Nanoporous Anodic Alumina (NAA) is formed by the electrochemical anodization of pure aluminum wafers and consists of a parallel array of pores surrounded by hexagonal cells of aluminum oxide (alumina).
- nanoporous anodic alumina
- nanoengineering
- nanostructures
- surface chemistry
1. Nanoporous Anodic Alumina (NAA): Definition and Formation Mechanism of the Porous Oxide
NAA is formed by the electrochemical anodization of pure aluminum wafers and consists of a parallel array of pores surrounded by hexagonal cells of aluminum oxide (alumina). Each cell is in a direct contact with six others forming a structure that resembles a honeycomb. The pores grow in depth perpendicularly to the metallic surface as the anodization advances. A standard NAA structure can be defined with three physical parameters: The pore diameter (dp), the interpore distance (dint), and the length of the pores (lp). These geometrical features of NAA are shown in Figure 1 [1].
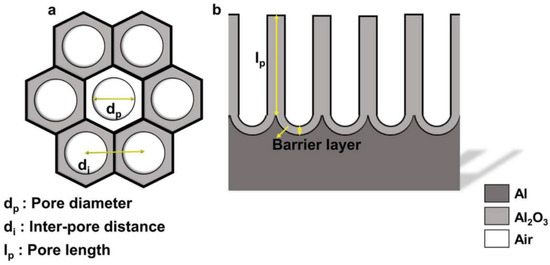
Figure 1. Schematic representation of nanoporous anodic alumina (NAA) structure. (a) Top-view with defined geometrical parameters; (b) cross-section with chemical composition. Reprinted with permission from [1]. Copyright 2017 Wiley.
The pore diameter can range from 8 to 500 nm for NAA structures and depends on the anodization process conditions such as the applied potential, the electrolyte, and its temperature [2][3][4][5].
The interpore distance dint indicates the average distance between the centers of two consecutive pores. This is an important value to determine the porosity of the structure since higher interpore distance translates to a smaller density of pores per surface unit. Higher concentration of the electrolyte and temperature seems to reduce the resulting interpore distance, while the applied potential was found to be in a positive correlation [6]. NAA presents high pore densities between 108–1011 cm−2 and pore length ranging from a few nanometers up to millimeter scale [7].
The length of the pore lp is proportional to the total current charge. During a standard anodization carried out under potentiostatic conditions, a brief initial rise and fall of the current precedes the stable plateau that enables to estimate thickness based on duration of the anodization [8][9]. Although the geometry of the pores remains constant during all the anodization process when the anodization parameters are kept invariant, it can be observed that pores tend to be wider close to the surface. It can be attributed to the longer exposure time, which causes slow oxide dissolution by the electrolyte. This may yield a certain degree of discrepancies as initial geometric features are determined by the process conditions. The dissolution rate of NAA in various acids is compared in the work of Poznyak et al. [10]. It was demonstrated to depend on the nature of the acid—the reactivity with respect to aluminum and the morphology of aluminum surface.
The influence of conditions that enable the precise tailoring of NAA’s geometrical features such as the electrolyte, its temperature and additives, the anodization potential and current, and post-processing treatments are described in detail in the sections below.
By chemical definition, NAA is an amorphous aluminum oxide with built up water and remains of the electrolyte ions incorporated during the anodization. The distribution of the impurities resembles layered onion-like structure with the highest concentration of ionic residues at the inner walls of the pores with a gradual decrease to the outside part of the pores [11][12][13]. This intake is an intrinsic factor partially responsible for the variations in the thermal conductivity of NAA reported in the literature. Recently, an attempt to systematize the observed effect was reported by Vera-Londono et al. [14]. It was established that thermal conductivity depends on the presence of ionic impurities—thus the electrolyte of choice—along with water built in the structure, and the crystalline form. As temperature increases to 100 °C, a drop in the thermal conductivity (~50%) is observed along with water loss. Later, however, continuous increase can be observed. Both removal of ionic impurities and transition into crystalline form result in higher thermal conductivity: 0.78 ± 0.19 W m−1 K−1 for the sulfuric NAA heated to 100 °C and reaching 4.16 ± 0.35 W m−1 K−1 when the sample was annealed at 1300 °C.
NAA is an attractive and interesting material that presents important features. One of the most prominent features of NAA is its optical responsiveness that can be tailored to act as a photonic crystal [13][15]. It exhibits a high degree of transmittance in the visible light region [16][17]. Additionally, NAA has photoluminescent properties [18]. The origin of the photoluminescent properties of NAA was examined by Cantelli et al. [19]. Recombination centers derived from oxygen defects were proposed as the origin of the emission. The outlined hypothesis pointed onto different current densities present during the anodization in different electrolytes as the contributing factor to the quantity of oxygen vacancies inside the NAA.
The material features also dielectric properties. The dielectric constant and loss are inversely proportional to the porosity of NAA and the applied alternating current frequency. The behavior of NAA is similar to other ceramic materials [20].
NAA is attractive not only due to its impressive enormous surface area combined with high chemical and thermal resistance, but due to the range of robust functionalization processes such as salinization [21], electrostatic interaction [22], and immune complexation [23] that allow versatile utility of such substrates: Sensors [24][25], templates [26], or drug delivery systems [27].
1.1. An Electrolytic Passivation of Aluminum
As mentioned before, NAA is obtained by the electrochemical etching of aluminum, however the electrochemical etching of aluminum does not always result in a porous structure of alumina: Different morphology of the grown oxide can be observed depending on the chemical character of the electrolyte. Electrolyte acidity is considered the major contribution factor to the distinct growth behavior. Anodization in a neutral electrolyte (borate, oxalate, citrate etc.; pH 5–7), that does not react with aluminum oxide yields a barrier-type anodic alumina as shown in Figure 2a [28]. However, an anodization performed in an acidic electrolyte (in which the oxide structure is slowly dissolved) results in the formation of a porous structure (Figure 2b) [29][30]. The most common electrolytes applied to create porous alumina are phosphorous, oxalic, and sulfuric acids—all featuring unique current/voltage parameters and structure geometry [31]. The anodization profile reflects the formation stage of the structure—the creation of a barrier layer and the growth of pores. In potentiostatic conditions, the formation of a barrier-type oxide follows exponential decrease of the current over time that goes along with the growth rate decrease. This retardation of the current flow is also reflected in a significant decrease of accessible oxide thickness as compared to the porous structure (Figure 2a). The formation of a nanoporous anodic alumina can be followed along with the current flow changes. Initial oscillations are related to the rearrangement of the structure with succeeding stabilization, when reaction reaches equilibrium. Then, quasi-stable current flow can be maintained for up to several days providing a steady growth rate (Figure 2b). A nanoporous film can reach even several hundreds of micrometers with a thickness linearly dependent on the applied current charge [3].
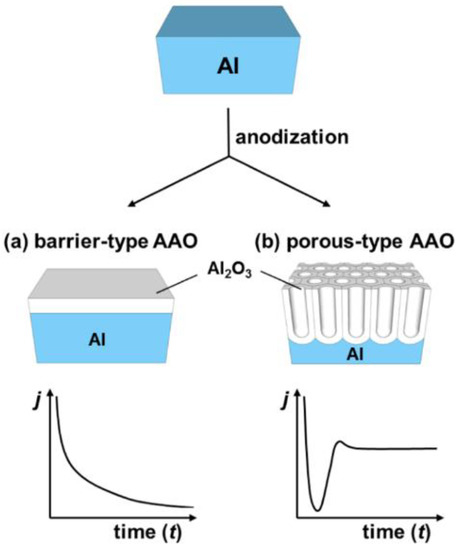
Figure 2. Types of anodic aluminum oxide and corresponding current-time transients. (a) Barrier type and (b) porous type. Reprinted with permission from [28]. Copyright 2014 American Chemical Society.
Through the radiotracer studies, it was possible to determine the exact place in which the formation of the oxide occurs in both scenarios. During the formation of a barrier oxide, growth occurs simultaneously at two interfaces: Oxide/electrolyte and metal/oxide. When a porous type alumina is formed, growth takes place only at the metal/oxide interface [32][33]. In further parts of the review, only porous structures will be discussed due to their unique, complex morphology in the micro- and nanoscale.
1.2. Pore Growth Mechanism and Spatial Ordering
While the word ‘formation’ may intuitively point to the one-way character of the process, it is in fact a result of several reactions occurring collaterally [34]. Pore formation during the anodization of aluminum is generally considered as a consequence of the equilibrium between two opposing changes in the structure: (i) Growth of the aluminum oxide at the metal/oxide interface and (ii) dissolution of the aluminum oxide at the oxide/electrolyte interface [35]. Simplified representation of the changes that occur during the anodization are visualized in Figure 3. When a constant anodic potential is applied, the entire surface of aluminum gets covered with a thin oxide barrier layer (I, Figure 3). As the process continues, electric resistance of the setup gradually increases along with the oxide layer growth resulting in the drop of the current flow, until it reaches a minimum value (II, Figure 3). O’Sullivan and Wood [6] suggested that at this stage the electric field concentrates on the local imperfections in the barrier layer. Different explanation, focusing on the local cracking in the barrier layer that facilitates the electrolyte penetration, was proposed by Thompson [36][37]. Polarization of Al-O bonds that goes along with the increased electric field can further promote the dissolution of the oxide. During this stage, a rapid increase of the current density can be observed (III, Figure 3) following a slight decrease and stabilization (IV, Figure 3). This slight drop has been related with a decrease in the pore density at the beginning of the process attributed to merging of pores.
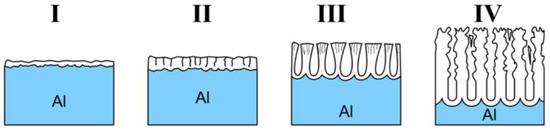
Figure 3. Scheme of step-by-step formation of porous oxide. (I) Formation of barrier layer, (II) formation of local cavities, (III) growth of porous structure, and (IV) schematic of nanoporous structure with initially disordered structure and following self-ordering. Reprinted (adapted) with permission from [28]. Copyright 2014 American Chemical Society.
The growth of highly organized structure from the very beginning of the process is possible only after certain preparations or pre-patterning. As shown in Figure 3, initial porous structure is disordered. However, as the process continues, the structure below becomes ordered. To avoid the presence of disordered pores at the outer surface, the golden standard utilized during NAA fabrication is the two-step anodization process [38]. Once the structure becomes regular during the first anodization step, the oxide layer is selectively removed—surface of the aluminum plate features cavities that mirrors geometry of the organized structure. Then, when the second step of anodization is applied, the growth immediately follows this geometry. It is important to note that high regularity can be obtained only under narrow sets of anodization conditions. There are many factors that affect the course of the anodization process: Potential, current flow, ion migration, local depletion, and in certain conditions, even electrical breakdown.
The extended investigation of the anodic alumina formation had led to the conclusion that growth and self-organization of alumina are significantly affected by the internal stress occurring during the formation process. A volume expansion is one of the crucial factors that have an impact on self-ordering. It was discovered [39] that the highest ordering occurs with a moderate volume expansion. Either the significant volume expansion or the contraction is accompanied by more disordered pores. Evolution of the internal stress that occurs during pores initiation and growth leads to the increase of the compressive stress with raising alumina thickness [40]. What is more, the extent of volume expansion can be linked to the regularity of the obtained structure. A simple way to quantify the volume expansion is by comparison between the final NAA volume to the initial thickness of aluminum. The relation between the spatial ordering and the observed volume expansion is investigated in the work of Jessensky [39]. Either contraction or higher expansion would result in a lower degree of ordering. These results were independently supported in the research of Nielsh et al. [41]. They proposed the 10% porosity rule as a requirement for the highest spatial ordering, which translates to the volume expansion of 1.23 independently of the anodization conditions. While in the most cases reported results remain in a good agreement with this implication, there are some exceptions. For example, it was possible to obtain highly ordered structures with 0.3 M selenic acid at much lower porosity of 0.8% [2].
Completely ordered NAA structures can be obtained for certain electrolytes under a narrow range of anodization conditions that are discussed in the next section.
1.3. Electrolyte Specific NAA Geometry
While development of the technology to increase the control over the process and reach high regularity is still an important research objective, decades ago its progress was significantly restricted by the existence of the initial, disordered layer of the alumina combined with a lack of sufficient tools to force the high ordering from the very beginning of the oxide growth. To date, the most frequently cited article involving nanoporous anodic alumina—the one that elevated the moderately attractive surface functionalization approach into a sophisticated nanotechnology tool—was the discovery done by Masuda and Fukuda [38]. The exponential growth of publications in two recent decades that followed the discovery is justified by the impressive regularity achievable with this new approach [42][43]. Properties of the grown NAA depend highly on the applied potential (with the resulting current) and a specific electrolyte. An initially disordered porous structure starts to form a regular array of hexagonal cells. The size of these cells is linearly dependent on the applied potential. However, it was observed that the highly regular morphology featuring low quantity of structural defects can be achieved only in narrow sets of conditions [44][45]. Each electrolyte utilized in the formation of nanoporous anodic alumina features a set of conditions: Potential, electrolyte concentration, and temperature in which high ordering can be achieved.
It was empirically established in many independent experiments, that interpore distance—or “cell size”—of the NAA structure depends directly on the applied voltage being defined as:
where Uan is an anodization voltage and k the constant that can be roughly estimated as k = 2.5 nm V−1, independently of the applied electrolyte [28][46]. However, self-ordered growth occurs only in a narrow range of anodization potential that is unique to particular ionic species. An incentive to seek for new electrolytes and self-ordering regimes is justified by the perspective to provide better covering of already accessible geometry [47].
A complete revision of the electrolytes reported in the literature that provide a high regularity of the porous structure and the corresponding range of obtainable pore distance is gathered in Table 1 and graphically represented in Figure 4. The value of k constant was based on calculation of all the values available in reports included in the Table 1. The presented value of k = 2.39 nm V−1 is close to the previously proposed simplified estimation [28][46].
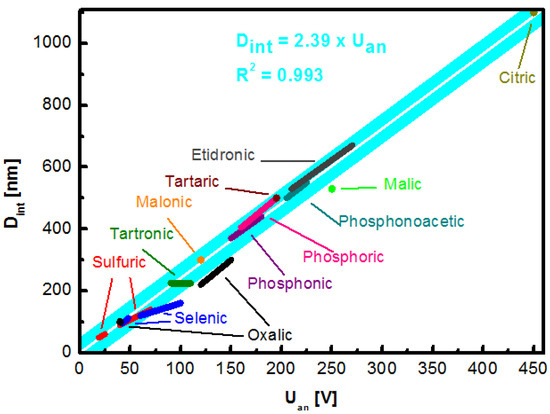
Figure 4. Linear relationship between anodization potential and interpore distance for self-ordered NAA formed during anodization in various electrolytes: Sulfuric, selenic, oxalic, tartronic, malonic, phosphonic, phosphoric, tartaric, phosphonoacetic, etidronic, malic, and citric acids.
The majority of research articles involves the fabrication of NAA grown in one of three acids considered “standard”: Sulfuric acid (H2SO4), oxalic (H2C2O4), and phosphoric (H3PO4) [6][31]. They are well-known and have been utilized for decades. Sulfuric acid was the first electrolyte used to yield an anodic alumina layer—initially with the intention to improve the corrosion resistance and hardness of the aluminum components. Amongst the aforementioned, it provides a triangular lattice with the highest density, which is a desirable feature in microelectronics [48][49]. What is more, the sulfuric-NAA provides the highest versatility in terms of an available cell size. Additionally, structures formed during the hard anodization exhibit high hardness of up to 400 Hv, attractive in terms of mechanical performance [50]. Even more impressive physical properties can be achieved with the novel discovery of the etidronic-NAA. The structures made with etidronic acid are 50% harder than with the sulfuric acid hard anodization (610 Hv) and can be further strengthened with thermal annealing (769 Hv) while featuring a low porosity of ~4%. What is more, the geometry of etidronic-NAA with near-subwavelength periodicity results in a significant reflection of light in the visible region: 490—760 nm [51]. Another phosphonate compound explored as an electrolyte for aluminum anodization is phosphonoacetic acid however, it has not been thoroughly investigated so far [52]. Different instance of a structure highly attractive in terms of optical properties is oxalic acid. The oxalic-NAA is often used to prepare photonic structures [53][54]. Fabrication of photonic crystals with improved color saturation preserving the aluminum substrate, which is possible due to introduction of short, high-voltage (250 V) anodization step subsequent to the conventional sinusoidal pulse anodization, was demonstrated by Sun et al. [55]. The oxalic-NAA is also one of the most prominent examples with regards to the photoluminescence performance, exceeding that of the sulfuric- and the phosphoric-NAA and can be adjusted with fabrication conditions [56][57]. Another example of a photoluminescence-active structure can be the arsenic-NAA [58]. This recent approach is characterized with a structure featuring much thicker skeleton of pure alumina as compared to other typical electrolytes. Moreover, the arsenic-NAA exhibits unique white photoluminescence emission (515 nm) under UV irradiation (254 nm). On the other hand, the tartaric-NAA features a broad spectrum of blue luminescence (400–750 nm). Detailed analysis of the as-prepared and annealed tartaric-NAA films revealed two sources of the emission [59]. The highest intensity peak with a maximum at 460 nm originated from bulk and adsorbed OH groups, while amorphous carbon derived from the electrolyte contributed to the peak at 550 nm. Practically, the tartaric acid-NAA remains unexplored with regards to nanotechnology, but the mixture of tartaric-sulfuric acid has been implemented as an alternative replacing chromic acid anodization for European aeronautical industry in 2014 [60]. Recently, a new electrolyte has been found to yield highly ordered porous structure. A high degree of self-ordering with malic acid was discovery by Zajączkowska et al. [61]. Additionally, it was observed that the repetition of anodization—applying the same external anodization parameters—affects the current-time transient. It was suggested that a high degree of malate ion incorporated into a NAA structure could promote attraction of these ions to the Al electrode further facilitating formation of the oxide structure—the first such observation reported in the literature so far.
Another acid that has been explored moderately recently (reported by Nishinaga et al. [2]) that can be used to yield a porous structure is selenic acid. The great potential of the selenic-NAA stems from its low porosity, colorlessness, and high transparency. Intriguingly, as compared to the NAA formed with organic electrolyte, it does not exhibit photoluminescence. It is also competitive with regards to the sulfuric-NAA due to rapid formation of the self-ordered structure of 10-nm pore diameter within 1 h. Similarl to sulfuric acid, self-ordering can be achieved for more than one potential range providing for higher fabrication versatility [2][62][63].
Table 1. Interpore distance reported for nanoporous anodic alumina anodized in various electrolytes.
| Interpore Distance [nm] |
Electrolyte | Concentration | Applied Potential [V] |
Temperature [°C] |
|---|---|---|---|---|
| 50–60 | Sulfuric acid | 0.3 M | 19–25 | 5 |
| 90–140 | Sulfuric acid | 10 wt % | 40–70 | 0.1 |
| 95–112 | Selenic acid | 0.3 M | 42–48 | 20 |
| 100 | Oxalic acid | 0.3 M | 40 | 5 |
| 120–160 | Selenic acid | 0.3 M | 60–100 | 0 |
| 225 | Tartronic acid | 0.3 M | 90–110 | 0.5 |
| 220–300 | Oxalic acid | 0.3 M | 120–150 | 1–2 |
| 300 | Malonic acid | 5.0 M | 120 | 0–1 |
| 370–440 | Phosphonic acid | 0.5–2.0 M | 150–180 | 0–20 |
| 405–500 | Phosphoric acid | 0.3 M | 160–195 | 5 |
| 500 | Tartaric acid | 2–4 wt % | 195 | 5 |
| 530 | Malic acid | 0.5 M | 230 | 5 |
| 500–550 | Phosphonoacetic acid | 0.1–0.9 M | 205–225 | 10 |
| 530–670 | Etidronic acid | 0.3 M | 210–270 | 0–40 |
| 1100 | Citric acid | 0.1–1 M | 260–450 | 10–30 |
Impact of Temperature and Additives
Temperature of the electrolyte during anodization is an important factor affecting the formation of NAA. Higher temperature is a convenient measure to accelerate the alumina growth and adjust the resultant pore diameter not affecting interpore distance simultaneously [64][65]. Evaluation how—independently of the electrolyte temperature—temperature of the aluminum anode can impact the formation of NAA was performed by Chernyakova et al. [66]. For the temperature increase between 5 °C and 60 °C dp and dint remain unchanged, while structural ordering has increased. Results indicate that the rate of the NAA chemical dissolution is not temperature dependent. Furthermore, above 60 °C the self-ordering drops and formed pores are 1.7 times broader. However, outcome of the anodization can be also altered using addition of various chemicals to the electrolyte.
Ethanol is a common electrolyte additive that enables to perform anodization below 0 °C reaching lower current densities. It allows to yield even smaller pores, for example 8 nm diameter pores for the sulfuric-NAA [5][67][68]. It was recently demonstrated that addition of ethanol improves the formation of NAA in sulfuric acid, suppressing its chemical dissolution and the anodization rate. What is more, the anodization was possible even with 50% ethanol content in the electrolyte [69]. Another common additive is ethylene glycol. It increases viscosity of the electrolyte decreasing the dissociation constant. Consequently, reduced electrolyte conductivity decreases current density. It is especially useful when anodizing low-purity aluminum as the increase of the current density may occur due to the localized impurities. Additionally, the anodization in a broader range of potential is possible without burning phenomenon. Yet, above certain critical potential values, hillocks and cracks occur decreasing the quality of resulting NAA [70]. The presence of ethylene glycol in the electrolyte for aluminum anodization facilitates oxidation of the intermetallic phase. Consequently, the connection between adjacent cells is weakened and voids form at the three cell junction—the effect intensifies along with raising ethylene glycol content [71]. Furthermore, intensified oxidation was linked with the increased incorporation of elements that does not originate from the electrolyte. It was established that increased intake derives from the elements present in the AA7075 alloy. Additionally, the growth rate had decreased as compared to the anodization without ethylene glycol [72]. When different alcohols are compared, effects are more pronounced for polyhydric alcohols [73]. However, not every aspect of electrolyte additives has been revealed so far. The challenge is their impact seems to be independently affected by other modifiers such as electrolyte, current density, pH, viscosity, etc. Anodization with addition of poly(ethylene glycol) (PEG) enables to alter pore diameter of the grown structure independently of the anodization voltage [74]. Authors attribute this behavior to the increase of the electric field strength (PEG has lower dielectric coefficient than water) and restricted chemical dissolution process. It was observed that immersion of the NAA films in the acid solution with 50% addition of PEG resulted in 4 times slower dissolution as compared to solution without PEG. As a result, it was possible to modulate the pore diameter of the structure using different content of PEG, while other parameters (acid concentration, anodization voltage, and electrolyte temperature) remained constant. The impact of PEG containing electrolyte on the morphology of NAA is shown in Figure 5.
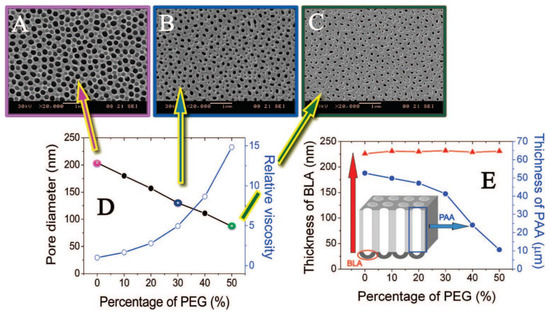
Figure 5. Impact of poly(ethylene glycol) (PEG) concentration on morphology of formed NAA films. Concentration of PEG in the electrolyte: (A) 0%, (B) 30%, (C) 50%, (D) influence of PEG content on the pore diameter and (E) the NAA film and barrier layer thickness in relation to PEG content in the electrolyte. Reprinted with permission from [74]. Copyright 2008 American Chemical Society.
Alternatively, an electrolyte additive may promote the incorporation of elements meant to improve properties of alumina matrix. The addition of lithium phosphate to the electrolyte successfully incorporating lithium ions into NAA during its formation is presented in [75]. The incorporation of metal ions may be potentially attractive with the intention to increase the conductivity of the material [76]. The interesting experiment involving the electrolyte composition has been presented by Christoulaki et al. [77]. Water in sulfuric and oxalic acid has been replaced with deuterated water, and in one case, the electrolyte composed of D2SO4 in D2O was used. Use of deuterated water resulted in the 20% reduction of the pore diameter, improved pore ordering and increased growth rate. Observed behavior has been attributed to the decrease of the alumina formation activation energy. Furthermore, such electrolytes enabled to analyze the incorporation of hydroxyl groups during the NAA formation and effects of prolonged immersion in the solvent. Small-Angle Neutron Scattering shown no significant difference in incorporation rate between H2O and D2O pointing on the weak OH incorporation, while the hydration through immersion shown to be a slow process.
1.4. Mild and Hard Anodization: Two Growth Regimes
For a long time, the default approach to the anodization of aluminum for the purpose of nanotechnology and research was the one performed under the moderate and constant potential—individually adjusted to each electrolyte—between the electrodes; under which the current flow is determined by reactions’ equilibrium. Due to the constant potential and the low current flow (that is typically below 30 mA cm−2) such a process is called potentiostatic mild anodization (MA). MA conditions result in a predictable course of the process and the stable growth rate of 1–3 µm h−1. However, several restrains of the process such as limited growth rate (the fabrication of self-standing membrane may require days of anodization), encouraged the exploration for a more practical, fast approach. What is more, self-ordering of NAA have narrow windows and discovery of new ordering regimes became the quest on its own.
The alternative approach commonly utilized in industry was left out of the scope in research field due to several restrains. Major characteristic of the process was a massive—as compared to the foremost—amount of the energy flow through the sample that is reflected in the widely used name: Hard anodization (HA). A basic constraint that limits access to certain benefits of the process is the amount of heat generated during formation of the alumina, related with the Joule’s effect. Reaching the critical point may result in the electric breakdown that can lead to the destruction of the sample [78]. The discovery of Lee and co-workers renewed the attention to HA [3]. Principle of the discovery was based on the formation of a thin—400 nm—layer of porous alumina prior to the introduction of the high potential. This ‘scaffold’ prevented the breakdown enabling the uniform NAA growth. It was hypothesized that such a pre-patterning promoted the uniform pore nucleation preventing catastrophic events and defects. Growth of the oxide film with this method was also much faster. Recently, even faster growth of NAA film in a process named ultra-hard anodization Noormohammadi et al. [79]. A 58 µm thick membrane was formed in 80 s: 30 times faster than during hard anodization and 450 times faster than with mild anodization. It was possible due to the high current density (2400 mA cm−2) combined with control of the barrier layer temperature and the diffusion length to mitigate burning and the dielectric breakdown.
The new self-ordering regime that exhibits different current/voltage over time characteristics brought questions about differences in the formation mechanism. Detailed analysis of both regimes using voltametric and microscopic methods was provided by Vega et al. [80]. They point out the difference in the local ion concentration as a major factor for the observed distinction in current characteristics. During mild anodization, the ionic concentration remains stable. However, rapid growth of the structure during hard anodization leads to the local depletion that is reflected in the gradually decreasing current when a constant potential is applied—with hard anodization being controlled by the diffusion. Intriguingly, the potential at which the breakdown occurs depends on the initial sample preparation and experimental conditions [3][81][80].
The significant contribution to the field was brought by the group of Napolskii and co-workers. They provided analysis that linked the impact of voltage ramp on the morphology and thickness homogeneity. It was demonstrated that when faster 5.0 V s−1 ramp was applied instead of conventional 0.5 V s−1, the significant reduction of morphological defects is observed [82]. While the occurrence of self-ordering regimes was well-known for spectrum of electrolytes, the question ‘why’ remained unaccounted. An attempt to empirically unravel this behavior was carried out by Roslyakov et al. [83]. They performed the anodization slowly raising the potential (0.5 V s−1 for 30–130 V for H2C2O4 and 50 mV s−1 for 15–60 V for H2SO4) and continued at every potential value so 105 C of charge can be utilized (corresponding to ~50 µm thick NAA) followed by the separation of alumina film into thin slices. Upon analysis of the NAA morphology, they proposed a model in which a high level of self-ordering can be achieved in two distinct regimes: The growth rate being restricted by ionic migration through the barrier layer or by diffusion inside the pore. Outside of these frames—during the “mixed” control—pore growth is disordered. Prevalence of the self-ordering depends on the applied voltage and is shown in Figure 6. The applied potential is linked with the formation efficiency, volume expansion, and content of the electrolyte impurities for 20–130 V anodization in 0.3 M H2C2O4 and 19–60 V 0.3 M H2SO4 [84]. The formation efficiency and the volume expansion were found proportional to the potential increase, while the degree of ion embedding was the highest for moderate values exceeding ones achieved with either low or high potential. Recently, detailed analysis of the electrolyte temperature effects on the alumina formation in oxalic acid at mild and hard anodization was provided [85]. Concluding the current profile, higher electrolyte temperature resulted in a shift of both kinetic and diffusion regimes to lower voltages (Figure 6). The temperature increase from 0 °C to 20 °C raised formation speed significantly: 3.8 times for mild anodization and 2.1 times for hard anodization. However, the faster growth was accompanied by the lower formation efficiency and the number of pores grown in hexagonal coordination. The most striking case was that of thicker films (above 20 µm) for 20 °C 40 V anodization as the formation switched into mixed regime explicitly retarding self-ordering.
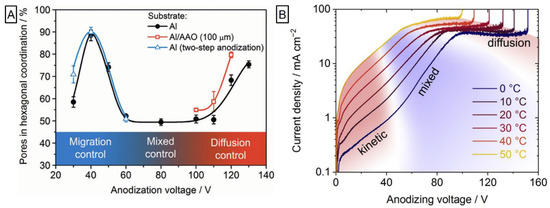
Figure 6. Anodization potential influence on degree of self-ordered growth and voltage/temperature dependent formation kinetics of 0.3 M H2C2O4 anodization of aluminum. (A) Dependence of pore ordering degree during formation of alumina structure with highlighted regions of different growth kinetics. Reprinted (adapted) with permission from [83]. Copyright 2017 Elsevier. (B) Linear staircase voltammograms with highlighted growth kinetics. Reprinted (adapted) with permission from [85]. Copyright 2019 Elsevier.
This entry is adapted from the peer-reviewed paper 10.3390/nano11020430
References
- Rajeev, G.; Simon, B.P.; Marsal, L.F.; Voelcker, N.H. Advances in Nanoporous Anodic Alumina-Based Biosensors to Detect Biomarkers of Clinical Significance: A Review. Adv. Healthc. Mater. 2018, 7, 1–18.
- Nishinaga, O.; Kikuchi, T.; Natsui, S.; Suzuki, R.O. Rapid fabrication of self-ordered porous alumina with 10-/sub-10-nm-scale nanostructures by selenic acid anodizing. Sci. Rep. 2013, 3, 1–6.
- Lee, W.; Ji, R.; Gösele, U.; Nielsch, K. Fast fabrication of long-range ordered porous alumina membranes by hard anodization. Nat. Mater. 2006, 5, 741–747.
- Yu, M.; Zhang, W.; Zhang, S.; Zhao, S.; Ai, F.; Zhu, X. Morphology evolution of porous anodic alumina in mixed H3PO4/NH4F electrolytes. Surf. Coat. Technol. 2018, 334, 500–508.
- Ribes, A.; Xifre-Perez, E.; Aznar, E.; Sancenon, F.; Pardo, T.; Marsal, L.F.; Martinez-Manez, R. Molecular gated nanoporous anodic alumina for the detection of cocaine. Sci. Rep. 2016, 6, 1–9.
- O’Sullivan, J.P.; Wood, G.C. Morphology and mechanism of formation of porous anodic films on aluminum. Proc. Roy. Soc. Ser. A Math. Phys. Sci. 1970, 317, 1731.
- Lin, Y.; Lin, Q.; Liu, X.; Gao, Y.; He, J.; Wang, W.; Fan, Z. A Highly Controllable Electrochemical Anodization Process to Fabricate Porous Anodic Aluminum Oxide Membranes. Nanoscale Res. Lett. 2015, 10, 1–8.
- Santos, A.; Ferré-Borrull, J.; Pallarès, J.; Marsal, L.F. Hierarchical nanoporous anodic alumina templates by asymmetric two-step anodization. Phys. Status Solidi Appl. Mater. Sci. 2011, 208, 668–674.
- Vojkuvka, L.; Marsal, L.F.; Ferré-Borrull, J.; Formentin, P.; Pallarés, J. Self-ordered porous alumina membranes with large lattice constant fabricated by hard anodization. Superlattices Microstruct. 2008, 44, 577–582.
- Poznyak, A.; Pligovka, A.; Turavets, U.; Norek, M. On-Aluminum and Barrier Anodic Oxide: Meeting Various Acids and Solutions. Coatings 2020, 10, 875.
- Han, H.; Park, S.J.; Jang, J.S.; Ryu, H.; Kim, K.J.; Baik, S.; Lee, W. In situ determination of the pore opening point during wet-chemical etching of the barrier layer of porous anodic aluminum oxide: Nonuniform Impurity Distribution in Anodic Oxide. ACS Appl. Mater. Interfaces 2013, 5, 3441–3448.
- Garcia-Vergara, S.J.; Habazaki, H.; Skeldon, P.; Thompson, G.E. Tracer studies relating to alloying element behaviour in porous anodic alumina formed in phosphoric acid. Electrochim. Acta 2010, 55, 3175–3184.
- Santos, A. Nanoporous anodic alumina photonic crystals: Fundamentals, developments and perspectives. J. Mater. Chem. C 2017, 5, 5581–5599.
- Vera-Londono, L.; Ruiz-Clavijo, A.; Caballero-Calero, O.; Martín-González, M. Understanding the thermal conductivity variations in nanoporous anodic aluminum oxide. Nanoscale Adv. 2020, 2, 4591–4603.
- Ferre-Borrull, J.; Xifre-Perez, E.; Pallares, J.; Marsal, L.F. Nanoporous Alumina; Losic, D., Santos, A., Eds.; Springer: Cham, Switzerland, 2015; Volume 219, pp. 185–217.
- Santos, A.; Balderrama, V.S.; Alba, M.; Formentin, P.; Ferre-Borrull, J.; Pallares, J.; Marsal, L.F. Tunable fabry-pérot interferometer based on nanoporous anodic alumina for optical biosensing purposes. Nanoscale Res. Lett. 2012, 7, 2–5.
- Kure-Chu, S.-Z.; Osaka, K.; Yashiro, H.; Wada, K.; Segawa, H.; Inoue, S. Facile Fabrication of Ordered Multi-Tiered Hierarchical Porous Alumina Nanostructures with Multiple and Fractional Ratios of Pore Interval toward Multifunctional Nanomaterials. ECS J. Solid State Sci. Technol. 2016, 5, 285–292.
- Li, Y.B.; Zheng, M.J.; Ma, L. High-speed growth and photoluminescence of porous anodic alumina films with controllable interpore distances over a large range. Appl. Phys. Lett. 2007, 91, 8–11.
- Cantelli, L.; Santos, J.S.; Silva, T.F.; Tabacniks, M.H.; Delgado-Silva, A.O.; Trivinho-Strixino, F. Unveiling the origin of photoluminescence in nanoporous anodic alumina (NAA) obtained by constant current regime. J. Lumin. 2019, 207, 63–69.
- Mir, M.A.; Shah, M.A.; Ganai, P.A. Dielectric study of nanoporous alumina fabricated by two-step anodization technique. Chem. Pap. 2020, 0123456789.
- Baranowska, M.; Slota, A.J.; Eravuchira, P.J.; Macias, G.; Xifre-Perez, E.; Pallares, J.; Ferre-Borrull, J.; Marsal, L.F. Protein attachment to nanoporous anodic alumina for biotechnological applications: Influence of pore size, protein size and functionalization path. Coll. Surf. B Biointerfaces 2014, 122, 375–383.
- Porta-i-batalla, M.; Eckstein, C.; Xifré-pérez, E.; Formentín, P.; Marsal, L.F. Sustained, Controlled and Stimuli- Responsive Drug Release Systems Based on Nanoporous Anodic Alumina with Layer-by-Layer Polyelectrolyte. Nanoscale Res. Lett. 2016, 11, 372.
- Eckstein, C.; Acosta, L.K.; Pol, L.; Xifre-Perez, E.; Pallares, J.; Ferre-Borrull, J.; Marsal, L.F. Nanoporous Anodic Alumina Surface Modification by Electrostatic, Covalent, and Immune Complexation Binding Investigated by Capillary Filling. ACS Appl. Mater. Interfaces 2018, 10, 10571–10579.
- Tabrizi, M.A.; Ferre-Borrull, J.; Marsal, L.F. Advances in optical biosensors and sensors using nanoporous anodic alumina. Sensors 2020, 20, 5068.
- Law, C.S.; Lim, S.Y.; Abell, A.D.; Voelcker, N.H.; Santos, A. Nanoporous Anodic Alumina Photonic Crystals for Optical Chemo- and Biosensing: Fundamentals, Advances, and Perspectives. Nanomaterials 2018, 8, 788.
- Zhou, Z.; Nonnenmann, S.S. Progress in nanoporous templates: Beyond anodic aluminum oxide and towards functional complex materials. Materials 2019, 12, 2535.
- Kapruwan, P.; Ferré-borrull, J.; Marsal, L.F. Nanoporous Anodic Alumina Platforms for Drug Delivery Applications: Recent Advances and Perspective. Adv. Mater. Interfaces 2020, 7, 1–17.
- Lee, W.; Park, S.J. Porous anodic aluminum oxide: Anodization and templated synthesis of functional nanostructures. Chem. Rev. 2014, 114, 7487–7556.
- Sato, Y.; Asoh, H.; Ono, S. Effects of electrolyte species and their combination on film structures and dielectric properties of crystalline anodic alumina films formed by two-step anodization. Mater. Trans. 2013, 54, 1993–1999.
- Yazdizadeh, M.; Yelon, A.; Ménard, D. Characterizing and optimizing the electropolishing and pore arrangement in porous anodic aluminum oxide (AAO). J. Porous Mater. 2020, 27, 995–1002.
- Mir, M.A.; Shah, M.A.; Ganai, P.A. Nanoporous anodic alumina (NAA) prepared in different electrolytes with different pore sizes for humidity sensing. J. Solid State Electrochem. 2020, 24, 1679–1686.
- Davies, J.A.; Domeij, B.; Pringle, J.P.S.; Brown, F. The Migration of Metal and Oxygen during Anodic Film Formation. J. Electrochem. Soc. 1965, 112, 675.
- Shimizu, K.; Thompson, G.E.; Wood, G.C.; Xu, Y. Direct observations of ion-implanted xenon marker layers in anodic barrier films on aluminium. Thin Solid Films 1982, 88, 255–262.
- Li, F.; Zhang, L.; Metzger, R.M. On the Growth of Highly Ordered Pores in Anodized Aluminum Oxide. Chem. Mater. 1998, 10, 2470–2480.
- Kumeria, T.; Santos, A. Nanoporous Anodic Alumina for Optical Biosensing. Springer Ser. Mater. Sci. 2015, 219, 293–318.
- Thompson, G.E.; Xu, Y.; Skeldon, P.; Shimizu, K.; Han, S.H.; Wood, G.C. Anodic oxidation of aluminium. Philos. Mag. B Phys. Condens. Matter. 1987, 55, 651–667.
- Thompson, G.E. Porous anodic alumina: Fabrication, characterization and applications. Thin Solid Films 1997, 297, 192–201.
- Masuda, H.; Fukuda, K. Ordered Metal Nanohole Arrays Made by a Two-Step Replication of Honeycomb Structures of Anodic Alumina. Science 1995, 268, 1466–1468.
- Jessensky, O.; Müller, F.; Gösele, U. Self-organized formation of hexagonal pore arrays in anodic alumina. Appl. Phys. Lett. 1998, 72, 1173–1175.
- Van Overmeere, Q.; Proost, J. Stress-affected and stress-affecting instabilities during the growth of anodic oxide films. Electrochim. Acta. 2011, 56, 10507–10515.
- Nielsch, K.; Choi, J.; Schwirn, K.; Wehrspohn, R.B. Self-ordering Regimes of Poorus Alumina: The 10% Porosity Rule. Nano Lett. 2002, 2, 1–4.
- Santos, A.; Kumeria, T.; Losic, D. Nanoporous anodic aluminum oxide for chemical sensing and biosensors. TrAC Trends Anal. Chem. 2013, 44, 25–38.
- Sousa, C.T.; Leitao, D.C.; Proenca, M.P.; Ventura, J.; Pereira, A.M.; Araujo, J.P. Nanoporous alumina as templates for multifunctional applications. Appl. Phys. Rev. 2014, 1, 031102.
- Houser, J.E.; Hebert, K.R. The role of viscous flow of oxide in the growth of self-ordered porous anodic alumina films. Nat. Mater. 2009, 8, 415–420.
- Hebert, K.R.; Albu, S.P.; Paramasivam, I.; Schmuki, P. Morphological instability leading to formation of porous anodic oxide films. Nat. Mater. 2012, 11, 162–166.
- Kikuchi, T.; Nishinaga, O.; Natsui, S.; Suzuki, R.O. Fabrication of self-ordered porous alumina via etidronic acid anodizing and structural color generation from submicrometer-scale dimple array. Electrochim. Acta 2015, 156, 235–243.
- Mirzoev, R.A.; Davydov, A.D.; Vystupov, S.I.; Kabanova, T.B. Conditions for self-ordering of porous structure of anodic aluminum oxide in weak and strong acids. Electrochim. Acta 2019, 294, 276–285.
- Masuda, H.; Hasegwa, F. Self-Ordering of Cell Arrangement of Anodic Porous Alumina Formed in Sulfuric Acid Solution. J. Electrochem. Soc. 1997, 144, L127.
- Sulka, G.D.; Parkoła, K.G. Anodising potential influence on well-ordered nanostructures formed by anodisation of aluminium in sulphuric acid. Thin Solid Films 2006, 515, 338–345.
- Raid, A.; Pavan, S.; Fridrici, V.; Poilane, C.; Kapsa, P. Temperature effect on the kinetic alumina layer growth on 5086 aluminum substrate. Mechanika 2017, 23, 923–930.
- Arango, F.; Sepúlveda, M.; Aguilar-Sierra, S.; Ricaurte, G.; Echeverría, F. Interferometric colours produced by anodised aluminium diffraction grating. Mater. Sci. Technol. 2020, 36, 1238–1244.
- Takenaga, A.; Kikuchi, T.; Natsui, S.; Suzuki, R.O. Self-ordered aluminum anodizing in phosphonoacetic acid and its structural coloration. ECS Solid State Lett. 2015, 4, P55–P58.
- Santos, A.; Balderrama, V.S.; Alba, M.; Formenting, P.; Ferre-Borrull, J.; Pallares, J.; Marsal, L.F. Nanoporous anodic alumina barcodes: Toward smart optical biosensors. Adv. Mater. 2012, 24, 1050–1054.
- Macias, G.; Ferré-Borrull, J.; Pallarès, J.; Marsal, L.F. 1-D nanoporous anodic alumina rugate filters by means of small current variations for real-time sensing applications. Nanoscale Res. Lett. 2014, 9, 1–6.
- Sun, C.; Hao, S.; Wang, Z.; Xu, Q.; Wang, Y.; Peng, Q.; Lan, T. Rapid fabrication of iridescent alumina films supported on an aluminium substrate by high voltage anodization. Opt. Mater. 2020, 104, 109937.
- Santos, A.; Alba, M.; Rahman, M.M.; Formentin, P.; Ferre-Borrull, J.; Pallares, J.; Marsal, L.F. Structural tuning of photoluminescence in nanoporous anodic alumina by hard anodization in oxalic and malonic acids. Nanoscale Res. Lett. 2012, 7, 1–11.
- Reddy, P.R.; Ajith, K.M.; Udayashankar, N.K. Effect of electrolyte concentration on morphological and photoluminescence properties of free standing porous anodic alumina membranes formed in oxalic acid. Mater. Sci. Semicond. Process. 2020, 106, 104755.
- Akiya, S.; Kikuchi, T.; Natsui, S.; Suzuki, R.O. Nanostructural characterization of large-scale porous alumina fabricated via anodizing in arsenic acid solution. Appl. Surf. Sci. 2017, 403, 652–661.
- Chernyakova, K.; Karpicz, R.; Zavadski, S.; Poklonskaya, O.; Jagminas, A.; Vrublevsky, I. Structural and fluorescence characterization of anodic alumina/carbon composites formed in tartaric acid solution. J. Lumin. 2017, 182, 233–239.
- González-Rovira, L.; González-Souto, L.; Astola, P.J.; Bravo-Benítez, C.; Botana, F.J. Assessment of the corrosion resistance of self-ordered anodic aluminum oxide (AAO) obtained in tartaric-sulfuric acid (TSA). Surf. Coat. Technol. 2020, 399, 126131.
- Zajączkowska, L.; Siemiaszko, D.; Norek, M. Towards Self-Organized Anodization of Aluminum in Malic Acid Solutions—New Aspects of Anodization in the Organic Acid. Materials 2020, 13, 3899.
- Gordeeva, E.O.; Roslyakov, I.V.; Napolskii, K.S. Aluminium anodizing in selenic acid: Electrochemical behaviour, porous structure, and ordering regimes. Electrochim. Acta 2019, 307, 13–19.
- Sadykov, A.I.; Kushnir, S.E.; Roslyakov, I.V.; Baranchikov, A.E.; Napolskii, K.S. Selenic acid anodizing of aluminium for preparation of 1D photonic crystals. Electrochem. Commun. 2019, 100, 104–107.
- Zaraska, L.; Stȩpniowski, W.J.; Ciepiela, E.; Sulka, G.D. The effect of anodizing temperature on structural features and hexagonal arrangement of nanopores in alumina synthesized by two-step anodizing in oxalic acid. Thin Solid Films 2013, 534, 155–161.
- Stȩpniowski, W.J.; Nowak-Stȩpniowska, A.; Presz, A.; Czujko, T.; Varin, R.A. The effects of time and temperature on the arrangement of anodic aluminum oxide nanopores. Mater. Charact. 2014, 91, 1–9.
- Chernyakova, K.; Tzaneva, B.; Vrublevsky, I.; Videkov, V. Effect of Aluminum Anode Temperature on Growth Rate and Structure of Nanoporous Anodic Alumina. J. Electrochem. Soc. 2020, 167, 103506.
- Resende, P.M.; Martín-González, M. Sub-10 nm porous alumina templates to produce sub-10 nm nanowires. Microporous Mesoporous Mater. 2019, 284, 198–204.
- Pla, L.; Xifre-Perez, E.; Ribes, A.; Aznar, E.; Marcos, D.; Marsal, L.F.; Martinez-Manez, R.; Sancenon, F. A Mycoplasma Genomic DNA Probe using Gated Nanoporous Anodic Alumina. ChemPlusChem 2017, 82, 337–341.
- Asoh, H.; Matsumoto, M.; Hashimoto, H. Effects of ethanol on the efficiency of the formation of anodic alumina in sulfuric acid. Surf. Coat. Technol. 2019, 378, 124947.
- Michalska-Domańska, M.; Stępniowski, W.J.; Jaroszewicz, L.R. Characterization of nanopores arrangement of anodic alumina layers synthesized on low-(AA1050) and high-purity aluminum by two-step anodizing in sulfuric acid with addition of ethylene glycol at low temperature. J. Porous Mater. 2017, 24, 779–786.
- Norek, M.; Zasada, D.; Siemiaszko, D. Systematic study on morphology of anodic alumina produced by hard anodization in the electrolytes modified with ethylene glycol. J. Nano Res. 2017, 46, 165–178.
- Farhan, M.; Anawati, A. Effect of additive ethylene glycol on morphology and mechanical hardness of anodic oxide film formed on AA7075. J. Phys. Conf. Ser. 2019, 1191, 012033.
- Matsumoto, M.; Hashimoto, H.; Asoh, H. Formation Efficiency of Anodic Porous Alumina in Sulfuric Acid Containing Alcohol: Comparison of the Effects of Monohydric and Polyhydric Alcohols as Additives. J. Electrochem. Soc. 2020, 167, 041504.
- Chen, W.; Wu, J.S.; Xia, X.H. Porous anodic alumina with continuously manipulated pore/cell size. ACS Nano 2008, 2, 959–965.
- Abd-Elnaiem, A.M.; Rashad, M. Morphology of anodic aluminum oxide anodized in a mixture of phosphoric acid and lithium phosphate monobasic. Mater. Res. Express 2019, 6, 016412.
- Maekawa, H.; Tanaka, R.; Sato, T.; Fujimaki, Y.; Yamamura, T. Size-dependent ionic conductivity observed for ordered mesoporous alumina-LiI composite. Solid State Ionics 2004, 175, 281–285.
- Christoulaki, A.; Moretti, C.; Chennevière, A.; Dubois, E.; Jouault, N. Improving structural features of nanoporous alumina using deuterated electrolytes. Microporous Mesoporous Mater. 2020, 303, 110201.
- Lämmel, C.; Schneider, M.; Heubner, C.; Beckert, W.; Michaelis, A. Investigations of burning phenomena during the hard anodising of aluminium by local in-operando temperature measurements. Electrochim. Acta 2017, 249, 271–277.
- Noormohammadi, M.; Arani, Z.S.; Ramazani, A.; Kashi, M.A.; Abbasimofrad, S. Super-fast fabrication of self-ordered nanoporous anodic alumina membranes by ultra-hard anodization. Electrochim. Acta 2020, 354, 136766.
- Vega, V.; Garcia, J.; Montero-Moreno, J.M.; Hernando, B.; Bachmann, J.; Prida, V.M.; Nielsch, K. Unveiling the Hard Anodization Regime of Aluminum: Insight into Nanopores Self-Organization and Growth Mechanism. ACS Appl. Mater. Interfaces 2015, 7, 28682–28692.
- Chu, S.Z.; Wada, K.; Inoue, S.; Isogai, M.; Yasumori, A. Fabrication of ideally ordered nanoporous alumina films and integrated alumina nanotubule arrays by high-field anodization. Adv. Mater. 2005, 17, 2115–2119.
- Roslyakov, I.V.; Kuratova, N.S.; Koshkodaev, D.S.; Merino, D.H.; Lukashin, A.V.; Napolskii, K.S. Morphology of anodic alumina films obtained by hard anodization: Influence of the rate of anodization voltage increase. J. Surf. Investig. X-ray Synchrotron Neutron Tech. 2016, 10, 191–197.
- Roslyakov, I.V.; Gordeeva, E.O.; Napolskii, K.S. Role of Electrode Reaction Kinetics in Self-Ordering of Porous Anodic Alumina. Electrochim. Acta 2017, 241, 362–369.
- Gordeeva, E.O.; Roslyakov, I.V.; Sadykov, A.I.; Suchkova, T.A.; Petukhov, D.I.; Shatalova, T.B.; Napolskii, K.S. Formation Efficiency of Porous Oxide Films in Aluminum Anodizing. Russ. J. Electrochem. 2018, 54, 990–998.
- Leontiev, A.P.; Roslyakov, I.V.; Napolskii, K.S. Complex influence of temperature on oxalic acid anodizing of aluminium. Electrochim. Acta 2019, 319, 88–94.
