1]. Global demand for hydrogen increases from year to year accompanying by an increase of the carbon dioxide emissions.
Despite hydrogen is a clean energy carrier its safe transportation and storage are rather complicated. Liquid organic hydrogen carriers (LOHCs) are used for safe storage and transportation of hydrogen [2][3]. They can be produced from biomass or CO2 thus avoiding the effect of the evolved CO2 for global warming. Formic acid (HCOOH) is an example of such a LOHC. It contains 53.4 g L−1 hydrogen (4.4 wt %), which is by a factor of 2 higher than the content of compressed hydrogen at 350 bar at the same volume. This amount corresponds to the energy density of 2.1 kWh L−1. In contrast to hydrogen, formic acid can be easily transported and stored and its application is much safer. An important feature of using formic acid is that it can be produced by catalytic hydrolysis/oxidation of biomass with high yields at low temperatures (<423 K) [4][5][6]. Hydrogen can be released from formic acid using catalysts at even lower temperatures (Figure 1). Thus, transformation of biomass to hydrogen through formic acid could be considered as an efficient route, because direct gasification of biomass also giving hydrogen demands very high temperatures (>900 K) (Figure 1). Recently, Zhang et al. [7] and Park et al. [8] demonstrated the proof of concept for such an approach.
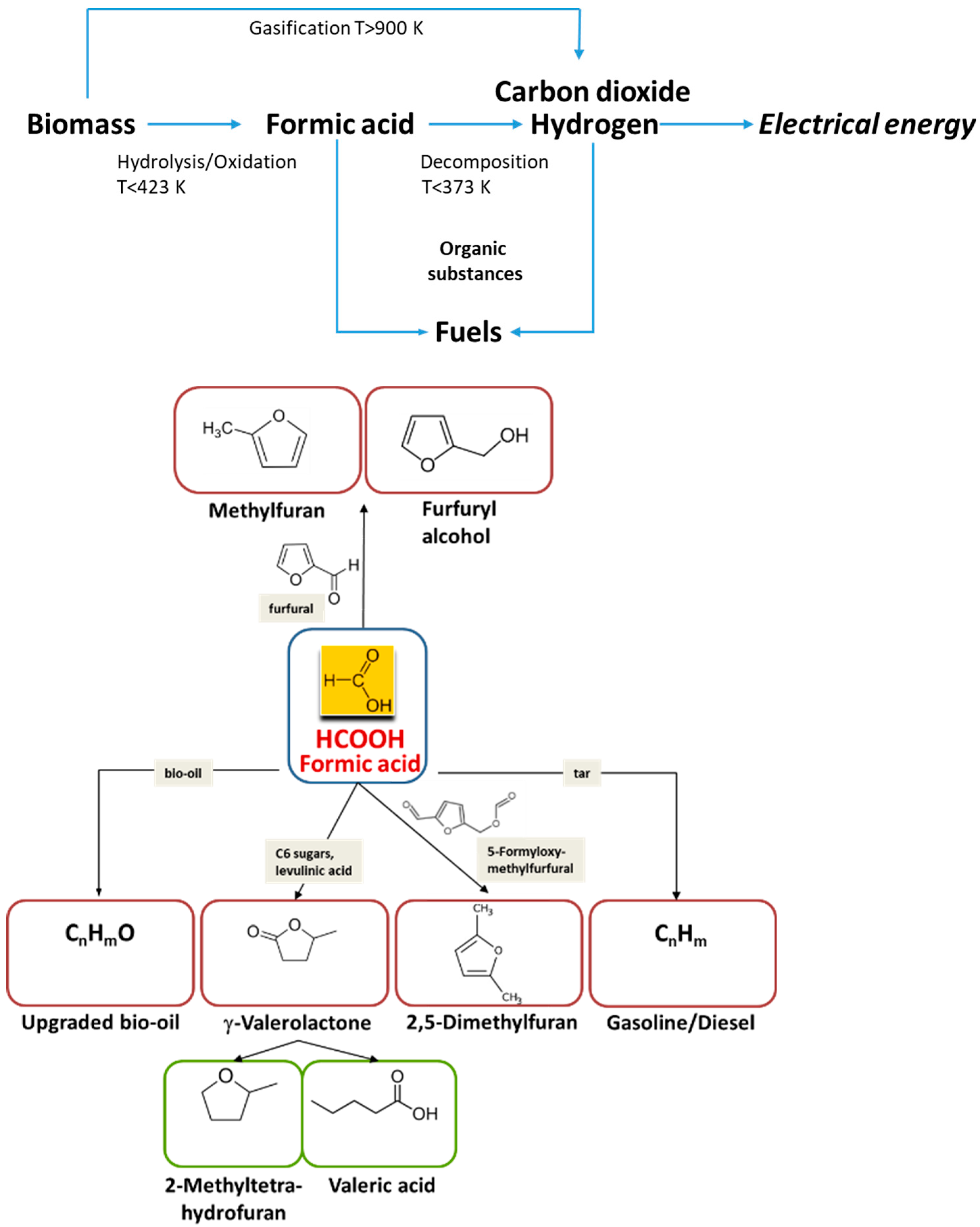
The hydrogen obtained from formic acid could be further transformed to electrical energy (Figure 1). The development of a compact integrated 25 kW system which converts formic acid to power has been discussed [9]. Formic acid could be used also as a donor of hydrogen instead of molecular hydrogen to hydrogenate different organic substances for production of fuels and intermediates for fuels [4][10]. Thus, it could be applied for synthesis of γ-valerolactone from C6 sugars and levulinic acid [11], 2,5-dimethylfuran from 5-formyloxymethylfurfural [12], furfuryl alcohol [13] and methylfuran [14] from furfural, upgraded bio-oil from bio-oil [15], and diesel/gasoline mixtures from tar [16] (Figure 1).
Supported catalysts with nanoparticles are traditional catalysts for the hydrogen production from formic acid in gas and liquid phase. Novel single atom metal catalysts supported on N-doped carbon may provide a higher activity in the formic acid decomposition than the activity of the catalysts with nanoparticles, but the difference is not so significant [17]. The activity of homogeneous metal complexes is often higher [18][19][20][21][22][23][24]. Hence, they could be used at lower temperatures. Metal complexes also represent more uniform active sites as compared to metal nanoparticles. Hence, basing on this knowledge the design of the catalyst could be facilitated. However, there are serious problems of application of homogeneous metal complexes as catalysts for different reactions limiting their industrial applications. They include difficulties in separation of a catalyst from the reaction medium and catalyst’s recovery, instability of homogeneous catalytic systems, as well as possible corrosive effects of catalyst solutions on the equipment [25].
Separation of the catalysts with noble metals could be important for production of hydrogen from reaction mixtures containing formic acid and obtained from biomass. To solve this problem, metal complexes could be supported on different supports. Serious efforts have been directed toward the immobilization of homogeneous catalysts on supports. Evidently, their catalytic properties could change due to a change of ligand environment, since after supporting the support surface sites become important ligands for metal atoms. These sites may have no direct analogs in solutions [25]. Their nature affects strongly the energy of interaction of metal complexes and resistance of the catalyst to leaching. Additionally, the support may provide surface sites for formic acid activation leading to its faster conversion.
Carbon dioxide is also produced as a by-product during the decomposition of formic acid; however, it can be further hydrogenated into formate salts at low temperatures [26][27]. Earlier, we have analyzed this reaction taking place on different catalysts, particularly on supported metal complexes [27]. In the present entry, we will consider in details the catalytic properties of supported Ru, Ir and Fe complexes in the hydrogen production from formic acid. This type of the catalysts shows excellent activity, selectivity and stability in the reaction and can be easily separated from the reaction mixture.
2. Progress in Catalytic Hydrogen Production from Formic Acid over Supported Metal Complexes
The summarized data for the key catalysts with supported Ru, Ir and Fe complexes for the hydrogen production from formic acid are shown in Table 1. Other metal complexes are almost not studied. The table can help to choose the optimal catalysts corresponding to certain conditions of the reaction. Basic additives to the reaction mixture or basic sites of the catalysts/supports are known to promote significantly the reaction. They deprotonate formic acid to formate species. Deprotonation of formic acid can be provided also by traditional oxide supports having basic sites [28] and by introduction of alkali metals promoters to supported metal catalysts [29][30][31]. Deprotonation provided by pyridinic N sites of N-doped carbon support was also reported for the catalysts with single metal atoms [32][33]. In this case, it reminds the effect of basic amine additives.
As for catalysts with nanoparticles, the steady-state turnover frequency (TOF) values obtained for the gas- phase reaction over a Pd/C catalyst doped with K ions with 3–4 nm Pd nanoparticles did not exceed 3600 h−1 at 353 K [29][30]. The TOF value corresponds to the number of hydrogen molecules obtained per one metal site per time unit. For the liquid-phase reaction and Pd nanoparticles (~1.4 nm) supported on N-doped carbon, the initial values were higher and reached 8414 h−1 at 333 K in the presence of sodium formate [22]. Some supported catalysts with nanoparticles (Ir/C [34] and Ru/C [35]) were used for comparison of the activity with supported metal complexes (Table 1). It was shown that their activity is negligible as compared with the activity of supported metal complexes.
It should be mentioned that some homogeneous complexes showed much higher TOFs than those of the supported metal complexes (Table 1). The values in the range 250,000–-322,000 h−1 for temperatures 363 and 373 K have been reported by a few groups of authors [21][23][24]. These complexes are also based on Ir [23][24] and Ru [21]. It would be useful to immobilize them on some supports in order to have an opportunity to separate easily the obtained catalyst from the reaction mixture.
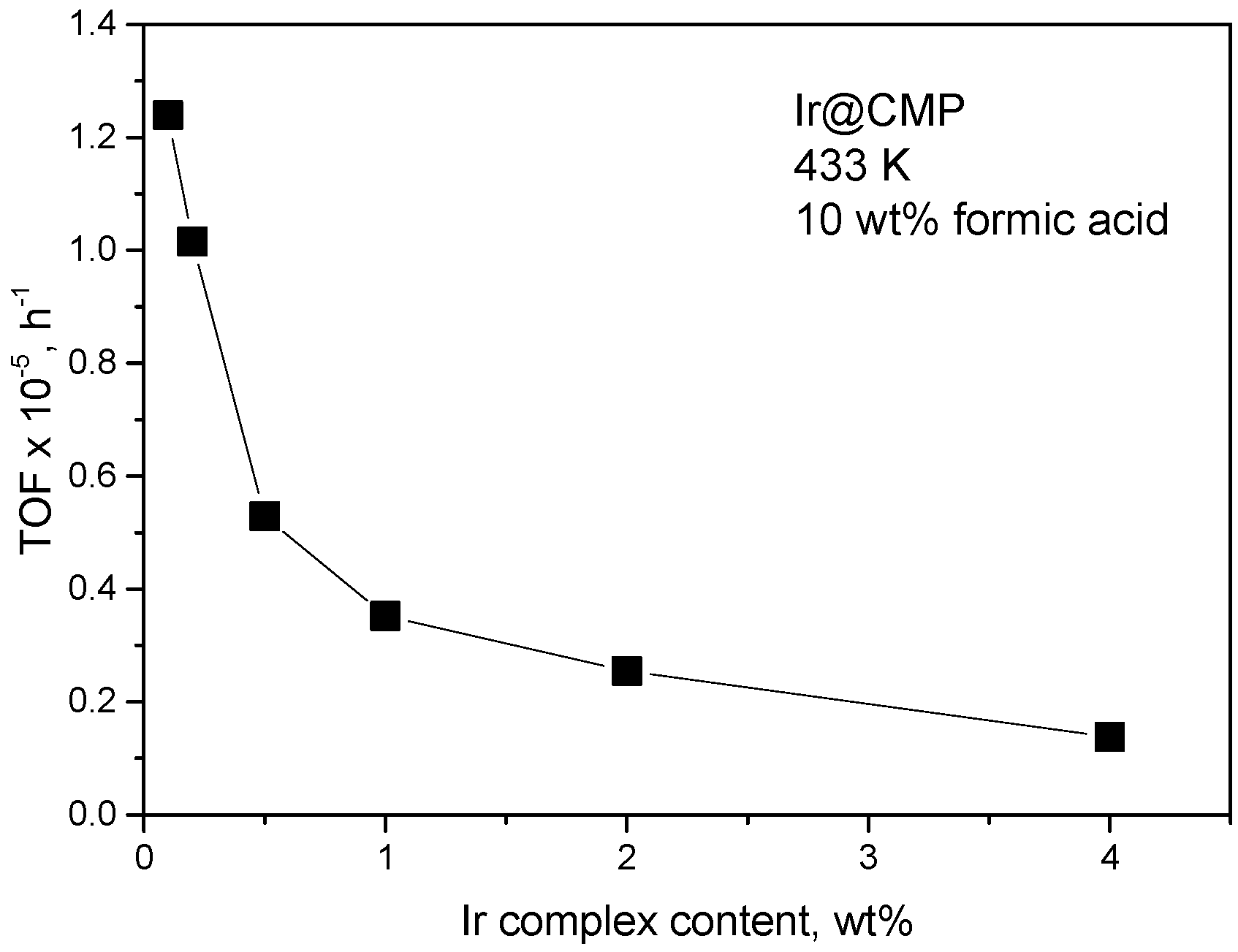
As it is shown in (Figure 2), concentration of a metal complex in the catalyst is an important factor determining TOFs. Interesting that at a lower concentration of a metal complex higher TOFs were observed. In this case, the most active sites could be stabilized by specific support sites. The progress in understanding of the nature of these sites may lead to development of a targeted synthesis of the catalysts with very active sites.
Table 1. Properties of the most active supported Ru, Ir and Fe complexes used for the hydrogen production from formic acid.
| Initial or Attached Complex | Catalyst Support | BET Surface Area of the Support, m2 g−1 | Active Metal Concentration, wt % | T, K | Concentration of Formic Acid and Sodium Formate | TOF, h−1 (Ea, kJ mol−1) |
Reference |
|---|---|---|---|---|---|---|---|
| Ru-mTPPTS | MCM41-Si(CH2)2PPh2 | - | 0.3 | 383 | 10 M (HCOOH+HCOONa, 9:1) | 2780 | [36] |
| RuII(η6-C6H6) | CTF500 | 1800 | 2.7 | 353 | 3 M | 4020 | [37] |
| RuCl2(p-cymene) | pDPPE | 33 | 1 | 433 | 2.2 M | 22,900 | [35] |
| RuCl2(p-cymene) | PPh2-MOF | 1075 to 161 (after reaction) | 0.7 | 418 | 5 vol% (gas phase reaction) | 2300 | [38] |
| IrIIICp* | CTF500 | 1800 | 0.2 | 353 | 3 M | 27,000 | [37] |
| [Cp*IrCl2]2 | bpy-CTF400 | 684 | 1.4 | 353 | 1 M | 2820 | [39] |
| [Cp*IrCl2]2 | bpy-CTF500 | 1566 | 11.3 | 363 | 1 M | 7930 | [39] |
| Cp*IrCl2 | polypyrrole | 51 | 4.3 | 333 | 1 M | 4060 | [40] |
| Cp*IrCl2 | polypyrrole | 51 | 4.3 | 363 | 2 M (HCOOH+HCOONa,1:1) | 46,000 (66) | [40] |
| [Cp*IrCl2]2 | CMP | 706 | 0.1 | 433 | 2.2 M | 123,894 (90) | [34] |
| Fe(BF4)2 | polyRPhphos@SiO2 | 502 | 0.8 | 363 | 7.6 M | 7600 (51) | [41] |
| Fe(BF4)2 | RPPh2@SiO2 | 531 | 0.9 | 363 | 7.6 M | 6396 (43) | [41] |
This entry is adapted from the peer-reviewed paper 10.3390/en14051334
References
- IEA. The Future of Hydrogen. Available online: (accessed on 26 February 2021).
- Preuster, P.; Papp, C.; Wasserscheid, P. Liquid Organic Hydrogen Carriers (LOHCs): Toward a Hydrogen-free Hydrogen Economy. Acc. Chem. Res. 2017, 50, 74–85.
- Rao, P.C.; Yoon, M. Potential Liquid-Organic Hydrogen Carrier (LOHC) Systems: A Review on Recent Progress. Energies 2020, 13, 6040.
- Bulushev, D.A.; Ross, J.R.H. Towards Sustainable Production of Formic Acid. ChemSusChem 2018, 11, 821–836.
- Gromov, N.V.; Medvedeva, T.B.; Rodikova, Y.A.; Babushkin, D.E.; Panchenko, V.N.; Timofeeva, M.N.; Zhizhina, E.G.; Taran, O.P.; Parmon, V.N. One-pot synthesis of formic acid via hydrolysis–oxidation of potato starch in the presence of cesium salts of heteropoly acid catalysts. RSC Adv. 2020, 10, 28856–28864.
- Preuster, P.; Albert, J. Biogenic Formic Acid as a Green Hydrogen Carrier. Energy Technol. 2018, 6, 501–509.
- Zhang, P.; Guo, Y.-J.; Chen, J.; Zhao, Y.-R.; Chang, J.; Junge, H.; Beller, M.; Li, Y. Streamlined hydrogen production from biomass. Nat. Catal. 2018, 1, 332–338.
- Park, J.-H.; Lee, D.-W.; Jin, M.-H.; Lee, Y.-J.; Song, G.-S.; Park, S.-J.; Jung, H.J.; Oh, K.K.; Choi, Y.-C. Biomass-formic acid-hydrogen conversion process with improved sustainability and formic acid yield: Combination of citric acid and mechanocatalytic depolymerization. Chem. Eng. J. 2020, 127827.
- Van Putten, R.; Wissink, T.; Swinkels, T.; Pidko, E.A. Fuelling the hydrogen economy: Scale-up of an integrated formic acid-to-power system. Int. J. Hydrogen Energy 2019, 44, 28533–28541.
- Nie, R.; Tao, Y.; Nie, Y.; Lu, T.; Wang, J.; Zhang, Y.; Lu, X.; Xu, C.C. Recent Advances in Catalytic Transfer Hydrogenation with Formic Acid over Heterogeneous Transition Metal Catalysts. ACS Catal. 2021, 11, 1071–1095.
- Heeres, H.; Handana, R.; Chunai, D.; Borromeus Rasrendra, C.; Girisuta, B.; Jan Heeres, H. Combined dehydration/(transfer)-hydrogenation of C6-sugars (D-glucose and D-fructose) to γ-valerolactone using ruthenium catalysts. Green Chem. 2009, 11, 1247–1255.
- Sun, Y.; Xiong, C.; Liu, Q.; Zhang, J.; Tang, X.; Zeng, X.; Liu, S.; Lin, L. Catalytic Transfer Hydrogenolysis/Hydrogenation of Biomass-Derived 5-Formyloxymethylfurfural to 2, 5-Dimethylfuran Over Ni–Cu Bimetallic Catalyst with Formic Acid As a Hydrogen Donor. Ind. Eng. Chem. Res. 2019, 58, 5414–5422.
- Nagaiah, P.; Gidyonu, P.; Ashokraju, M.; Rao, M.V.; Challa, P.; Burri, D.R.; Kamaraju, S.R.R. Magnesium Aluminate Supported Cu Catalyst for Selective Transfer Hydrogenation of Biomass Derived Furfural to Furfuryl Alcohol with Formic Acid as Hydrogen Donor. ChemistrySelect 2019, 4, 145–151.
- Fu, Z.; Wang, Z.; Lin, W.; Song, W.; Li, S. High efficient conversion of furfural to 2-methylfuran over Ni-Cu/Al2O3 catalyst with formic acid as a hydrogen donor. Appl. Catal. A Gen. 2017, 547, 248–255.
- Bulushev, D.A.; Ross, J.R.H. Catalysis for conversion of biomass to fuels via pyrolysis and gasification: A review. Catal. Today 2011, 171, 1–13.
- Chesnokov, V.V.; Dik, P.P.; Chichkan, A.S. Formic Acid as a Hydrogen Donor for Catalytic Transformations of Tar. Energies 2020, 13, 4515.
- Bulushev, D.A.; Bulusheva, L.G. Catalysts with single metal atoms for the hydrogen production from formic acid. Catal. Rev. 2021, 1–40.
- Li, Z.; Xu, Q. Metal-Nanoparticle-Catalyzed Hydrogen Generation from Formic Acid. Acc. Chem. Res. 2017, 50, 1449–1458.
- Stathi, P.; Solakidou, M.; Louloudi, M.; Deligiannakis, Y. From Homogeneous to Heterogenized Molecular Catalysts for H2 Production by Formic Acid Dehydrogenation: Mechanistic Aspects, Role of Additives, and Co-Catalysts. Energies 2020, 13, 733.
- Laurenczy, G.; Dyson, P.J. Homogeneous Catalytic Dehydrogenation of Formic Acid: Progress Towards a Hydrogen-Based Economy. J. Braz. Chem. Soc. 2014, 25, 2157–2163.
- Filonenko, G.A.; van Putten, R.; Schulpen, E.N.; Hensen, E.J.M.; Pidko, E.A. Highly Efficient Reversible Hydrogenation of Carbon Dioxide to Formates Using a Ruthenium PNP-Pincer Catalyst. ChemCatChem 2014, 6, 1526–1530.
- Onishi, N.; Iguchi, M.; Yang, X.; Kanega, R.; Kawanami, H.; Xu, Q.; Himeda, Y. Development of Effective Catalysts for Hydrogen Storage Technology Using Formic Acid. Adv. Energy Mater. 2019, 9, 1801275.
- Papp, G.; Ölveti, G.; Horváth, H.; Kathó, Á.; Joó, F. Highly efficient dehydrogenation of formic acid in aqueous solution catalysed by an easily available water-soluble iridium(iii) dihydride. Dalton Trans. 2016, 45, 14516–14519.
- Wang, W.-H.; Ertem, M.Z.; Xu, S.; Onishi, N.; Manaka, Y.; Suna, Y.; Kambayashi, H.; Muckerman, J.T.; Fujita, E.; Himeda, Y. Highly Robust Hydrogen Generation by Bioinspired Ir Complexes for Dehydrogenation of Formic Acid in Water: Experimental and Theoretical Mechanistic Investigations at Different pH. ACS Catal. 2015, 5, 5496–5504.
- Yermakov, Y.I.; Kuznetsov, B.N.; Zakharov, V.A. Chapter 1: Introduction to the Field of Catalysis by Supported Complexes. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1981; Volume 8, pp. 1–58.
- Álvarez, A.; Bansode, A.; Urakawa, A.; Bavykina, A.V.; Wezendonk, T.A.; Makkee, M.; Gascon, J.; Kapteijn, F. Challenges in the Greener Production of Formates/Formic Acid, Methanol, and DME by Heterogeneously Catalyzed CO2 Hydrogenation Processes. Chem. Rev. 2017, 117, 9804–9838.
- Bulushev, D.A.; Ross, J.R.H. Heterogeneous catalysts for hydrogenation of CO2 and bicarbonates to formic acid and formates. Catal. Rev. 2018, 60, 566–593.
- Zacharska, M.; Chuvilin, A.L.; Kriventsov, V.V.; Beloshapkin, S.; Estrada, M.; Simakov, A.; Bulushev, D.A. Support effect for nanosized Au catalysts in hydrogen production from formic acid decomposition. Catal. Sci. Technol. 2016, 6, 6853–6860.
- Jia, L.; Bulushev, D.A.; Beloshapkin, S.; Ross, J.R.H. Hydrogen production from formic acid vapour over a Pd/C catalyst promoted by potassium salts: Evidence for participation of buffer-like solution in the pores of the catalyst. Appl. Catal. B-Environ. 2014, 160, 35–43.
- Jia, L.; Bulushev, D.A.; Ross, J.R.H. Formic acid decomposition over palladium based catalysts doped by potassium carbonate. Catal. Today 2016, 259, 453–459.
- Bulushev, D.A.; Zacharska, M.; Guo, Y.; Beloshapkin, S.; Simakov, A. CO-free hydrogen production from decomposition of formic acid over Au/Al2O3 catalysts doped with potassium ions. Catal. Commun. 2017, 92, 86–89.
- Bing, Q.M.; Liu, W.; Yi, W.C.; Liu, J.Y. Ni anchored C2N monolayers as low-cost and efficient catalysts for hydrogen production from formic acid. J. Power Sources 2019, 413, 399–407.
- Bulushev, D.A.; Sobolev, V.I.; Pirutko, L.V.; Starostina, A.V.; Asanov, I.P.; Modin, E.; Chuvilin, A.L.; Gupta, N.; Okotrub, A.V.; Bulusheva, L.G. Hydrogen Production from Formic Acid over Au Catalysts Supported on Carbon: Comparison with Au Catalysts Supported on SiO2 and Al2O3. Catalysts 2019, 9, 376.
- Broicher, C.; Foit, S.R.; Rose, M.; Hausoul, P.J.C.; Palkovits, R. A Bipyridine-Based Conjugated Microporous Polymer for the Ir-Catalyzed Dehydrogenation of Formic Acid. ACS Catal. 2017, 7, 8413–8419.
- Hausoul, P.J.C.; Broicher, C.; Vegliante, R.; Göb, C.; Palkovits, R. Solid Molecular Phosphine Catalysts for Formic Acid Decomposition in the Biorefinery. Angew. Chem. Int. Ed. 2016, 55, 5597–5601.
- Gan, W.; Dyson, P.J.; Laurenczy, G. Hydrogen storage and delivery: Immobilization of a highly active homogeneous catalyst for the decomposition of formic acid to hydrogen and carbon dioxide. React. Kinet. Catal. Lett. 2009, 98, 205.
- Bavykina, A.V.; Goesten, M.G.; Kapteijn, F.; Makkee, M.; Gascon, J. Efficient production of hydrogen from formic acid using a Covalent Triazine Framework supported molecular catalyst. ChemSusChem 2015, 8, 809–812.
- Beloqui Redondo, A.; Morel, F.L.; Ranocchiari, M.; van Bokhoven, J.A. Functionalized Ruthenium–Phosphine Metal–Organic Framework for Continuous Vapor-Phase Dehydrogenation of Formic Acid. ACS Catal. 2015, 5, 7099–7103.
- Gunasekar, G.H.; Kim, H.; Yoon, S. Dehydrogenation of formic acid using molecular Rh and Ir catalysts immobilized on bipyridine-based covalent triazine frameworks. Sustain. Energy Fuels 2019, 3, 1042–1047.
- Shen, Y.; Zhan, Y.; Bai, C.; Ning, F.; Wang, H.; Wei, J.; Lv, G.; Zhou, X. Immobilized iridium complexes for hydrogen evolution from formic acid dehydrogenation. Sustain. Energy Fuels 2020, 4, 2519–2526.
- Stathi, P.; Deligiannakis, Y.; Avgouropoulos, G.; Louloudi, M. Efficient H2 production from formic acid by a supported iron catalyst on silica. Appl. Catal. A Gen. 2015, 498, 176–184.
