Static (SO) and dynamic (DO) osteogenesis are two very different types of osteogenesis, which are thus named because the former is characterized by pluristratified cords of unexpectedly stationary osteoblasts which differentiate at a fairly constant distance from the blood capillaries and transform into osteocytes without moving from the onset site, while the latter is distinguished by the well-known typical monostratified laminae of movable osteoblasts, which secrete bone moving towards the vessels.
- static osteogenesis
- dynamic osteogenesis
- osteoblasts
- osteocytes
- intramembranous ossification
1. Introduction
It is generally accepted that bone matrix deposition depends on the secretory activity of osteoblasts arranged in monostratified laminae, where all secreting cells are synchronized by side-to-side and gap junctions [1][2][3] and are polarized towards the same direction, i.e., the osteogenic surface. Moreover, according to the classical view, it is considered that as osteoid seam secretion proceeds, the osteoblastic laminae move away from the osteogenic surface towards the vessels, and those osteoblasts selected to differentiate into osteocytes remain entrapped within the preosseus matrix by the enlargement of their secretory territory (Figure 1) [4]. As a consequence, it has been clearly demonstrated that the rate of osteoblast movement is a function of the V/ST ratio (where V is the protoplasmic volume and ST is the secretory territory of the osteoblast). In other words, the osteoid appositional growth rate of each osteoblast depends on the proportion of its protoplasm engaged on a given bone surface [5]. In 1999, Marotti and coworkers [6] suggested the term “dynamic osteogenesis” to indicate this well-known type of bone formation (involving osteoblast movement) to distinguish it from another (until then) unknown type of bone deposition discovered for the first time by analyzing intramembranous ossification in detail. This new type of osteogenesis was named “static osteogenesis”, because it is performed by stationary osteoblasts that transform into osteocytes at the same site where they differentiate without moving. Later, exhaustive structural and ultrastructural documentation on the morpho-functional differences between these two types of bone formation was provided [7].
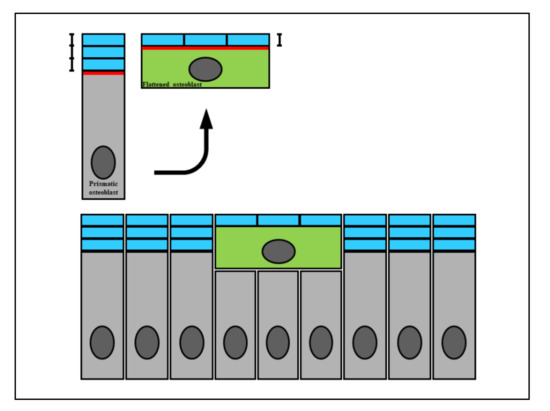
Figure 1. A diagram showing how the wideness of osteoblast secretory territory (red line) varies during its recruitment (green osteoblast), since the produced osteoid seam (blue bars) stratifies on a larger surface according to the morphological modification from a prismatic to a flattened shape. This fact explains the incorporation of recruited osteoblasts transforming into osteocytes (see text for explanation).
2. Structural and Functional Differences between SO and DO
Static and dynamic osteogenesis in various aspects in terms fo their structure, timing, inductive conditioning factors, and meaning. For clarity only we will firstly describe the characteristics of DO (which is classically well known) and then those of SO (which was only recently discovered), even if, when both occur, they take place in a time sequence (first SO and then DO).
In particular, with regard to morphology they present consistent differences in: (1) the mobility, arrangement, movement, and polarization of osteoblasts; (2) the morphology and distribution of the resulting osteocytes; and (3) the collagen texture of the bone matrix (Table 1).
Table 1. Main morpho-functional differences between SO and DO.
| STATIC OSTEOGENESIS | DYNAMIC OSTEOGENESIS | |
|---|---|---|
| at the beginning of the process of bone formation (fast) | TIME OF OCCURRENCE | soon after the primary thin SO-trabeculae (slow) |
| cytokines issued by vessels | CONDITIONING FACTORS | osteocyte signaling (due to mechanical stress) |
| cords | ARRANGEMENTS OF OSTEOBLAST | monostratified laminae |
| various directions | POLARIZATION OF OSTEOBLASTS | same direction (i.e. towards the pre-existent bone) |
| stationary osteoblasts | OSTEOBLAST MOTION | movable osteoblasts |
| haphazardly, often in confluent lacunae (high cellularity) | DISTRIBUTION OF OSTEOCYTES | orderly manner–the osteocytes are recruited in planes (lower cellularity) |
| mostly woven bone | TEXTURE | mostly lamellar bone |
| bad (due to the high cellularity and woven texture) | MECHANICAL PROPERTIES | good (osteocytes located in planes and lamellar texture) -more suitable to loading |
| to provide a preliminary rigid scaffold, on which DO can occur | AIMS | to produce bone tissue according the mechanical/metabolic needs |
In DO, bone deposition occurs by means of monostratified laminae in which prismatic osteoblasts are arranged in epithelial-like manner, all polarized in the same side and sharing the same osteogenic direction, i.e., towards the growing osteogenic surface. As is well known, each osteoblast displays an ill-defined euchromatic nucleus and an organellar machinery which is characteristically highly developed and located in polarized secretory cells. A distinctive feature of osteoblastic laminae during DO is their location in an “asymmetrical” environment, between the mineralized front (from which they move away as they secrete the preosseous matrix) and the vascular surface (towards which they migrate, thus maintaining a favorable metabolic situation) (Figure 2). With regard to the DO osteocyte morphology, there are dendritic elements displaying an almond-like shape in the cell body, in which cytoplasmic processes occur in an asynchronous and asymmetrical manner (first short “mineral-facing” dendrites, then long/slender “vascular-facing” ones) [8] in concomitance with, and depending on, the recruitment in the plane of the “committed” osteoblasts transforming into osteocytes. Such recruitment, in turn, influences the collagen texture, giving rise mostly to lamellar bone. During DO, gap and adherent junctions are observed between movable osteoblasts inside the lamina, in addition to gap junctions or simple contacts between osteoblasts and DO osteocyte dendrites as well as between osteocytes in cytoplasmic processes [3].
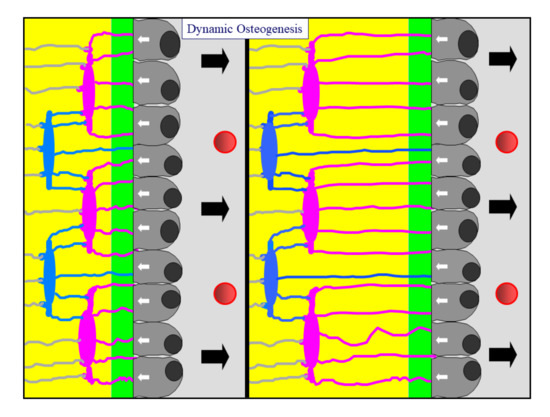
Figure 2. A diagram showing an osteoblastic lamina during DO. From left to right, the osteoblastic lamina moves away (as indicated by black arrows) from the mineralized front (green bar) to the vessels (red circles). The white arrows indicate the osteoblast polarization side. Two generations of almond-like shaped osteocytes (located in planes) are enclosed inside the bone (see text for further details).
In SO, bone deposition occurs by means of unmovable (i.e., stationary) osteoblasts irregularly arranged in cords that differentiate among the vessels (see below); inside the cords, formed by 2–3 layers of cells, osteoblasts are polarized in different directions with respect to the adjacent ones, and give rise to osteocytes at the same site where they previously differentiated (Figure 3). A further distinctive feature of these osteoblasts is their presence in a “symmetrical” environment: this differentiates them from those arranged in the typical laminae during DO, which are located in an “asymmetrical” domain (see above). With regard to morphology, SO osteocytes retain approximately the same globous shape and ultrastructure of the parental stationary osteoblasts; moreover, they are often located inside confluent lacunae in the core of the thin bony trabeculae (about 10–15 µm thick), formed only by 2–3 irregular rows/groups of osteocytes. Their dendrites are also shorter than those of DO osteocytes and radiate simultaneously (not in an asynchronous manner as in DO) all around the cell body (Figure 4). At first, the dendrites are so short as to look like spines; afterward, some of them elongate, but only marginally, because their parental osteoblasts are practically stationary, withdrawing from one another by just a few micra. The resulting osteocyte arrangement, which is haphazard and clustered, is dependent on the peculiar recruitment in groups and, in turn, influences the collagen texture, resulting in woven bone (Figure 5). Because the secretory territories of SO stationary osteoblasts are randomly oriented, the matrix is highly porous, and the orientation of the collagen fibers, while uniform at the cellular level [9], is random at larger scales. Gap junctions or simple contacts are observed between stationary osteoblasts and between the spines and dendrites of SO osteocytes [10].
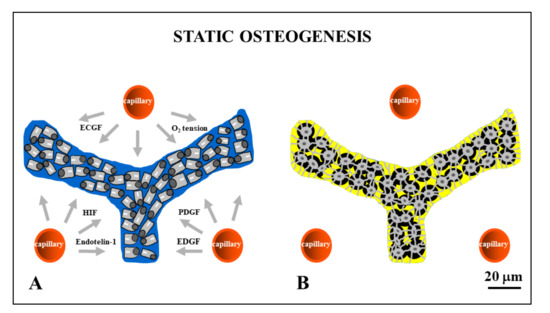
Figure 3. Drawings showing stationary osteoblasts, irregularly arranged in cords, that differentiate among the vessels (red circles). Inside the cords, osteoblasts are polarized in different directions with respect to the adjacent ones (A) and give rise to osteocytes (B) at the same site where they previously differentiated. ECGF: endothelial cell growth factor; HIF: hypoxia-inducible factor; PDGF: platelet-derived growth factor; EDGF: endothelial-derived growth factor.
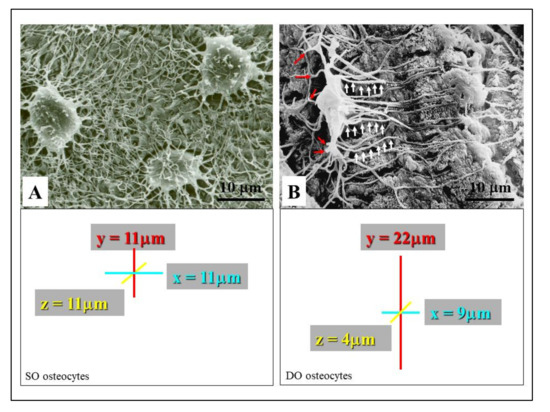
Figure 4. SEM micrographs showing the morphology of SO/DO osteocytes. (A) SO osteocytes have a globous shape, and their short dendrites radiate all around the cell body. (B) DO osteocytes display an almond-like shaped cell body and two different types of cytoplasmic processes: the short “mineral-facing” dendrites (red arrows) and the long/slender “vascular-facing” dendrites (white arrows). The square areas show the mean values of osteocyte cell body axes; the almost spherical morphology of SO osteocytes with respect to the triaxial ellipsoidal shape of the DO osteocytes can be seen.
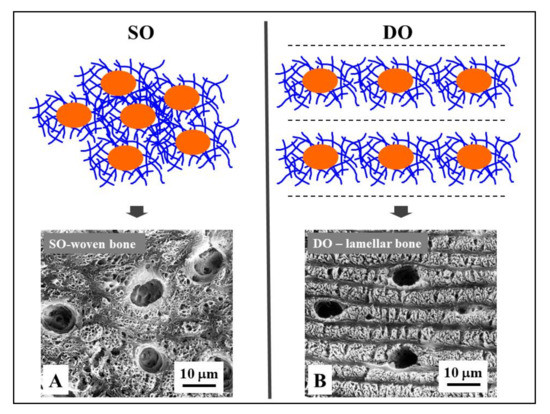
Figure 5. Osteocyte arrangements in static and dynamic osteogenesis (top): Osteocytes (orange) are always found within a pericellular loose collagen texture (blue) in both SO (osteocytes arranged in group) and DO (osteocytes recruited on a plane). Depending on the cell arrangement, woven bone texture forms in SO, while lamellar bone forms in DO. (bottom) SEM micrographs (A, B) of osteocyte lacunae in SO (right) and DO (left).
In addition to the prior differences, SO and DO have different conditioning factors. An intriguing problem is the differentiation of stationary osteoblasts at a certain distance from the vessels; SO allows the formation of a preliminary trabecular bony framework enclosing the blood vessels. This is essential for the subsequent bone apposition by DO. In a previous paper of ours [11], when comparing intramembranous and endochondral ossifications, the following crucial differences between SO and DO clearly emerged. During intramembranous ossification center development, bone growth occurs by progressive extension of stationary osteoblastic cords inside the surrounding mesenchyme, fairly constantly at about midway between blood capillaries (mean distance of cords from vessels, 28 ± 0.4 μm). Recently, in a study on regeneration with bone substitutes, the intimate functional connection between blood vessels and bone formation highlighted the combined interaction between the mesenchymal stem cell secretome (extracellular vesicles) and microRNAs [12]. However, this fact does not explain the mechanism by which mesenchymal cells (transforming into preosteoblasts) “sense” the position of the vessels. Several factors may be involved, such as O2 tension [13][14], endothelial cell-derived cytokines (i.e., endothelin-1) [15][16][17], and growth factors (EDGF, ECGF) [18][19][20][21]. In particular, vascular endothelial growth factor (VEGF), a main player in angiogenesis, is capable of provoking the migration and proliferation of endothelial cells and indirectly stimulating osteogenesis through the regulation of the osteogenic growth factors released through paracrine signaling [12]. Moreover, in studies on bone healing, it was shown that osteoblast differentiation could also depend on platelet-derived growth factor (PDGF) [22][23][24]. In a study concerning genetic manipulations in mice, Schipani and coworkers [25] recently highlighted the critical role of the hypoxia-inducible-factor/vascular endothelial growth factor (HIF) pathway in coupling angiogenesis and osteogenesis.
Briefly, SO is triggered by inductive stimuli. Since during SO the bone matrix is laid down without preexistent osteocytes, we believe that the irregular arrangement and polarization of the osteoblasts in stationary osteogenic cords probably depends on a lack of osteocyte guidance. Only after SO, does DO occurs by means of movable osteoblastic laminae. These DO osteoblasts are likely guided by SO osteocytes (inside the preliminary SO bony trabeculae) and are also capable of “sensing” the vessels (towards which they move) through the stromal cells located between the migrating laminae of the bone-growing surfaces and the vascular endothelia [26].
To summarize, SO is in fact a form of neo-osteogenesis, in the sense that it occurs in mesenchymal tissue (for example during intramembranous ossification of the cranial vault) in which pre-existing bone is lacking and therefore there are no osteocytes that can act as mechanosensors to guide the subsequent osteogenesis. In tissues where SO occurs, the absence of mechano-sensors makes mechanical stresses completely irrelevant since they cannot be perceived. It follows that the SO can only be induced by cytokines released by the endothelial cells; it is therefore to be considered a typical process of osseoinduction which is mechanically independent.
Conversely, DO occurs on the surfaces of a pre-existing bone containing osteocytes (Figure 6), which perceive the deformations induced in the bone matrix by mechanical loading. Therefore, in DO mechanical factors can play a pivotal role in triggering and directing the activity of osteoblasts (Figure 7). It is thus to be considered a process of osseoconduction which is strongly influenced by mechanical stress [27].
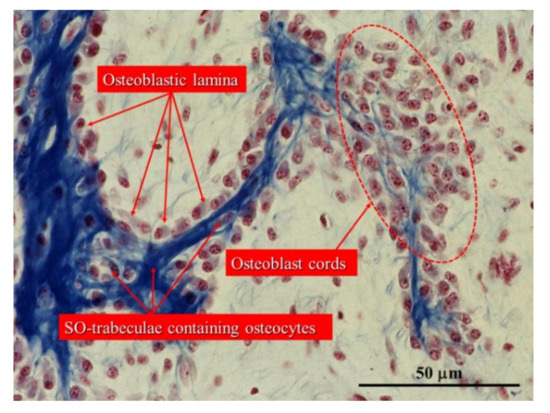
Figure 6. Histological section of an intramembranous ossification center showing a cord of stationary osteoblasts (red circles) and osteoblastic laminae on the surfaces of pre-existing bone containing SO osteocytes. (Light microscope photo from new-born rabbit calvaria).
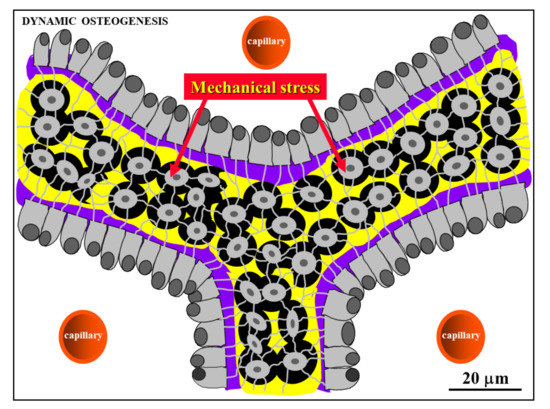
Figure 7. Schematic drawing showing laminae of movable osteoblasts laying down bone matrix (purple) on the surfaces of SO trabeculae (see text for further explanation).
Besides the described differences, SO and DO also occur with diverse speeds: in SO, bone matrix is generally produced very rapidly, while in DO the events proceed over a longer period of time. Thus, the former allows for the formation of a preliminary network of trabecular woven bone (surrounding wide primitive vascular spaces), which has a supporting function for subsequent lamellar bone apposition by typical movable osteoblasts (Figure 8); this in turn allows SO trabeculae thickening by DO and consequently the narrowing of the primitive vascular haversian spaces, giving rise to primary osteons (i.e., bone compaction) (Figure 9).
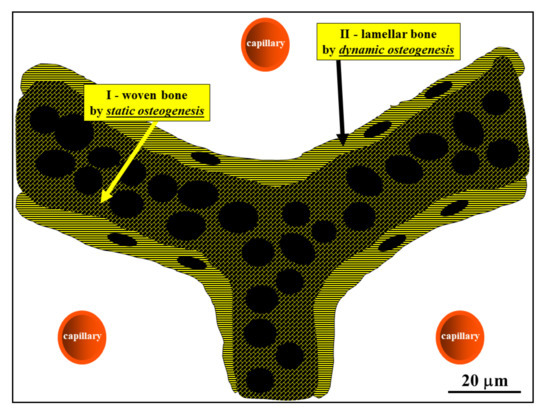
Figure 8. Schematic drawing showing the woven bone (by SO) forming the core of preliminary trabeculae for successive lamellar bone apposition by typical movable osteoblasts (in DO). (See SEM micrographs in Figure 5).
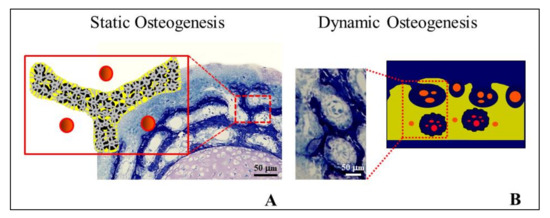
Figure 9. Cross-sections at the mid shaft level of the cartilaginous bud of a chick embryo showing: (A) SO trabeculae surrounding primary haversian spaces (the schematic drawing depicted in the insert represents the field of the square area in the histological section); (B) primitive vascular haversian spaces narrowed by means of DO (i.e., bone compaction), thickening SO trabeculae and giving rise to primary osteons (the histological section shows the field in the square in the schematic drawing).
In this regard, it should be underlined that SO and DO also differ in the involvement of the apoptosis occurring adjacent to the sites of osteogenesis: this is more frequently observed during DO than during SO, because in this context apoptosis is mainly dedicated to making space for advancing bone osteogenesis. This is in line with the progression modes of the two processes, because DO is an invasive process involving the invasion of mesenchyme, filling the primary haversian spaces, while SO proceeds following a non-invasive pattern [28].
Osteocytes derived by both static and dynamic osteogenesis remain in contact with each other by gap junctions, forming a functional syncytium (i.e., a network of strain-sensitive cells throughout the developing bone tissue) which also stays in contact with the osteoblasts covering the bone growth surfaces [7][10] (Figure 10). It is of note that the stationary osteoblasts allow for the “introduction” of the strain-sensitive osteocytes capable of sensing and responding to mechanical signals from the inception of bone formation.
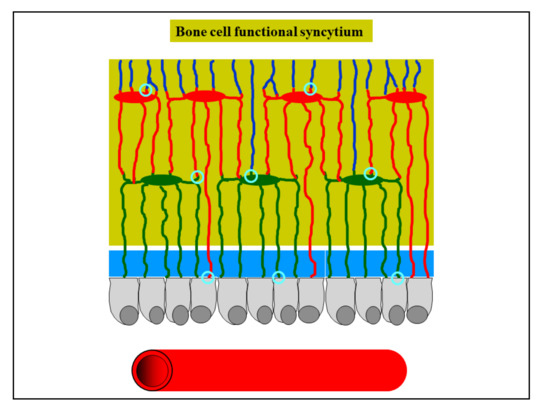
Figure 10. Schematic drawing showing osteocytes remaining in contact with each other by gap junctions (light blue circles), forming a functional syncytium which also stays in contact with the osteoblasts covering the bone growth surfaces (see text for further explanation).
In contrast to intramembranous ossification, SO never takes place during endochondral ossification [11]. In this case, DO occurs directly close to the remnants of calcifying cartilage; in fact, the remnants of calcifying cartilage have the same supporting function of the preliminary trabeculae in SO forming at the onset of intramembranous ossification (Figure 11).
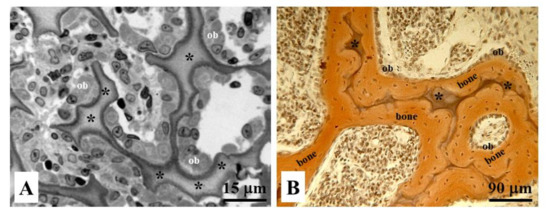
Figure 11. Histological sections of endochondral ossification in (A) osteoblastic laminae (ob) differentiated close to the remnants of calcified cartilage (asterisks), and in (B) bone deposition occurring through movable osteoblastic laminae forming and then compacting the primary haversian systems by dynamic osteogenesis.
3. Experimental Evidence for SO and DO Occurrence
The occurrence of SO and DO explains varied evidence both in experimental conditions and in pathological situations.
In one of our experimental studies [35] concerning the timing and manner of bone healing in rat femurs (after drilling transcortical holes, with/without contemporary administration of parathyroid hormone, PTH (1-34)), the interpretation of the results clearly underlined the correlation with the sequential occurrence of the two types of osteogenesis. The final goal was to identify the phase of osteogenesis (static versus dynamic) in which PTH (1-34) can most significantly exert its eventual effect, in order to determine how and when to use the drug with maximum efficacy in improving bone lesion repair strategies. Histomorphometric analysis revealed that 10 days after drilling, despite the holes being temporarily filled by the same amount of newly formed trabecular bone by static osteogenesis independent of the treatment, the extent of the surface of movable osteoblast laminae (covering the SO-trabecular surface) was statistically greater in animals which underwent PTH (1-34) administration as compared to controls. This datum strongly suggests the effect of PTH (1-34) in anticipating the occurrence of dynamic osteogenesis involved in the production of good-quality bone (i.e., lamellar bone, with a more ordered collagen texture, instead of woven-fibered bone) more suitable for mechanical loading. With regard to the translational aspect of basic research, the authors wanted to stress the importance of the two types of osteogenesis in the clinical research to define the best therapeutic approaches/strategies for drug use in orthopedics for recovery from severe skeletal lesions.
The two types of osteogenesis were found to be crucial in data interpretation in two of our (successive) investigations [36,37] for determining whether PTH (1-34) administration during normal diet restoration (after a calcium-free diet) can influence the amounts and sites of bone mass recovery. Besides the importance of the interplay between mineral homeostasis and skeletal homeostasis in modulating and guiding bone response to dietary/metabolic alterations, evidence emerged that the most-involved bony architecture is trabecular bone, as it is the most susceptible to the dynamical balance between the two types of homeostasis. Trabecular bone has the ability to respond more readily to mineral homeostasis. This ability of trabecular bone led to: (1) higher bone loss of less-loaded bony trabeculae as a consequence of a calcium-free diet in response to mineral homeostasis, and (2) new bone deposition on the surface of the few remaining overloaded trabeculae by means of dynamic (instead of static) osteogenesis to respond to skeletal homeostasis during normal diet restoration. Once again, the kind of osteogenesis recruited in bone healing is not irrelevant.
On the contrary, in some pathologies like human facet joint osteoarthritis (FJOA), remodeling of the subchondral trabecular bone compartment is characterized by increased trabecular number, rather than trabecular thickening [38]; this observation can be explained by the impairment of viability of osteocytes (i.e., the bone mechanosensors) inside the trabecular bone due to osteoarthritis, where static (as compared to dynamic) osteogenesis underpins specific bone remodeling and is activated through the recruitment of osteoprogenitor cells by endothelial-derived growth factors, giving rise to the formation of new trabeculae. The same authors also commented that a histological analysis of osteocyte lacunae in healthy and osteoarthritic specimens could provide further support for the role of static osteogenesis in the pathogenesis of FJOA.
Another pathology where static and dynamic osteogenesis explains some evidence is osteogenesis imperfecta (OI), as described by Shapiro et al. in 2020 [39]. The terms “static” and “dynamic” osteogenesis correspond to the definitions previously reported by Shapiro [40], i.e., with regard to the differences in osteoblast location/function in “mesenchymal osteoblasts” (MOBLs) that produce woven bone and “surface osteoblasts” (SOBLs) that produce lamellar bone. After the production of woven bone by MOBLs, SOBLs continued to secrete lamellar bone close to the woven scaffold. Shapiro and coworkers [39] observed in OI that “the more severe the variant of OI is, the greater the persistence of woven bone and the more immature the structural pattern; the pattern shifts to a structurally stronger lamellar arrangement once a threshold accumulation for an adequate scaffold of woven bone has been reached”. The authors correlated SO and DO with the subsequent temporal phases of woven (first) and lamellar (later) bone deposition by MOBLs and SOBLs, respectively, in the production of normal/healed/pathological bone. Lamellar bone organization increases as the disease resolves. In OI, the hypercellularity of bone depends on the relatively reduced matrix synthesis; however, the woven and lamellar bone segments are hypercellular to differing extents both in relation to each other and to normal bone. This is mainly due to woven bone rather than lamellar bone (which contains fewer cells as compared to normal woven bone).
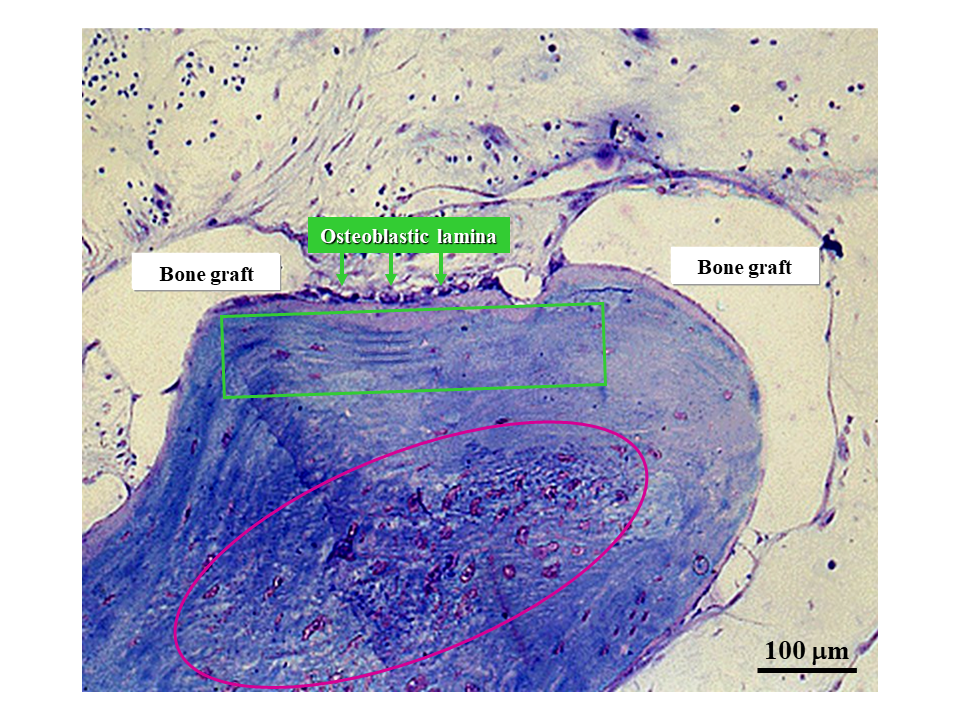
During bone healing induced by dental bone implants and in biomaterial osseointegration [33], SO and DO are crucial for evaluating the fate of implanted materials (Figure 12). In fact, the same sequence of events occurs as during intramembranous ossification in skeletal organogenesis, with firstly SO (depending on inductive stimuli) and then DO (which is mechanically driven). As a consequence of the different levels of quality of the derived bone tissue texture (poor in SO and mechanically valid in DO), the clinical implications of these findings are related to the time of load application after prosthesis/biomaterial implantation. This fact is empirically applied in the clinical practice of both orthopedic and maxillo-facial surgeons. For this reason, it is crucial to determine the timing of the transition between SO and DO in post-surgical and implant behavior in order to establish when poor-quality bone can be loaded after reinforcement/replacement with bone actually capable of resisting mechanical loading has taken place.
Figure 12. Micrograph under LM in ordinary light showing graft osseointegration. The purple circle outlines the core of a SO trabecula made up of woven bone containing many globous osteocytes. The green square outlines the lamellar bone subsequently laid down by DO.
The aspects concerning inductive/mechanical stimuli and bone texture quality also acquire particular importance during healing of cranial linear osteotomies with different devices; this has been experimentally induced in rabbits to test the validity of traditional procedures versus piezosurgery [41,42]: the observations with regard to bone gap healing are in line with our previous observations [7] on the steps occurring in a sequence during bone histogenesis.
4. Conclusions and Future Perspectives
In conclusion, the morpho-functional aspects of SO and DO, which have been well-investigated during both skeletal organogenesis and bone regeneration, could acquire particular relevance in clinical practice as far as their translational significance is concerned.
As previously reported, in bone healing the first stages of bone deposition produce poor-quality bone due to inductive stimuli so that loading appears to be useless or sometimes even dangerous during static osteogenesis; on the contrary, mechanical loading, which is known to greatly enhance osteoblast activity, becomes very important after the onset of dynamic osteogenesis. Therefore, it could become crucial in clinical practice to determine for how long SO continues before DO starts, primarily to establish when poor-quality bone is reinforced with (or replaced by) bone which is actually capable of resisting mechanical loading (Figure 13). At the moment, functional investigations are in progress to better elucidate the transition time and manner from SO to DO.
Figure 13. Schematic drawing with regard to the translational meaning of SO versus DO (see text for explanation).
A key aspect to be clarified in the near future is the duration time, in different conditions, of SO before the initiation of DO, for instance during critical-size bone defect healing, biomaterial/prosthetic osseo-integration, and bone loss recovery after severe metabolic pathologies.
References
- Jeansonne, B.G.; Feafin, F.F.; McMinn, R.W.; Scoemaker, R.L.; Rehm, V.S. Cell-to-cell comunication of osteoblasts. Dent. Res. 1979, 58, 1415–1423, doi:10.1177/00220345790580042101.
- Doty, S.B. Morphological evidence of gap junctions between bone cells. Calcif Tissue Int 1981, 33(1), 509-512. doi:10.1007/bf02409482.
- Palumbo, C.; Palazzini, S.; Marotti, G. Morphological study of intercellular junctions during osteocyte differentiation. Bone 1990, 11, 401–406, doi:10.1016/8756-328290134-k.
- Marotti, G.; Ferretti, M.; Muglia, M.A.; Palumbo, C.; Palazzini, S. A quantitative evaluation of osteoblast-osteocyte relationships on growing endosteal surface of rabbit tibiae. Bone 1992, 13, 363–368, doi:10.1016/8756-328290452-3.
- Marotti, G. Decrement in volume of osteoblasts during osteon formation and its effects on the size of the corresponding osteocytes. In Bone Histomorphometry; Meunier, P.J., Ed.; Armour Montagu: Paris, French, 1976; pp 385–
- Marotti, G.; Ferretti, M.; Palumbo, C.; Benincasa, M. Static and dynamic bone formation and the mechanism of collagen fiber orientation. Bone 1999, 25, 156.
- Ferretti, M.; Palumbo, C.; Contri, M.; Marotti, G. Static and dynamic osteogenesis: Two different types of bone formation. Embryol. 2002, 206, 21–29, doi:10.1007/s00429-002-0265-6.
- Palumbo, C.; Palazzini, S.; Zaffe, D.; Marotti, G. Osteocyte differentiation in the tibia of newborn rabbit: An ultrastructural study of the formation of cytoplasmic processes. Acta Anat. (Basel) 1990, 137, 350–358, doi:10.1159/000146907.
- Kerschnitzki, M.; Wagermaier, W.; Roschger, P.; Seto, J.; Shahar, R.; Duda, G.N.; Mundlos, S.; Fratzl, P. The organization of the osteocyte network mirrors the extracellular matrix orientation in bone. Struct. Biol. 2011, 173, 303–311, doi: 10.1016/j.jsb.2010.11.014.
- Palumbo, C.; Ferretti, M.; Marotti, G. Osteocyte dendrogenesis in static and dynamic bone formation: An ultrastructural study. Rec. A 2004, 278, 474–480, doi:10.1002/ar.a.20032.
- Ferretti, M.; Palumbo, C.; Bertoni, L.; Cavani, F.; Marotti, G. Does static precede dynamic osteogenesis in endochondral ossification as occurs in intramembranous ossification? Rec. A 2006, 288A, 1158-1162, doi:10.1002/ar.a.20386.
- Diomede, F.; Marconi, G.D.; Fonticoli, L.; Pizzicanella, J.; Merciaro, I.; Bramanti, P.; Mazzon, E.; Trubiani, O. Functional Relationship between Osteogenesis and Angiogenesis in Tissue Regeneration. J. Mol. Sci. 2020, 21, 3242, doi:10.3390/ijms21093242.
- Brighton, C.T.; Schaffer, J.L.; Shapiro, D.B.; Tang, J.J.; Clark, C.C. Proliferation and macromolecular synthesis by rat calvarial bone cells grown in various oxygen tensions. Orthop. Res. 1991, 9, 847–854, doi:10.1002/jor.1100090610.
- Lennon, D.P.; Edmison, J.M.; Caplan, A.I. Cultivation of rat marrow-derived mesenchimal stem cells in reduced oxygen tension: Effects on in vitro and in vivo osteochondrogenesis. Cell Physiol. 2001, 187, 345–355, doi:10.1002/jcp.1081.
- Sasaki, T.; Hong, M.H. Endothelin-1 localization in bone cells and vascular endothelial cells in bone marrow. Anat Rec 1993, 237, 332–337, doi:10.1002/ar.1092370306.
- Kasperk, C.H.; Borcsok, I.; Schairer, H.U.; Schneider, U.; Nawroth, P.P.; Niethard, F.U.; Ziegler, R. Endothelin-1 is a potent regulator of human bone cell metabolism in vitro. Tissue Int. 1997, 60, 368–374, doi:10.1007/s002239900245.
- Inoue, A.; Kamiya, A.; Ishiji, A.; Hiruma, Y.; Hirose, S.; Hagiwara, H. Vasoactive peptide-regulated gene expression during osteoblastic differentiation. Cardiovasc. Pharmacol. 2000, 36(5 Suppl. 1), S286–S289, doi:10.1097/00005344-200036051-00084.
- Guenther, H.L.; Fleisch, H.; Sorgente, N. Endothelial cells in culture synthesize a potent bone cell active mitogen. Endocrinology 1986, 119, 193–201, doi:1210/endo-119-1-193.
- Canalis, E.; McCarthy, T.; Centrella, M. The regulation of bone formation by local growth factors. In Bone and Mineral Research; Peck, W.A., Ed.; Elsevier: Amsterdam, The Netherlands, 1989, 6, pp 27–
- Streeten, A.; Brandi, M.L. Biology of the bone endothelial cells. Bone Miner. 1990, 10, 85–94, doi:10.1016/0169-600990084-s.
- Villanueva, J.E.; Nimni, M.E. Promotion of calvarial cell osteogenesis by endothelial cells. Bone Min. Res. 1990, 5, 733–739.
- Zheng, M.H., Wood, D.J.; Papadimitriou, J.M. What’s new in the role of cytokines on osteoblast proliferation and differentiation? Res. Pract. 1992, 188, 1104–1121, doi:10.1016/S0344-033881263-X.
- Lind, M. Growth factor stimulation of bone healing. Effects on osteoblasts, osteomies, andimpiants fixation. Acta Orthop. Scand. 1998, 283, 2–37.
- Chaudhary, L.R.; Hofmeister, A.M.; Hruska, K.A. Differential growth factor control of bone formation through osteoprogenitor differentiation. Bone 2004, 34, 402–411, doi: 10.1016/j.bone.2003.11.014.
- Schipani,E.; Maes, C.; Carmeliet, G.; Semenza, G.L. Perspective Regulation of osteogenesis-angiogenesis coupling by HIFs and VEGF. Bone Min. Res. 2009, 24, 1347–1353, doi:10.1359/JBMR.090602.
- Palazzini, S.; Palumbo, C.; Ferretti, M.; Marotti, G. Stromal cell structure and relationships in perimedullary spaces of chick embryo shaft bones. Anat Embryol. 1998, 197, 349–357, doi:10.1007/s004290050145.
- Marotti, G. Static and Dynamic osteogenesis in the processes on bone repair. I.O.T. 2004, 30 (Suppl. 1), 1–5.
- Palumbo, C.; Ferretti, M.; De Pol, A. Apoptosis during intramembranous ossification. Anat. 2003, 203, 589–598, doi:10.1046/j.1469-7580.2003.00247.x.
- Traini, T. The development of the alveolar bone by static osteogenesis: Microanatomy and clinical implications. Oral Surg. 2013, 4, 29–37.
- Stein, K.; Prondvai, E. Rethinking the nature of fibrolamellar bone: An integrative biological revision of sauropod plexiform bone formation. Rev. 2014, 89, 24–47, doi:10.1111/brv.12041.
- Prondvai, E.; Stein, K.H.W.; De Ricqlès, A.; Cubo, J. Development-based revision of bone tissue classification: The importance of semantics for science. J. Linn. Soc. Lond. 2014, 112, 799–816.
- Cubo; J., Hui, M., Clarac, F., Quilhac, A. Static osteogenesis does not precede dynamic osteogenesis in periosteal ossification of Pleurodeles (Caudata, Amphibia) and Pogona (Squamata, Lepidosauria). Morphol. 2017, 278, 621–628, doi:10.1002/jmor.20659.
- Marotti, G.; Zaffe, D.; Ferretti, M.; Palumbo, C. Static osteogenesis and dynamic osteogenesis: Their relevance in dental bone implants and biomaterial osseointegration. JOB 2010, 1, 133–139.
- Cubo, J.; Legendre, P.; de Ricqles, A.; Montes, L.; de Margerie, E.; Castanet, J.; Desdevises, Y. Phylogenetic, functional, and structural components of variation in bone growth rate of amniotes. Dev. 2008, 10, 217–227.
- Cavani, F.; Ferretti, M.; Smargiassi, A.; Palumbo, C. PTH(1-34) effects on repairing experimentally drilled holes in rat femur: Novel aspects—qualitative vs. quantitative improvement of osteogenesis. Anat. 2017, 230, 75–84, doi:10.1111/joa.12533.
- Ferretti, M.; Cavani, F.; Smargiassi, A.; Roli, L.; Palumbo, C. Mineral and Skeletal Homeostasis Influence the Manner of Bone Loss in Metabolic Osteoporosis due to Calcium-Deprived Diet in Different Sites of Rat Vertebra and Femur. Res. Int. 2015, 304178, doi:10.1155/2015/304178.
- Ferretti, M.; Cavani, F.; Roli, L.; Checchi, M.; Magarò, M.S.; Bertacchini, J.; Palumbo, C. Interplay among calcium diet content, PTH(1-34) treatment and balance of bone homeostases in rat model: The trabecular bone as keystone. J. Mol. Sci. 2019, 20, 753, doi:10.3390/ijms20030753.
- Netzer, C.; Distel, P.; Wolfram, U.; Deyhle, H.; Jost, G.F.; Schären, S.; Geurts, J. Comparative Analysis of Bone Structural Parameters Reveals Subchondral Cortical Plate Resorption and Increased Trabecular Bone Remodeling in Human Facet Joint Osteoarthritis. J. Mol. Sci. 2018, 19, 845, doi:10.3390/ijms19030845.
- Shapiro, F.; Maguire, K.; Swami, S.; Zhu, H.; Flynn, E.; Wang, J.; Wu, J.Y. Histopathology of osteogenesis imperfecta bone. Supramolecular assessment of cells and matrices in the context of woven and lamellar bone formation using light, polarization and ultrastructural microscopy. Bone Rep. 2020. PII: S2352-187230494-0, doi: 10.1016/j.bonr.2020.100734.
- Shapiro, F. Bone development and its relation to fracture repair. The role of mesenchymal osteoblasts and surface osteoblasts. Cells Mater 2008, 15, 53–76, doi:10.22203/ecm.v015a05.
- Anesi, A.; Ferretti, M.; Cavani, F.; Salvatori, R.; Bianchi, M.; Russo, A.; Chiarini, L.; Palumbo, C. Structural and ultrastructural analyses of bone regeneration in rabbit cranial osteotomy: Piezosurgery versus traditional osteotomes. Cranio-Maxillofac. Surg. 2018, 46, 107–118, doi: 10.1016/j.jcms.2017.10.004.
- Anesi, A.; Di Bartolomeo, M.; Pellacani, A.; Ferretti, M.; Cavani, F.; Salvatori, R.; Nocini, R.; Palumbo, C.; Chiarini, L. Bone Healing Evaluation Following Different Osteotomic Techniques in Animal Models: A Suitable Method for Clinical Insights. Sci. 2020, 10, 7165, doi:10.3390/app10207165.
- Suda, T.; Takahashi, N.; Udagawa, N.; Jimi, E.; Gillespie, M.T.; Martin, T.J. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Rev. 1999, 20, 345–357, doi:10.1210/edrv.20.3.0367.
- Palumbo, C.; Ferretti, M.; Ardizzoni, A.; Zaffe, D.; Marotti, G. Osteocyte-osteoclast morphological relationships and the putative role of osteocytes in bone remodeling. Musculoskel. Neuron. Interact. 2001, 1, 327–332.
- Toscani, D.; Bolzoni, M.; Ferretti, M.; Palumbo, C.; Giuliani, N. Role of osteocytes in myeloma bone disease: Anti-sclerostin antibody as new therapeutic strategy. Immunol. 2018, 9, 2467, doi:10.3389/fimmu.2018.02467.
- Delaisse, J.M. The reversal phase of the bone-remodeling cycle: Cellular prerequisites for coupling resorption and formation. Bonekey Rep. 2014, 3, 561, doi:10.1038/bonekey.2014.56.
- Everts, V.; Delaissé, J.M.; Korper, W.; Jansen, D.C.; Tigchelaar-Gutter, W.; Saftig, P.; Beertsen, W. The bone lining cell: Its role in cleaning Howship’s lacunae and initiating bone formation. Bone Miner. Res. 2002, 17, 77–90, doi:10.1359/jbmr.2002.17.1.77.
- Raggatt, L.J.; Partridge, N.C. Cellular and molecular mechanisms of bone remodeling. Biol. Chem. 2010, 285, 25103–25108, doi:10.1074/jbc.R109.041087.
- Zhao, C.; Irie, N.; Takada, Y.; Shimoda, K.; Miyamoto, T.; Nishiwaki. ; Suda,T.; Matsuo, K. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 2006, 4, 111–121, doi: 10.1016/j.cmet.2006.05.012.
- Sims, A.; Martin, T.J. Osteoclasts provide coupling signals to osteoblast lineage cells through multiple mechanisms. Annu. Rev. Physiol. 2020, 82, 507–529, doi:10.1146/annurev-physiol-021119-034425.
- Roodman, G.D. Pathogenesis of myeloma bone disease. Leukemia 2009, 23, 435–441, doi:10.1038/leu.2008.336.
- Yaccoby, S. Advances in the understanding of myeloma bone disease and tumour growth. J. Haematol. 2010, 149, 311–321, doi:10.1111/j.1365-2141.2010.08141.x.
- Giuliani, N.; Colla, S.; Morandi, F.; Lazzaretti, M.; Sala, R.; Bonomini, S.; Grano, M.; Colucci, S.; Svaldi, M.; Rizzoli, V. Myeloma cells block RUNX2/CBFA1 activity in human bone marrow osteoblast progenitors and inhibit osteoblast formation and differentiation. Blood 2005, 106, 2472–2483, doi:10.1182/blood-2004-12-4986.
This entry is adapted from the peer-reviewed paper 10.3390/app11052025
References
- Jeansonne, B.G.; Feafin, F.F.; McMinn, R.W.; Scoemaker, R.L.; Rehm, V.S. Cell-to-cell comunication of osteoblasts. J. Dent. Res. 1979, 58, 1415–1423.
- Doty, S.B. Morphological evidence of gap junctions between bone cells. Calcif. Tissue Int. 1981, 33, 509–512.
- Palumbo, C.; Palazzini, S.; Marotti, G. Morphological study of intercellular junctions during osteocyte differentiation. Bone 1990, 11, 401–406.
- Marotti, G.; Ferretti, M.; Muglia, M.A.; Palumbo, C.; Palazzini, S. A quantitative evaluation of osteoblast-osteocyte relationships on growing endosteal surface of rabbit tibiae. Bone 1992, 13, 363–368.
- Marotti, G. Decrement in volume of osteoblasts during osteon formation and its effects on the size of the corresponding osteocytes. In Bone Histomorphometry; Meunier, P.J., Ed.; Armour Montagu: Paris, France, 1976; pp. 385–397.
- Marotti, G.; Ferretti, M.; Palumbo, C.; Benincasa, M. Static and dynamic bone formation and the mechanism of collagen fiber orientation. Bone 1999, 25, 156.
- Ferretti, M.; Palumbo, C.; Contri, M.; Marotti, G. Static and dynamic osteogenesis: Two different types of bone formation. Anat. Embryol. 2002, 206, 21–29.
- Palumbo, C.; Palazzini, S.; Zaffe, D.; Marotti, G. Osteocyte differentiation in the tibia of newborn rabbit: An ultrastructural study of the formation of cytoplasmic processes. Acta Anat. 1990, 137, 350–358.
- Kerschnitzki, M.; Wagermaier, W.; Roschger, P.; Seto, J.; Shahar, R.; Duda, G.N.; Mundlos, S.; Fratzl, P. The organization of the osteocyte network mirrors the extracellular matrix orientation in bone. J. Struct. Biol. 2011, 173, 303–311.
- Palumbo, C.; Ferretti, M.; Marotti, G. Osteocyte dendrogenesis in static and dynamic bone formation: An ultrastructural study. Anat. Rec. A 2004, 278, 474–480.
- Ferretti, M.; Palumbo, C.; Bertoni, L.; Cavani, F.; Marotti, G. Does static precede dynamic osteogenesis in endochondral ossification as occurs in intramembranous ossification? Anat. Rec. A 2006, 288A, 1158–1162.
- Diomede, F.; Marconi, G.D.; Fonticoli, L.; Pizzicanella, J.; Merciaro, I.; Bramanti, P.; Mazzon, E.; Trubiani, O. Functional Relationship between Osteogenesis and Angiogenesis in Tissue Regeneration. Int. J. Mol. Sci. 2020, 21, 3242.
- Brighton, C.T.; Schaffer, J.L.; Shapiro, D.B.; Tang, J.J.; Clark, C.C. Proliferation and macromolecular synthesis by rat calvarial bone cells grown in various oxygen tensions. J. Orthop. Res. 1991, 9, 847–854.
- Lennon, D.P.; Edmison, J.M.; Caplan, A.I. Cultivation of rat marrow-derived mesenchimal stem cells in reduced oxygen tension: Effects on in vitro and in vivo osteochondrogenesis. J. Cell Physiol. 2001, 187, 345–355.
- Sasaki, T.; Hong, M.H. Endothelin-1 localization in bone cells and vascular endothelial cells in bone marrow. Anat. Rec. 1993, 237, 332–337.
- Kasperk, C.H.; Borcsok, I.; Schairer, H.U.; Schneider, U.; Nawroth, P.P.; Niethard, F.U.; Ziegler, R. Endothelin-1 is a potent regulator of human bone cell metabolism in vitro. Calcif. Tissue Int. 1997, 60, 368–374.
- Inoue, A.; Kamiya, A.; Ishiji, A.; Hiruma, Y.; Hirose, S.; Hagiwara, H. Vasoactive peptide-regulated gene expression during osteoblastic differentiation. J. Cardiovasc. Pharmacol. 2000, 36 5 Suppl. 1, S286–S289.
- Guenther, H.L.; Fleisch, H.; Sorgente, N. Endothelial cells in culture synthesize a potent bone cell active mitogen. Endocrinology 1986, 119, 193–201.
- Canalis, E.; McCarthy, T.; Centrella, M. The regulation of bone formation by local growth factors. In Bone and Mineral Research; Peck, W.A., Ed.; Elsevier: Amsterdam, The Netherlands, 1989; Volume 6, pp. 27–56.
- Streeten, E.A.; Brandi, M.L. Biology of the bone endothelial cells. Bone Miner. 1990, 10, 85–94.
- Villanueva, J.E.; Nimni, M.E. Promotion of calvarial cell osteogenesis by endothelial cells. J. Bone Min. Res. 1990, 5, 733–739.
- Zheng, M.H.; Wood, D.J.; Papadimitriou, J.M. What’s new in the role of cytokines on osteoblast proliferation and differentiation? Phatol. Res. Pract. 1992, 188, 1104–1121.
- Lind, M. Growth factor stimulation of bone healing. Effects on osteoblasts, osteomies, andimpiants fixation. Acta Orthop. Scand. 1998, 283, 2–37.
- Chaudhary, L.R.; Hofmeister, A.M.; Hruska, K.A. Differential growth factor control of bone formation through osteoprogenitor differentiation. Bone 2004, 34, 402–411.
- Schipani, E.; Maes, C.; Carmeliet, G.; Semenza, G.L. Perspective Regulation of osteogenesis-angiogenesis coupling by HIFs and VEGF. J. Bone Min. Res. 2009, 24, 1347–1353.
- Palazzini, S.; Palumbo, C.; Ferretti, M.; Marotti, G. Stromal cell structure and relationships in perimedullary spaces of chick embryo shaft bones. Anat. Embryol. 1998, 197, 349–357.
- Marotti, G. Static and Dynamic osteogenesis in the processes on bone repair. G.I.O.T. 2004, 30 (Suppl. 1), 1–5.
- Palumbo, C.; Ferretti, M.; De Pol, A. Apoptosis during intramembranous ossification. J. Anat. 2003, 203, 589–598.