Nuclear domains 10 (ND10), a.k.a. promyelocytic leukemia nuclear bodies (PML-NBs), are membraneless subnuclear domains that are highly dynamic in their protein composition in response to cellular cues. They are known to be involved in many key cellular processes including DNA damage response, transcription regulation, apoptosis, oncogenesis, and antiviral defenses.
- herpesvirus
- ND10
- PML nuclear bodies
- virus-host interaction
- antiviral defense
1. Introduction
Nuclear domains 10 (ND10) are dynamic macromolecular structures that assemble in the nucleoplasm of many types of mammalian cells [1][2]. They were first identified as unique nuclear dots recognized by autoimmune antibodies that were distinguishable from other known nuclear bodies [3]. These discrete spherical dots were designated as ND10 based on their average number of 10 loci per nucleus in various cultured cells [3][4]. More interestingly, the size and number of ND10 were found to increase with interferon (IFN) treatment and diminish upon herpes simplex virus (HSV) infection [4][5]. These discoveries immediately linked this new type of nuclear body to host antiviral response and raised enormous interest in the field of virology [1][6][7][8].
In that same era, parallel studies investigating the acute promyelocytic leukemia (APL) etiology revealed that a chromosome t(15;17) translocation that led to a fusion of promyelocytic leukemia (PML) protein to the retinoic acid receptor α (RARα) was the direct cause of APL [9][10]. Studies on PML quickly identified PML nuclear bodies (PML-NBs), in which the wild-type PML was a constant constituent. Further experiments showed that the introduction of the PML/RARα fusion protein to normal cells disrupted PML-NBs, whereas treatment with the pharmacological doses of retinoic acid (RA) restored PML-NBs in the APL-derived NB4 cells [11][12]. Since the clinical RA administration was known to induce blast differentiation and complete remission in APL patients, PML was thereby classified as a tumor suppressor, and oncogenic regulation has since been considered an important function of PML-NBs [13][14][15][16].
With the extensive interest invested in characterizing the composition and functions of these novel nuclear bodies, people quickly realized that ND10 bodies and PML-NBs represented the same set of macromolecular structures, for which we follow the herpes virologists’ tradition and call them ND10 from this point on. Besides their implication in antiviral defense and tumor suppression, the ND10 structures are known to function in many cellular pathways, including transcription/translation regulation, DNA damage response, cell cycle regulation, and apoptosis and senescence regulation [1][17][18][19]. So far, more than 150 component proteins are known to associate with ND10 bodies [20]. Among them, some proteins are permanent residents such as PML, whereas many others are transiently associated with ND10 in response to various cellular cues [20][21]. The majority of ND10 components are regulatory proteins with multiple functions in key cellular pathways. The diversity and importance of ND10 proteins in a cell’s life have kept these nuclear bodies in the scientific spotlight for decades, yet how ND10 bodies dynamically shift the components to orchestrate a specific function is still not well understood. Recent studies on both the virology and cancer biology fronts have implied that ND10 may play dual roles and serve as a frenemy in establishing the viral replication and tumor malignancy [22][23], which have attracted further interest in the complex functions of ND10.
Theoretically, viruses depend on the host cell machineries to complete their life cycles, so they must have developed delicate means to differentiate friends from foes in the host. ND10 nuclear bodies, with the diverse array of positive and negative gene regulators enriched in the loci, are known to mount defenses to downregulate viral expression, but they also contain abundant gene activators waiting to be harnessed by the virus. The aggregation of both beneficial and inimical factors within ND10 provides us a unique opportunity to understand the intimate frenemy relation of a host to the virus, which is the molecular foundation for viral infection and pathogenesis.
2. ND10 Composition and Biogenesis
As mentioned earlier, the ever-growing list of functionally diverse proteins associated with ND10 has implied promiscuous functions for these nuclear bodies. The constant ND10 constituents are likely the leading factors that organize the various functions. Here we briefly introduce the few well-known ND10 constituents most relevant to many virology studies discussed in this review.
PML, also known as TRIM19, is a member of the tripartite motif (TRIM)-containing protein family that serves as the ND10 organizer. The conserved TRIM located in the N-terminus of PML, also known as the RING-BOX-Coiled Coil (RBCC) domain [24], mediates the PML oligomerization and is the foundation for ND10 formation [25]. PML has at least seven isoforms generated by alternative splicing of a single gene. All PML isoforms share the RBCC containing N-terminus but vary in their unique C-termini [24]. While all PML isoforms can be induced by an IFN treatment [26], the expression profile of these isoforms is not uniform, with PML I and II at levels higher than the others [27]. All isoforms except PML VII contain a conserved nuclear localization signal (NLS) and work as nuclear proteins. PML is post-translationally modified by small ubiquitin-like modifier (SUMO) on several lysine residues in a cell-cycle-dependent manner [28]. It also has a SUMO interaction motif (SIM) that allows the protein to interact with SUMOylated partners. The SUMO–SIM interactions among ND10 components are the main driving forces that mediate the protein networking within ND10 [2][29]. Since the discovery of PML as a tumor suppressor, many important cellular functions have been associated with this protein. Among them, PML is known to be involved in cell cycle regulation, senescence, apoptosis, regulation of angiogenesis, regulation of mRNA translation, chromatin remodeling, and antiviral defenses [21][30][31][32]. Lately, increasing evidence has shown differential functions for the individual PML isoforms. For example, in the gene locus of major histocompatibility class (MHC) I, PML interacts with SATB1 (specific AT-rich binding protein 1), a protein involved in the organization of higher-order chromatin architecture through loop formation. Knockdown of individual PML isoforms differentially changes the chromatin loop formation and subsequently leads to activation, suppression, or no change in the expression of genes located in the MHC I locus [33] (for details on the differential functions of PML isoforms, see Reference [34]).
Members of the speckled protein (SP) family are another group of dominant residents in the ND10 bodies [35][36], including Sp100, Sp110, and Sp140, all of which have different isoforms resulting from alternative splicing [37]. Similar to PML, SPs are IFN-stimulated genes (ISGs), function in the nucleus, and can be modified by SUMOylation for their recruitment to ND10 [5][38][39][40]. Additional functional domains of SPs include a SAND domain that binds to DNA, a plant homeodomain (PHD) that recognizes histone methylation, and a bromodomain (BRD) that reacts to histone acetylation [37]. Because of their ability to interact with DNA and the modified histones, SPs are known as chromatin readers in gene transcriptional regulation and are believed to participate mainly in immune response. For example, patients with primary biliary cholangitis have autoimmune antibodies against Sp100 in their sera [41], whereas loss-of-function mutations of Sp110 are linked to immunodeficiency [42]. SPs likely regulate the immune response through chromatin remodeling, but the exact mechanisms are still elusive. Similar to PML isoforms, SP isoforms also have differential functions in immune regulation, inasmuch as Sp110 B and C are found to suppress the NF-κB responsive promoters, while Sp110 A can enhance them [43].
Another classical component of ND10 is the death domain associated protein (Daxx) [44]. Daxx has two SIM domains located in the N- and C-termini that mediate its interaction with SUMOylated proteins such as PML [45][46]. Besides the full-length Daxx, two C-terminal alternatively spliced isoforms, Daxx-β and Daxx-γ, exist that are less localized to the ND10 bodies due to the loss of the C-terminal SIM [47][48]. Daxx is a well-known H3.3 histone chaperone that interacts with the histones H3.3/H4 dimer through its histone binding domain (HBD) [49][50]. In its central region, Daxx has a four-helix bundle (4HB) that allows the protein to interact with many partners such as transcription factors, chromatin remodelers, and histone modifiers [51]. One of these partners is the chromatin remodeler alpha-thalassemia/mental retardation X-linked syndrome protein (ATRX) [52]. The Daxx/ATRX complex can deposit H3.3 into the heterochromatin regions and participate in the regulation of apoptosis and DNA damage response [51][53]. Dysregulation of Daxx/ATRX has been observed in many types of cancer (for a detailed review, see Reference [51]).
PML plays an essential structural role in the formation of ND10 and is therefore regarded as the ND10 organizer or ND10 scaffold protein [2]. In mouse PML-/- embryonic fibroblasts, Sp100 is found dispersed throughout the nucleus, while Daxx aggregates at condensed chromatin that is morphologically different from the ND10 structures [45]. However, in human PML-/- epithelial cells, Xu et al. found that Sp100 and Daxx colocalized with each other but in condensates that did not resemble ND10 in either size or number [22], suggesting that the ND10 formation and ND10 dynamics vary in different species or types of cells. Further analyses have shown that for ND10 to take shape, PML molecules first form an outer shell via the self-oligomerization mediated by the RBCC domain [25][40] and then strengthen the PML–PML intermolecular interactions through SUMO–SIM interactions [54]. The secondary enhancement of SUMO–SIM interactions between the PML proteins is dispensable in the nucleation of ND10 formation because a PML mutant lacking the SUMO/SIM sites is still able to form the spherical shell of ND10 [40]. However, SUMO–SIM interactions are required for the recruitment of other components to the inner core of ND10 [40][55]. The recent discovery of multivalent SUMO–SIM interactions as a driving force for the formation of membraneless condensates via liquid-liquid phase separation (LLPS) [56][57] has triggered a hypothesis that ND10 bodies most likely form through LLPS [13][56][58]. Since the SUMO–SIM interactions are more important for the inner core of ND10 rather than the outer shell, the biogenesis of ND10 may be more complicated than can be explained by LLPS. Interestingly, many viral proteins such as ICP0 of HSV-1 [59], IE1 of HCMV [60], BZLF1 of EBV [61], and Rta of KHSV [62], all of which target ND10 to counteract the suppression on viral genome, are SUMOylated or contain SIMs and rely on the SUMO–SIM interactions to be recruited to ND10.
3. Brief Overview of the Dual Role of ND10 in Tumorigenesis
The lack of functional PML and the abnormality of ND10 have been found not only in hematologic cancers but also in the various solid tumors including breast, colorectal, prostate, lung, and bladder cancers [63][64][65], suggesting the critical role of ND10 in tumor suppression. Much of the tumor suppression activity of ND10 is mediated through the numerous permanent and transient components of ND10 that regulate the cell cycle, DNA damage response, apoptosis, and senescence [1][17][18][19]. For example, the aforementioned Daxx protein promotes apoptosis and suppresses cell proliferation by repressing the expression of anti-apoptotic genes including Bcl2 and survivin through enhancing the heterochromatin marks on their promoters [51][66]. Furthermore, the first-ever identified tumor suppressor, p53, and the p53 modifiers such as the p53 activating kinase HIPK2, the p53 E3 ubiquitin ligase MDM2, and the p53 deacetylase SIRT1 are transient components recruited to ND10 upon specific inductions [67][68][69], suggesting that the critical role of p53 in apoptosis and cell cycle checkpoint is regulated through cross-talking with ND10. For example, upon DNA damage, ND10 nuclear bodies sequestrate MDM2 and prevent the MDM2-mediated degradation of p53. Consequently, the upregulation of p53 mediated by ND10 promotes apoptosis and inhibits cell proliferation [69][70]. Additional ND10 components associated with the heterochromatin formation and cell senescence regulation, such as retinoblastoma protein (pRb) and HIRA, are also known to inhibit cell proliferation by inducing senescence and further underpin the tumor suppression role of the ND10 nuclear bodies [71][72].
Although the complete or partial loss of functional PML is a hallmark for various tumors [63][65], higher expression of PML has also been reported in tumors such as ovarian carcinoma and triple-negative breast cancer (TNBC) [23][73]. Interestingly, recent results showed that PML knockdown in cultured cells derived from these tumors induced apoptosis in the ovarian carcinoma cells and senescence response in the TNBC cells and thereby inhibited the cell proliferation in tissue culture or mouse xenograft [23][73], suggesting the pro-tumor roles of PML and ND10 in these particular cancers. Further investigation in the TNBC cells demonstrated that arsenic treatment, which triggers PML degradation [74], did not elicit the senescence response like that of PML knockdown [23], indicating that distinctive methods of removing PML can lead to differential cellular response, something analogous to what has been shown in the HSV-1 studies, which are discussed in the next section. More complex pro-cancer functions of PML have been found in the maintenance of leukemia stem cells, neuroblast migration, and TNBC metastasis [75][76][77]. Seemingly, the diverse ND10 components may regulate the different aspects of tumorigenesis in a cancer-type-dependent manner.
As in cancer research, ND10 nuclear bodies are traditionally considered as antiviral restrictions, in which the main components such as PML, Sp100, and the Daxx/ATRX complex are involved in inhibiting viral DNA transcription/replication via IFN response or chromatin repression. However, the fact that viral gene expression starts with the viral DNA located at ND10 [78] has long raised the suspicion that ND10 nuclear bodies may also provide functions favorable to viral replication. Here we summarize recent literature about ND10 effects in human herpesviruses to delineate the dual functions of ND10 in the virus-host interactions.
4. Dual Role of ND10 in α-Herpesvirus Infection
4.1. HSV Lytic Infection
Herpes simplex viruses 1 and 2 are members of the Alphaherpesvirinae subfamily that establish lytic infection in epithelial cells and then the lifelong latency in neuronal cells. Although the prevalence and latency sites show some differences between HSV-1 and HSV-2, many of the molecular events found in HSV-1 can be recapitulated in HSV-2. As mentioned above, the antiviral functions of ND10 are first implied by the disappearance of ND10 in HSV-1 infection [4]. Most of the mechanistic studies of ND10 functions also come from understanding the relations between HSV-1 and ND10. First, HSV-1 DNA, upon entering the nucleus, is immediately found in the vicinity of ND10 loci in the high multiplicity of infection (MOI) [79]. However, results at low MOI showed that in newly infected cells at the edge of a developing plaque, components of ND10 relocated to the viral DNA entry site near the nuclear periphery [80][81]. The convergence of ND10 proteins towards the incoming DNA upon its entry reflects the host effort to immediately dampen the expression of invading DNA as part of the cell’s intrinsic antiviral defenses. Interestingly, nascent ICP0 (infected cell protein 0), an immediate-early (IE) protein of HSV-1, also colocalizes with ND10 upon its synthesis [82]. ICP0 has an E3 ubiquitin ligase activity, which recognizes PML and Sp100 and targets them for proteasomal degradation [83][84][85]. The loss of the ND10 organizer, PML, leads to the disruption of ND10 structures and the dispersal of ND10 components [84][85][86]. The ICP0-mediated ND10 destruction is a critical step in early HSV-1 infection, inasmuch as the replication of an ICP0-null virus or a recombinant virus with ICP0 incapable of ND10 colocalization is largely defective in viral growth at low MOI [87][88][89]. Moreover, Everett et al. showed that depletion of PML, Sp100, Daxx, or ATRX, individually or in combination, improved the growth of the ICP0-null virus [90][91][92][93], indicating that ND10 components are repressive to viral transcription/replication and ICP0 is a major viral counteraction that alleviates the ND10 restrictions.
Although ND10 nuclear bodies are viewed as a part of the cellular antiviral defense systems, a few recent studies have demonstrated a more complex side of ND10. For example, an independent PML knockdown assay conducted by Knipe and colleagues showed a decrease of viral yield for an ICP0-null virus derived from a different HSV-1 strain [94], contradictory to the results of Everett et al. as described above [90]. The contradiction may be the reflection of a strain-dependency in the ICP0–ND10 interaction, or more likely, experimental variations in the two different knockdown methods used in those reports, where the Everett group used shRNA and the Knipe group used a pool of four siRNAs. Both knockdown methods targeted all PML isoforms at the common N-terminus but by different sequences. It has been reported that different PML isoforms are known to be recognized and degraded by ICP0 via totally distinct biochemical mechanisms [83][95][96]. Gu and colleagues showed that in HSV-1 infected cells, a single SIM located at ICP0 residues 362-364 was essential for the degradation of PML isoforms II, IV, and VI. However, for the degradation of PML I, this SIM was found dispensable. Instead, PML I-interaction domains located within ICP0 residues 1–83 and 245–474 redundantly facilitate the interaction between ICP0 and PML and the degradation of PML I [95][96]. The obvious differential recognition of PML isoforms by ICP0 suggests that PML isoforms may play distinctive roles in HSV-1 infection. With that in mind, a plausible explanation that can reconcile these contradictory results from the Everett and Knipe reports is that PML isoforms may be targeted with different efficiency when the sequences and amounts of siRNA are different. This may further alter the balance in the protein networking of PML within ND10 and thereby lead to the opposite results.
Another study implying a promotive role of ND10 in HSV-1 replication comes from the Roizman group, in which a CRISPR/Cas knockout of the PML gene caused a significant reduction in the replication of the wild-type HSV-1 at low MOI [22][97]. Roizman and colleagues further characterized these PML-/- cells and found that Sp100 was less induced upon IFN treatment compared to the parental cells. ICP0 was also less produced and did not properly colocalize with the Sp100/Daxx aggregates, which resulted in an ineffective Sp100 degradation in the PML-/- cells [22]. These results have revealed an effector role of PML that mediates the IFN response in HSV-1 infection but also indicated the dependence of HSV-1 on PML for a more effective replication. PML isoform II has been shown to specifically interact with the transcription factors NFκB, IRF3, and STAT1 to promote the expression of IFNβ and ISGs [98], suggesting that different PML isoforms can mediate specific reactions in cellular response triggered by HSV-1 infection. Whether these reactions are intertwined with other regulatory pathways via the PML associated proteins in ND10 will need further investigation.
Studies on additional ND10 components have shown that ND10 functions in HSV-1 infection are associated with the chromatin remodeling of viral DNA. HSV-1 ejects naked DNA into the nucleus. The incoming DNA immediately encounters histone deposition to form a chromatin-like viral genome [99][100][101]. Cabral et al. showed that ATRX was required in the maintenance of viral chromatin stability but not involved in the initial viral chromatin formation [102]. In another study, HIRA (histone cell cycle regulator), a histone H3.3 chaperone, was found to be recruited to ND10 and bind to the HSV-1 genome to mediate the H3.3 deposition early in HSV-1 infection [103][104]. HIRA localization to ND10 is cell-type dependent [103][104]. Therefore, it is not yet clear whether the HIRA-dependent histone deposition and the ATRX-mediated chromatin maintenance are sequential events or whether they have any cooperativity in the early infection. A chromatin corepressor, CoREST, and its associated proteins are also recruited to ND10 in early HSV-1 infection [105]. In all of the above cases, chromatin formation and repressor association of the HSV-1 genome, which bring inhibitions to the viral gene expression, are regarded as parts of the host’s intrinsic antiviral defenses. The degradation of PML and the disruption of ND10 by ICP0 is considered a key viral countermeasure to release the chromatin repression imposed by ND10. Additional viral counteractions to release the ND10 restrictions include viral microRNAs that downregulate the ATRX expression [106]. An interesting phenomenon associated with the triangular relation of HSV DNA, ND10, and viral counteractions is that HSV-1 replication compartments evolve at the ND10 foci after the disruption of ND10 [79][107][108]. The observation that CoREST and CoREST-associated proteins are also found in the replication compartments [109] raises further questions for the roles of proteins recruited to ND10 during the HSV-1 infection. Is it possible that some of these proteins are recruited to ND10 in order to mediate some of the pro-viral functions of ND10 in the establishment of HSV-1 transcription/replication? An overview of ND10-HSV-1 interactions in the lytic cycle is depicted in Figure 1.
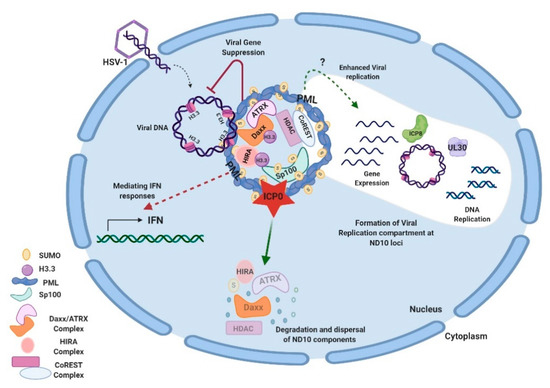
Figure 1. Interactions between HSV-1 and ND10 in the lytic cycle. HSV-1 injects its naked double-stranded DNA into the nucleus of the infected cell. ND10 components converge to the incoming DNA and impose suppression on the viral DNA. Specifically, Daxx/ATRX, and HIRA complexes deposit H3.3 histones on viral DNA to create the heterochromatin silencing. PML is an effector to mediate the IFN response in HSV-1 infection. Newly expressed ICP0 counteracts gene repression by degrading PML and Sp100 and dispersing ND10. Viral genes get expressed and replication compartments form at the ND10 loci after the ND10 dispersal. Viral proteins such as ICP8 (ssDNA binding protein) and UL30 (viral DNA polymerase) are typical markers for the replication compartments. The efficiency of viral replication can be reduced or enhanced by a PML depletion, depending on experimental conditions. Components of the CoREST complex are recruited to ND10 and reside in the viral replication compartments. This figure was created with BioRender.com.
4.2. HSV Latent Infection
Besides the lytic infection phase, HSV-1 and HSV-2 establish lifelong latent infection in ganglia neurons, which is the etiological cause of recurrent infections in HSV pathogenesis. Latent HSV infection can be experimentally reproduced in animals [110]. However, due to the limited availability of ganglia neurons and the infection heterogeneity in these neurons, ND10 functions in HSV latently infected animals are largely unclear. Much of the latency studies are performed with HSV-1 and remain descriptive. Fluorescent staining showed that in a latently infected ganglion, about half of the infected neurons contained one single spot of HSV-1 DNA enwrapped by the donut-shaped ND10 body, where the latency-associated transcript (LAT) was undetectable. The other half of the infected neurons in the same ganglion contained multiple spots of HSV-1 DNA, where at least one of the spots was colocalized with ND10, and the LAT expression spread out to overlap with all spots [111]. Although viral mRNA profiles in these heterogeneously infected neurons have not yet been reported, it is quite reasonable to postulate that viral gene expression will be different around the HSV genome within the distinct spot patterns. Whether and how ND10 nuclear bodies in these spot patterns play differential roles in HSV latency maintenance or latency reactivation remain to be seen.
HSV IE-deficient viruses can form quiescent infection in cultured fibroblasts, in which the viral genome persists in cells for a long period of time but the viral genes are tightly silenced. The quiescent genome does not respond to the general reactivation stimuli but can be reactivated by the introduction of IE proteins such as ICP0 or by the treatments that perturb heterochromatin. Because of the experimental simplicity and reproducibility in the quiescent infection it is considered an alternative model to the animal latency model and a good tool to mimic the molecular relationship between the latent HSV genome and ND10 [112]. With this system, Cohen et al. showed that the quiescent viral genome of HSV-1 was associated with two histone H3.3 chaperone complexes, Daxx/ATRX and HIRA, in multiple loci. They redundantly chromatinized all classes of viral genes including the LAT locus with the histone H3.3 modified by the repressive K9 trimethylation mark [113]. The heterochromatinization of LAT in this study is in accordance with the previous observation that a latent genome associated with an ND10 body in a single spot pattern silences the LAT expression in vivo [111]. However, how this quiescence is related to the heterogeneous spot pattern in mice remains unanswered. PML was shown to play an important role in the silencing of the quiescent genome. In its absence, the colocalization of Daxx/ATRX with the quiescent viral genome was reduced, and the H3.3 load on the multiple loci was significantly decreased [113]. The disruption of ND10 integrity in cells harboring the quiescent viral DNA by the ectopic expression of ICP0 or by the treatment of histone deacetylase (HDAC) inhibitors led to the reactivation of the quiescent genome [113][114]. These results confirmed the repressive function of ND10 in the quiescent infection. With the obvious lack of neuronal supportive cells and surrounding tissues, the quiescent infection in monolayer fibroblasts cannot completely recapitulate the HSV-1 latency. However, the results are still indicative although do not necessarily reflect the full picture. The newly developed technology of 3D organoids derived from iPSC (induced pluripotent stem cells) [115] may become a better model for the mechanistic studies of HSV-1 latency, and a comprehensive role of ND10 in the latent HSV-1 infection remains to be seen.
4.3. VZV
Varicella-zoster virus (VZV) is the third human α-herpesvirus that is restricted by ND10 [116]. Results showed that PML and Daxx knockdown increased virus production [116], whereas overexpression of PML isoform IV inhibited it [117]. ORF61, the VZV ortholog of ICP0, was capable of disrupting ND10 via the SIMs located in its N- and C-termini [118]. ORF61 of VZV does have a RING finger domain [119], but it does not mediate PML degradation [116]. This means HSV-1 and VZV use different mechanisms to overcome the ND10 restrictions. Interestingly, PML IV inhibited viral yield by forming a cage-like structure around the newly formed viral capsids, likely to prevent the nuclear egress process [117], reflecting a role for ND10 in virus assembly and maturation. The relationship between VZV and the other PML isoforms is not clear, whereas beneficial roles of ND10 have not been reported in VZV infection.
This entry is adapted from the peer-reviewed paper 10.3390/v13020239
References
- Lallemand-Breitenbach, V.; de Thé, H. PML nuclear bodies: From architecture to function. Curr. Opin. Cell Biol. 2018, 52, 154–161.
- Corpet, A.; Kleijwegt, C.; Roubille, S.; Juillard, F.; Jacquet, K.; Texier, P.; Lomonte, P. PML nuclear bodies and chromatin dynamics: Catch me if you can! Nucleic. Acids Res. 2020, 48, 11890–11912.
- Ascoli, C.A.; Maul, G.G. Identification of a novel nuclear domain. J. Cell Biol. 1991, 112, 785–795.
- Maul, G.G.; Guldner, H.H.; Spivack, J.G. Modification of discrete nuclear domains induced by herpes simplex virus type 1 immediate early gene 1 product (ICP0). J. Gen. Virol. 1993, 74, 2679–2690.
- Guldner, H.H.; Szostecki, C.; Grötzinger, T.; Will, H. IFN enhance expression of Sp100, an autoantigen in primary biliary cirrhosis. J. Immunol. 1992, 149, 4067–4073.
- Everett, R.D.; Chelbi-Alix, M.K. PML and PML nuclear bodies: Implications in antiviral defence. Biochimie 2007, 89, 819–830.
- Tavalai, N.; Stamminger, T. New insights into the role of the subnuclear structure ND10 for viral infection. Biochim. Biophys. Acta 2008, 1783, 2207–2221.
- Geoffroy, M.C.; Chelbi-Alix, M.K. Role of promyelocytic leukemia protein in host antiviral defense. J. Interferon Cytokine Res. 2011, 31, 145–158.
- de Thé, H.; Chomienne, C.; Lanotte, M.; Degos, L.; Dejean, A. The t(15;17) translocation of acute promyelocytic leukaemia fuses the retinoic acid receptor alpha gene to a novel transcribed locus. Nature 1990, 347, 558–561.
- Borrow, J.; Goddard, A.; Sheer, D.; Solomon, E. Molecular analysis of acute promyelocytic leukemia breakpoint cluster region on chromosome 17. Science 1990, 249, 1577–1580.
- Koken, M.H.; Puvion-Dutilleul, F.; Guillemin, M.C.; Viron, A.; Linares-Cruz, G.; Stuurman, N.; de Jong, L.; Szostecki, C.; Calvo, F.; Chomienne, C.; et al. The t(15;17) translocation alters a nuclear body in a retinoic acid-reversible fashion. EMBO J. 1994, 13, 1073–1083.
- Daniel, M.T.; Koken, M.; Romagné, O.; Barbey, S.; Bazarbachi, A.; Stadler, M.; Guillemin, M.C.; Degos, L.; Chomienne, C.; de Thé, H. PML protein expression in hematopoietic and acute promyelocytic leukemia cells. Blood 1993, 82, 1858–1867.
- Li, Y.; Ma, X.; Wu, W.; Chen, Z.; Meng, G. PML Nuclear Body Biogenesis, Carcinogenesis, and Targeted Therapy. Trends Cancer 2020, 6, 889–906.
- Salomoni, P.; Dvorkina, M.; Michod, D. Role of the promyelocytic leukaemia protein in cell death regulation. Cell Death Dis. 2012, 3, e247.
- Guan, D.; Kao, H.Y. The function, regulation and therapeutic implications of the tumor suppressor protein, PML. Cell Biosci. 2015, 5, 60.
- Bernardi, R.; Papa, A.; Pandolfi, P.P. Regulation of apoptosis by PML and the PML-NBs. Oncogene 2008, 27, 6299–6312.
- Sahin, U.; Lallemand-Breitenbach, V.; de Thé, H. PML nuclear bodies: Regulation, function and therapeutic perspectives. J. Pathol. 2014, 234, 289–291.
- Chang, H.R.; Munkhjargal, A.; Kim, M.-J.; Park, S.Y.; Jung, E.; Ryu, J.-H.; Yang, Y.; Lim, J.-S.; Kim, Y. The functional roles of PML nuclear bodies in genome maintenance. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 2018, 809, 99–107.
- Gu, H.; Zheng, Y. Role of ND10 nuclear bodies in the chromatin repression of HSV-1. Virol. J. 2016, 13, 62.
- Van Damme, E.; Laukens, K.; Dang, T.H.; Van Ostade, X. A manually curated network of the PML nuclear body interactome reveals an important role for PML-NBs in SUMOylation dynamics. Int. J. Biol. Sci. 2010, 6, 51–67.
- Pearson, M.; Pelicci, P.G. PML interaction with p53 and its role in apoptosis and replicative senescence. Oncogene 2001, 20, 7250–7256.
- Xu, P.; Mallon, S.; Roizman, B. PML plays both inimical and beneficial roles in HSV-1 replication. Proc. Natl. Acad. Sci. USA 2016, 113, e3022–e3028.
- Arreal, L.; Piva, M.; Fernández, S.; Revandkar, A.; Schaub-Clerigué, A.; Villanueva, J.; Zabala-Letona, A.; Pujana, M.; Astobiza, I.; Cortazar, A.R.; et al. Targeting PML in triple negative breast cancer elicits growth suppression and senescence. Cell Death Differ. 2020, 27, 1186–1199.
- Nisole, S.; Stoye, J.P.; Saïb, A. TRIM family proteins: Retroviral restriction and antiviral defence. Nat. Rev. Microbiol. 2005, 3, 799–808.
- Li, Y.; Ma, X.; Chen, Z.; Wu, H.; Wang, P.; Wu, W.; Cheng, N.; Zeng, L.; Zhang, H.; Cai, X.; et al. B1 oligomerization regulates PML nuclear body biogenesis and leukemogenesis. Nat. Commun. 2019, 10, 3789.
- Grötzinger, T.; Sternsdorf, T.; Jensen, K.; Will, H. Interferon-modulated expression of genes encoding the nuclear-dot-associated proteins Sp100 and promyelocytic leukemia protein (PML). Eur. J. Biochem. 1996, 238, 554–560.
- Condemine, W.; Takahashi, Y.; Zhu, J.; Puvion-Dutilleul, F.; Guegan, S.; Janin, A.; de Thé, H. Characterization of Endogenous Human Promyelocytic Leukemia Isoforms. Cancer Res. 2006, 66, 6192–6198.
- Everett, R.D.; Lomonte, P.; Sternsdorf, T.; van Driel, R.; Orr, A. Cell cycle regulation of PML modification and ND10 composition. J. Cell Sci. 1999, 112, 4581–4588.
- Bernardi, R.; Pandolfi, P.P. Structure, dynamics and functions of promyelocytic leukaemia nuclear bodies. Nat. Rev. Mol. Cell Biol. 2007, 8, 1006–1016.
- Salomoni, P.; Pandolfi, P.P. The Role of PML in Tumor Suppression. Cell 2002, 108, 165–170.
- Bernardi, R.; Guernah, I.; Jin, D.; Grisendi, S.; Alimonti, A.; Teruya-Feldstein, J.; Cordon-Cardo, C.; Simon, M.C.; Rafii, S.; Pandolfi, P.P. PML inhibits HIF-1alpha translation and neoangiogenesis through repression of mTOR. Nature 2006, 442, 779–785.
- Hsu, K.-S.; Kao, H.-Y. PML: Regulation and multifaceted function beyond tumor suppression. Cell Biosci. 2018, 8, 5.
- Kumar, P.P.; Bischof, O.; Purbey, P.K.; Notani, D.; Urlaub, H.; Dejean, A.; Galande, S. Functional interaction between PML and SATB1 regulates chromatin-loop architecture and transcription of the MHC class I locus. Nat. Cell Biol. 2007, 9, 45–56.
- Nisole, S.; Maroui, M.A.; Mascle, X.H.; Aubry, M.; Chelbi-Alix, M.K. Differential Roles of PML Isoforms. Front. Oncol. 2013, 3, 125.
- Szostecki, C.; Guldner, H.H.; Netter, H.J.; Will, H. Isolation and characterization of cDNA encoding a human nuclear antigen predominantly recognized by autoantibodies from patients with primary biliary cirrhosis. J. Immunol. 1990, 145, 4338–4347.
- Bloch, D.B.; Nakajima, A.; Gulick, T.; Chiche, J.D.; Orth, D.; de La Monte, S.M.; Bloch, K.D. Sp110 localizes to the PML-Sp100 nuclear body and may function as a nuclear hormone receptor transcriptional coactivator. Mol. Cell. Biol. 2000, 20, 6138–6146.
- Fraschilla, I.; Jeffrey, K.L. The Speckled Protein (SP) Family: Immunity’s Chromatin Readers. Trends Immunol. 2020, 41, 572–585.
- Sternsdorf, T.; Jensen, K.; Will, H. Evidence for Covalent Modification of the Nuclear Dot–associated Proteins PML and Sp100 by PIC1/SUMO-1. J. Cell Biol. 1997, 139, 1621–1634.
- Seeler, J.-S.; Marchio, A.; Losson, R.; Desterro, J.M.P.; Hay, R.T.; Chambon, P.; Dejean, A. Common Properties of Nuclear Body Protein SP100 and TIF1α Chromatin Factor: Role of SUMO Modification. Mol. Cell. Biol. 2001, 21, 3314–3324.
- Sahin, U.; Ferhi, O.; Jeanne, M.; Benhenda, S.; Berthier, C.; Jollivet, F.; Niwa-Kawakita, M.; Faklaris, O.; Setterblad, N.; de Thé, H.; et al. Oxidative stress-induced assembly of PML nuclear bodies controls sumoylation of partner proteins. J. Cell Biol. 2014, 204, 931–945.
- Szostecki, C.; Krippner, H.; Penner, E.; Bautz, F.A. Autoimmune sera recognize a 100 kD nuclear protein antigen (sp-100). Clin. Exp. Immunol. 1987, 68, 108–116.
- Roscioli, T.; Cliffe, S.T.; Bloch, D.B.; Bell, C.G.; Mullan, G.; Taylor, P.J.; Sarris, M.; Wang, J.; Donald, J.A.; Kirk, E.P.; et al. Mutations in the gene encoding the PML nuclear body protein Sp110 are associated with immunodeficiency and hepatic veno-occlusive disease. Nat. Genet. 2006, 38, 620–622.
- Leu, J.-S.; Chang, S.-Y.; Mu, C.-Y.; Chen, M.-L.; Yan, B.-S. Functional domains of SP110 that modulate its transcriptional regulatory function and cellular translocation. J. Biomed. Sci. 2018, 25, 34.
- Torii, S.; Egan, D.A.; Evans, R.A.; Reed, J.C. Human Daxx regulates Fas-induced apoptosis from nuclear PML oncogenic domains (PODs). Embo. J. 1999, 18, 6037–6049.
- Ishov, A.M.; Sotnikov, A.G.; Negorev, D.; Vladimirova, O.V.; Neff, N.; Kamitani, T.; Yeh, E.T.; Strauss, J.F., 3rd; Maul, G.G. PML is critical for ND10 formation and recruits the PML-interacting protein daxx to this nuclear structure when modified by SUMO-1. J. Cell Biol. 1999, 147, 221–234.
- Santiago, A.; Godsey, A.C.; Hossain, J.; Zhao, L.Y.; Liao, D. Identification of two independent SUMO-interacting motifs in Daxx: Evolutionary conservation from Drosophila to humans and their biochemical functions. Cell Cycle 2009, 8, 76–87.
- Lin, D.Y.; Huang, Y.S.; Jeng, J.C.; Kuo, H.Y.; Chang, C.C.; Chao, T.T.; Ho, C.C.; Chen, Y.C.; Lin, T.P.; Fang, H.I.; et al. Role of SUMO-interacting motif in Daxx SUMO modification, subnuclear localization, and repression of sumoylated transcription factors. Mol. Cell 2006, 24, 341–354.
- Wethkamp, N.; Hanenberg, H.; Funke, S.; Suschek, C.V.; Wetzel, W.; Heikaus, S.; Grinstein, E.; Ramp, U.; Engers, R.; Gabbert, H.E.; et al. Daxx-beta and Daxx-gamma, two novel splice variants of the transcriptional co-repressor Daxx. J. Biol. Chem. 2011, 286, 19576–19588.
- Elsässer, S.J.; Huang, H.; Lewis, P.W.; Chin, J.W.; Allis, C.D.; Patel, D.J. DAXX envelops a histone H3.3-H4 dimer for H3.3-specific recognition. Nature 2012, 491, 560–565.
- Lewis, P.W.; Elsaesser, S.J.; Noh, K.-M.; Stadler, S.C.; Allis, C.D. Daxx is an H3.3-specific histone chaperone and cooperates with ATRX in replication-independent chromatin assembly at telomeres. Proc. Natl. Acad. Sci. USA 2010, 107, 14075–14080.
- Mahmud, I.; Liao, D. DAXX in cancer: Phenomena, processes, mechanisms and regulation. Nucleic. Acids Res. 2019, 47, 7734–7752.
- Tang, J.; Wu, S.; Liu, H.; Stratt, R.; Barak, O.G.; Shiekhattar, R.; Picketts, D.J.; Yang, X. A Novel Transcription Regulatory Complex Containing Death Domain-associated Protein and the ATR-X Syndrome Protein. J. Biol. Chem. 2004, 279, 20369–20377.
- Zink, L.M.; Delbarre, E.; Eberl, H.C.; Keilhauer, E.C.; Bönisch, C.; Pünzeler, S.; Bartkuhn, M.; Collas, P.; Mann, M.; Hake, S.B. H3.Y discriminates between HIRA and DAXX chaperone complexes and reveals unexpected insights into human DAXX-H3.3-H4 binding and deposition requirements. Nucleic. Acids Res. 2017, 45, 5691–5706.
- Wang, P.; Benhenda, S.; Wu, H.; Lallemand-Breitenbach, V.; Zhen, T.; Jollivet, F.; Peres, L.; Li, Y.; Chen, S.-J.; Chen, Z.; et al. RING tetramerization is required for nuclear body biogenesis and PML sumoylation. Nat. Commun. 2018, 9, 1277.
- Sahin, U.; de Thé, H.; Lallemand-Breitenbach, V. PML nuclear bodies: Assembly and oxidative stress-sensitive sumoylation. Nucleus 2014, 5, 499–507.
- Banani, S.F.; Rice, A.M.; Peeples, W.B.; Lin, Y.; Jain, S.; Parker, R.; Rosen, M.K. Compositional Control of Phase-Separated Cellular Bodies. Cell 2016, 166, 651–663.
- Zhang, H.; Zhao, R.; Tones, J.; Liu, M.; Dilley, R.L.; Chenoweth, D.M.; Greenberg, R.A.; Lampson, M.A. Nuclear body phase separation drives telomere clustering in ALT cancer cells. Mol. Biol. Cell 2020, 31, 2048–2056.
- Mediani, L.; Guillén-Boixet, J.; Alberti, S.; Carra, S. Nucleoli and Promyelocytic Leukemia Protein (PML) bodies are phase separated nuclear protein quality control compartments for misfolded proteins. Mol. Cell Oncol. 2019, 6, e1415624.
- Everett, R.D.; Boutell, C.; Pheasant, K.; Cuchet-Lourenço, D.; Orr, A. Sequences related to SUMO interaction motifs in herpes simplex virus 1 protein ICP0 act cooperatively to stimulate virus infection. J. Virol. 2014, 88, 2763–2774.
- Paulus, C.; Harwardt, T.; Walter, B.; Marxreiter, A.; Zenger, M.; Reuschel, E.; Nevels, M.M. Revisiting promyelocytic leukemia protein targeting by human cytomegalovirus immediate-early protein 1. PLoS Pathog. 2020, 16, e1008537.
- Adamson, A.L.; Kenney, S. Epstein-barr virus immediate-early protein BZLF1 is SUMO-1 modified and disrupts promyelocytic leukemia bodies. J. Virol. 2001, 75, 2388–2399.
- Izumiya, Y.; Kobayashi, K.; Kim, K.Y.; Pochampalli, M.; Izumiya, C.; Shevchenko, B.; Wang, D.H.; Huerta, S.B.; Martinez, A.; Campbell, M.; et al. Kaposi’s sarcoma-associated herpesvirus K-Rta exhibits SUMO-targeting ubiquitin ligase (STUbL) like activity and is essential for viral reactivation. PLoS Pathog. 2013, 9, e1003506.
- Gurrieri, C.; Capodieci, P.; Bernardi, R.; Scaglioni, P.P.; Nafa, K.; Rush, L.J.; Verbel, D.A.; Cordon-Cardo, C.; Pandolfi, P.P. Loss of the Tumor Suppressor PML in Human Cancers of Multiple Histologic Origins. J. Natl. Cancer Inst. 2004, 96, 269–279.
- Mazza, M.; Pelicci, P.G. Is PML a Tumor Suppressor? Front. Oncol. 2013, 3, 174.
- Reineke, E.L.; Lam, M.; Liu, Q.; Liu, Y.; Stanya, K.J.; Chang, K.S.; Means, A.R.; Kao, H.Y. Degradation of the tumor suppressor PML by Pin1 contributes to the cancer phenotype of breast cancer MDA-MB-231 cells. Mol. Cell Biol. 2008, 28, 997–1006.
- Rapkin, L.M.; Ahmed, K.; Dulev, S.; Li, R.; Kimura, H.; Ishov, A.M.; Bazett-Jones, D.P. The histone chaperone DAXX maintains the structural organization of heterochromatin domains. Epigenetics Chromatin. 2015, 8, 44.
- Crawford, L.V.; Pim, D.C.; Gurney, E.G.; Goodfellow, P.; Taylor-Papadimitriou, J. Detection of a common feature in several human tumor cell lines—A 53,000-dalton protein. Proc. Natl. Acad. Sci. USA 1981, 78, 41–45.
- Matt, S.; Hofmann, T.G. Crosstalk between p53 modifiers at PML bodies. Mol. Cell Oncol. 2018, 5, e1074335.
- Bernardi, R.; Scaglioni, P.P.; Bergmann, S.; Horn, H.F.; Vousden, K.H.; Pandolfi, P.P. PML regulates p53 stability by sequestering Mdm2 to the nucleolus. Nat. Cell Biol. 2004, 6, 665–672.
- Kurki, S.; Latonen, L.; Laiho, M. Cellular stress and DNA damage invoke temporally distinct Mdm2, p53 and PML complexes and damage-specific nuclear relocalization. J. Cell Sci. 2003, 116, 3917–3925.
- Rai, T.S.; Cole, J.J.; Nelson, D.M.; Dikovskaya, D.; Faller, W.J.; Vizioli, M.G.; Hewitt, R.N.; Anannya, O.; McBryan, T.; Manoharan, I.; et al. HIRA orchestrates a dynamic chromatin landscape in senescence and is required for suppression of neoplasia. Genes Dev. 2014, 28, 2712–2725.
- Ye, X.; Zerlanko, B.; Zhang, R.; Somaiah, N.; Lipinski, M.; Salomoni, P.; Adams, P.D. Definition of pRB- and p53-dependent and -independent steps in HIRA/ASF1a-mediated formation of senescence-associated heterochromatin foci. Mol. Cell Biol. 2007, 27, 2452–2465.
- Liu, S.B.; Shen, Z.F.; Guo, Y.J.; Cao, L.X.; Xu, Y. PML silencing inhibits cell proliferation and induces DNA damage in cultured ovarian cancer cells. Biomed. Rep. 2017, 7, 29–35.
- Chen, G.Q.; Zhu, J.; Shi, X.G.; Ni, J.H.; Zhong, H.J.; Si, G.Y.; Jin, X.L.; Tang, W.; Li, X.S.; Xong, S.M.; et al. In Vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-RAR alpha/PML proteins. Blood 1996, 88, 1052–1061.
- Ito, K.; Bernardi, R.; Morotti, A.; Matsuoka, S.; Saglio, G.; Ikeda, Y.; Rosenblatt, J.; Avigan, D.E.; Teruya-Feldstein, J.; Pandolfi, P.P. PML targeting eradicates quiescent leukaemia-initiating cells. Nature 2008, 453, 1072–1078.
- Amodeo, V.; Deli, A.; Betts, J.; Bartesaghi, S.; Zhang, Y.; Richard-Londt, A.; Ellis, M.; Roshani, R.; Vouri, M.; Galavotti, S.; et al. A PML/Slit Axis Controls Physiological Cell Migration and Cancer Invasion in the CNS. Cell Rep. 2017, 20, 411–426.
- Ponente, M.; Campanini, L.; Cuttano, R.; Piunti, A.; Delledonne, G.A.; Coltella, N.; Valsecchi, R.; Villa, A.; Cavallaro, U.; Pattini, L.; et al. PML promotes metastasis of triple-negative breast cancer through transcriptional regulation of HIF1A target genes. JCI Insight 2017, 2, e87380.
- Maul, G.G. Nuclear domain 10, the site of DNA virus transcription and replication. BioEssays 1998, 20, 660–667.
- Maul, G.G.; Ishov, A.M.; Everett, R.D. Nuclear Domain 10 as Preexisting Potential Replication Start Sites of Herpes Simplex Virus Type-1. Virology 1996, 217, 67–75.
- Everett, R.D.; Murray, J. ND10 components relocate to sites associated with herpes simplex virus type 1 nucleoprotein complexes during virus infection. J. Virol. 2005, 79, 5078–5089.
- Alandijany, T.; Roberts, A.P.E.; Conn, K.L.; Loney, C.; McFarlane, S.; Orr, A.; Boutell, C. Distinct temporal roles for the promyelocytic leukaemia (PML) protein in the sequential regulation of intracellular host immunity to HSV-1 infection. PLoS Pathog. 2018, 14, e1006769.
- Maul, G.G.; Everett, R.D. The nuclear location of PML, a cellular member of the C3HC4 zinc-binding domain protein family, is rearranged during herpes simplex virus infection by the C3HC4 viral protein ICP0. J. Gen. Virol. 1994, 75, 1223–1233.
- Boutell, C.; Cuchet-Lourenço, D.; Vanni, E.; Orr, A.; Glass, M.; McFarlane, S.; Everett, R.D. A viral ubiquitin ligase has substrate preferential SUMO targeted ubiquitin ligase activity that counteracts intrinsic antiviral defence. PLoS Pathog. 2011, 7, e1002245.
- Müller, S.; Dejean, A. Viral immediate-early proteins abrogate the modification by SUMO-1 of PML and Sp100 proteins, correlating with nuclear body disruption. J. Virol. 1999, 73, 5137–5143.
- Chelbi-Alix, M.K.; de Thé, H. Herpes virus induced proteasome-dependent degradation of the nuclear bodies-associated PML and Sp100 proteins. Oncogene 1999, 18, 935–941.
- Everett, R.D.; Freemont, P.; Saitoh, H.; Dasso, M.; Orr, A.; Kathoria, M.; Parkinson, J. The disruption of ND10 during herpes simplex virus infection correlates with the Vmw110- and proteasome-dependent loss of several PML isoforms. J. Virol. 1998, 72, 6581–6591.
- Lium, E.K.; Silverstein, S. Mutational analysis of the herpes simplex virus type 1 ICP0 C3HC4 zinc ring finger reveals a requirement for ICP0 in the expression of the essential alpha27 gene. J. Virol. 1997, 71, 8602–8614.
- Gu, H.; Zheng, Y.; Roizman, B. Interaction of herpes simplex virus ICP0 with ND10 bodies: A sequential process of adhesion, fusion, and retention. J. Virol. 2013, 87, 10244–10254.
- Stow, N.D.; Stow, E.C. Isolation and characterization of a herpes simplex virus type 1 mutant containing a deletion within the gene encoding the immediate early polypeptide Vmw110. J. Gen. Virol 1986, 67, 2571–2585.
- Everett, R.D.; Parada, C.; Gripon, P.; Sirma, H.; Orr, A. Replication of ICP0-null mutant herpes simplex virus type 1 is restricted by both PML and Sp100. J. Virol. 2008, 82, 2661–2672.
- Lukashchuk, V.; Everett, R.D. Regulation of ICP0-null mutant herpes simplex virus type 1 infection by ND10 components ATRX and hDaxx. J. Virol. 2010, 84, 4026–4040.
- Glass, M.; Everett, R.D. Components of Promyelocytic Leukemia Nuclear Bodies (ND10) Act Cooperatively To Repress Herpesvirus Infection. J. Virol. 2013, 87, 2174–2185.
- Everett, R.D.; Rechter, S.; Papior, P.; Tavalai, N.; Stamminger, T.; Orr, A. PML contributes to a cellular mechanism of repression of herpes simplex virus type 1 infection that is inactivated by ICP0. J. Virol. 2006, 80, 7995–8005.
- Merkl, P.E.; Orzalli, M.H.; Knipe, D.M. Mechanisms of Host IFI16, PML, and Daxx Protein Restriction of Herpes Simplex Virus 1 Replication. J. Virol. 2018, 92.
- Zheng, Y.; Samrat, S.K.; Gu, H. A Tale of Two PMLs: Elements Regulating a Differential Substrate Recognition by the ICP0 E3 Ubiquitin Ligase of Herpes Simplex Virus 1. J. Virol. 2016, 90, 10875–10885.
- Jan Fada, B.; Kaadi, E.; Samrat, S.K.; Zheng, Y.; Gu, H. Effect of SUMO-SIM Interaction on the ICP0-Mediated Degradation of PML Isoform II and Its Associated Proteins in Herpes Simplex Virus 1 Infection. J. Virol 2020, 94.
- Xu, P.; Roizman, B. The SP100 component of ND10 enhances accumulation of PML and suppresses replication and the assembly of HSV replication compartments. Proc. Natl. Acad. Sci. USA 2017, 114, e3823–e3829.
- Chen, Y.; Wright, J.; Meng, X.; Leppard, K.N. Promyelocytic Leukemia Protein Isoform II Promotes Transcription Factor Recruitment To Activate Interferon Beta and Interferon-Responsive Gene Expression. Mol. Cell Biol. 2015, 35, 1660–1672.
- Cereghini, S.; Yaniv, M. Assembly of transfected DNA into chromatin: Structural changes in the origin-promoter-enhancer region upon replication. Embo. J. 1984, 3, 1243–1253.
- Kent, J.R.; Zeng, P.Y.; Atanasiu, D.; Gardner, J.; Fraser, N.W.; Berger, S.L. During lytic infection herpes simplex virus type 1 is associated with histones bearing modifications that correlate with active transcription. J. Virol. 2004, 78, 10178–10186.
- Knipe, D.M.; Cliffe, A. Chromatin control of herpes simplex virus lytic and latent infection. Nat. Rev. Microbiol. 2008, 6, 211–221.
- Cabral, J.M.; Oh, H.S.; Knipe, D.M. ATRX promotes maintenance of herpes simplex virus heterochromatin during chromatin stress. Elife 2018, 7.
- McFarlane, S.; Orr, A.; Roberts, A.P.E.; Conn, K.L.; Iliev, V.; Loney, C.; da Silva Filipe, A.; Smollett, K.; Gu, Q.; Robertson, N.; et al. The histone chaperone HIRA promotes the induction of host innate immune defences in response to HSV-1 infection. PLoS Pathog. 2019, 15, e1007667.
- Rai, T.S.; Glass, M.; Cole, J.J.; Rather, M.I.; Marsden, M.; Neilson, M.; Brock, C.; Humphreys, I.R.; Everett, R.D.; Adams, P.D. Histone chaperone HIRA deposits histone H3.3 onto foreign viral DNA and contributes to anti-viral intrinsic immunity. Nucleic. Acids Res. 2017, 45, 11673–11683.
- Gu, H.; Liang, Y.; Mandel, G.; Roizman, B. Components of the REST/CoREST/histone deacetylase repressor complex are disrupted, modified, and translocated in HSV-1-infected cells. Proc. Natl. Acad. Sci. USA 2005, 102, 7571–7576.
- Jurak, I.; Silverstein, L.B.; Sharma, M.; Coen, D.M. Herpes simplex virus is equipped with RNA- and protein-based mechanisms to repress expression of ATRX, an effector of intrinsic immunity. J. Virol. 2012, 86, 10093–10102.
- Burkham, J.; Coen, D.M.; Weller, S.K. ND10 protein PML is recruited to herpes simplex virus type 1 prereplicative sites and replication compartments in the presence of viral DNA polymerase. J. Virol. 1998, 72, 10100–10107.
- Sourvinos, G.; Everett, R.D. Visualization of parental HSV-1 genomes and replication compartments in association with ND10 in live infected cells. Embo. J. 2002, 21, 4989–4997.
- Gu, H.; Roizman, B. Engagement of the lysine-specific demethylase/HDAC1/CoREST/REST complex by herpes simplex virus 1. J. Virol. 2009, 83, 4376–4385.
- Preston, C.M.; Efstathiou, S. Molecular Basis of HSV Latency and Reactivation. In Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis; Arvin, A., Campadelli-Fiume, G., Mocarski, E., Moore, P.S., Roizman, B., Whitley, R., Yamanishi, K., Eds.; Cambridge University Press: Cambridge, UK, 2007.
- Catez, F.; Picard, C.; Held, K.; Gross, S.; Rousseau, A.; Theil, D.; Sawtell, N.; Labetoulle, M.; Lomonte, P. HSV-1 Genome Subnuclear Positioning and Associations with Host-Cell PML-NBs and Centromeres Regulate LAT Locus Transcription during Latency in Neurons. PLOS Pathog. 2012, 8, e1002852.
- Everett, R.D.; Murray, J.; Orr, A.; Preston, C.M. Herpes simplex virus type 1 genomes are associated with ND10 nuclear substructures in quiescently infected human fibroblasts. J. Virol. 2007, 81, 10991–11004.
- Cohen, C.; Corpet, A.; Roubille, S.; Maroui, M.A.; Poccardi, N.; Rousseau, A.; Kleijwegt, C.; Binda, O.; Texier, P.; Sawtell, N.; et al. Promyelocytic leukemia (PML) nuclear bodies (NBs) induce latent/quiescent HSV-1 genomes chromatinization through a PML NB/Histone H3.3/H3.3 Chaperone Axis. PLoS Pathog. 2018, 14, e1007313.
- Maroui, M.A.; Callé, A.; Cohen, C.; Streichenberger, N.; Texier, P.; Takissian, J.; Rousseau, A.; Poccardi, N.; Welsch, J.; Corpet, A.; et al. Latency Entry of Herpes Simplex Virus 1 Is Determined by the Interaction of Its Genome with the Nuclear Environment. PLoS Pathog. 2016, 12, e1005834.
- D’Aiuto, L.; Bloom, D.C.; Naciri, J.N.; Smith, A.; Edwards, T.G.; McClain, L.; Callio, J.A.; Jessup, M.; Wood, J.; Chowdari, K.; et al. Modeling Herpes Simplex Virus 1 Infections in Human Central Nervous System Neuronal Cells Using Two- and Three-Dimensional Cultures Derived from Induced Pluripotent Stem Cells. J. Virol. 2019, 93, e00111–e00119.
- Kyratsous, C.A.; Silverstein, S.J. Components of nuclear domain 10 bodies regulate varicella-zoster virus replication. J. Virol. 2009, 83, 4262–4274.
- Reichelt, M.; Wang, L.; Sommer, M.; Perrino, J.; Nour, A.M.; Sen, N.; Baiker, A.; Zerboni, L.; Arvin, A.M. Entrapment of viral capsids in nuclear PML cages is an intrinsic antiviral host defense against varicella-zoster virus. PLoS Pathog. 2011, 7, e1001266.
- Wang, L.; Oliver, S.L.; Sommer, M.; Rajamani, J.; Reichelt, M.; Arvin, A.M. Disruption of PML nuclear bodies is mediated by ORF61 SUMO-interacting motifs and required for varicella-zoster virus pathogenesis in skin. PLoS Pathog. 2011, 7, e1002157.
- Walters, M.S.; Kyratsous, C.A.; Silverstein, S.J. The RING finger domain of Varicella-Zoster virus ORF61p has E3 ubiquitin ligase activity that is essential for efficient autoubiquitination and dispersion of Sp100-containing nuclear bodies. J. Virol. 2010, 84, 6861–6865.
