Gliomas are a group of primary tumors of the central nervous system (CNS) originating from glial cells. It was estimated that gliomas account for nearly a quarter of all primary CNS tumors. The management of malignant gliomas poses several challenges, in part due to the heterogeneous and resistant nature of neoplasm, as well as the obstacles faced when administering high-dose radiation and chemotherapy in tissue as vulnerable as that of the CNS. These difficulties are due to the tumor’s aggressiveness and the adverse effects of radio/chemotherapy on the brain. Stem cell therapy is an exciting area of research being explored for several medical issues.
- Gliomas
- Stem cell
- neural stem cell
1. Introduction
Gliomas are a group of primary tumors of the central nervous system (CNS) originating from glial cells [1]. According to the Central Brain Tumor Registry of the United States (CBTRUS), gliomas account for around 25% of all primary brain tumors, with glioblastoma being the most common glioma, as well as the most common malignant CNS tumor [2]. Histologically, gliomas originate from three types of glial cells: Oligodendrocytes, ependymal cells, and astrocytes. The most common histological variant of glioma is astrocytic tumors accounting for over 70% of all gliomas [2]. The most recent World Health Organization (WHO) classification of gliomas is based on both histological—according to their cell of origin (e.g., astrocytoma, ependymoma)—and molecular—according to specific acquired mutations (e.g., diffuse astrocytoma, IDH-mutant)—characteristics [3]. Tumors are also graded on a scale from one to four, with grade IV glioblastomas being the most invasive and lethal [3]. In contrast to low-grade gliomas, in which concomitant chemotherapy is not always required [4][5], the infiltrative and diffuse nature of high-grade “malignant” gliomas mandates the use of chemotherapy [6].
The management of malignant gliomas poses several challenges, in part due to the heterogeneous and resistant nature of neoplasm, as well as the obstacles faced when administering high-dose radiation and chemotherapy in tissue as vulnerable as that of the CNS. Limitations to therapy also include the unfavorable pharmacokinetics of chemotherapeutic drugs, which prevent them from efficiently penetrating the blood–brain barrier and frequent relapses due to the metastatic seeding associated with glioblastoma [7][8]. Shortcomings in the current treatment options for malignant gliomas have sparked an interest in the search for novel techniques such as direct receptor antagonists, immune therapy, and stem cell therapy.
Stem cells (SCs) are precursor cells that retain the capacity to differentiate into various types of tissues. Stem cells are classified according to their origin; however, adult stem cells, such as mesenchymal stem cells (MSCs), are the most commonly used therapeutically. MSCs are multipotent stem cells that can differentiate into all cells of a mesenchymal lineage [9][10] and are isolated from the bone marrow, adipose tissue, umbilical cord, and dental pulp. Neural stem cells (NSC) are specific types of adult stem cells found in the subependymal zone and the dentate gyrus, and are responsible for the regeneration of neurons, astrocytes, and oligodendrocytes [11].
Stem cell therapy involves either the administration of exogenous stem cells or the mobilization of endogenous stem cells. Stem cell mobilization is an important approach in the management of degenerative disorders, whereas the administration of exogenous stem cells is more pertinent in the management of malignant gliomas. Several studies have demonstrated the ability of stem cells to target brain pathologies, such as areas of demyelination, ischemia, and neoplasms [7][12][13]. Both MSCs and NSCs were found to have high tropism to malignant gliomas due to the overexpression of cell surface markers, as well as the secretion of molecular signals in the tumor’s microenvironment [14][15]. Factors such as cytokines (e.g., tumor necrosis factor-alpha “TNF-α,” interleukin-8 “IL-8,” and stromal cell-derived factor alpha “SDF-α”) [14][16][17][18], hypoxia-inducible factor-1a, hepatocyte growth factor, and vascular endothelial growth factor have all been implicated in stem cell migration toward neoplasms [19][20], as well as tumor extracellular matrix components such as tenascin-C, laminin, and inhibitor of matrix metalloproteinase-1 [19][21].
This intrinsic property of stem cells has prodded interest in their ability to serve as drug delivery systems, which could potentially circumvent the blood–brain barrier. In vivo animal studies have shown that the modification of stem cells can directly target neoplasms through various mechanisms, decrease the tumor burden, and thus prolong survival. Molecularly engineered stem cells can be modified to (1) prevent angiogenesis, (2) deliver inflammatory cytokines and mediate immune response, (3) initiate ligand-activated anti-tumor pathways, (4) compete for certain pro-proliferative ligands and thus inhibit tumor growth, (5) release anti-tumor toxins, (6) induce “cell suicide” through well-established enzyme-prodrug systems, (7) deliver nanoparticles and oncogenic viral particles, and finally, (8) release vesicles containing anti-tumor microRNA (miRNA) (Figure 1) [7].
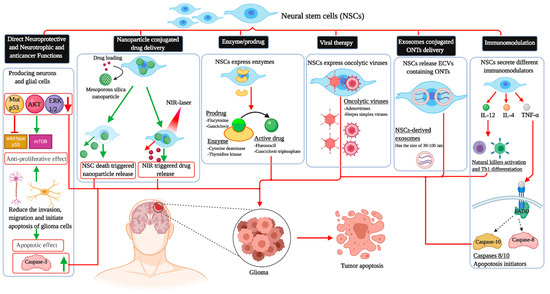
Figure 1. The different applications of neural stem cells in killing glioblastoma cells. NSCs: Neural stem cell; AKT: Protein kinase B; ERK1/2: Extracellular-regulated kinase; TNF-α: Tumor necrosis factor-alpha; IL-4: Interkelukin-4; IL-12: Interkelukin-12; FADD: Fas-associated protein with death domain; ONTs: Oligonucleotide therapeutics; Th1: T helper 1; mTOR: Mammalian target of rapamycin; NIR: Near-infrared; ECVs: Extracellular vesicles.
2. Tropism, Migration, and Tumor Homing Properties of Neural Stem Cells
NSCs are mainly detected in the hippocampus and the subventricular zone situated in the dentate gyrus of the brain [22][23]. Thanks to their tropic properties, they can serve as delivery vehicles of a variety of elements such as antitumor drugs and suicide genes in a selective way to the tumoral mass [24]. Therefore, NSCs have been extensively investigated in drug and oncolytic viruses delivery in brain malignancies, especially medulloblastomas [25] and gliomas [26][27][28]. This tropism was explored in rodent brains through the simultaneous NSCs and glioblastoma cells implementation [29]. NSCs have the ability to migrate toward malignant brain masses of glial origin and tumors of other origins such as medulloblastoma and metastatic cancers such as melanoma and breast neoplasms [25][30][31].
The migratory movement of NSCs starts about 50 min after their transplantation, and the number of stem cells in the tumor site increases slowly up to 5 days in the region, with a significant expansion up to 15 days later [32]. This migration of NCSs to the malignant mass may progress in a dose-dependent manner [33], and it is under the influence of the tumoral microenvironment components [34]. Furthermore, the killing capacity may also be influenced by the distance between the delivery site and the tumor. A recent report showed that direct injection of the potent stem cells into the tumor foci led to a rapid decrease in tumor growth with a reduction in the mass volume to sub-detection levels after ten days post-NCSs delivery, whereas implementation at a distance of two millimeters far from the mass was associated with a significant attenuation of tumor proliferation by day 14 and a reduction in the mass to sub-detection level by day 21 after NSC delivery [34].
The migratory capacity of NCSs is dependent on chemotactic factors. Accordingly, the presence of multifocal masses may reduce the killing capacities of NSC therapies due to the decrease in the amount of NSCs reaching each mass, as each focus releases chemotactic factors and thus may dilute the NSC dose per tumor mass [34]. The exact mechanistic pathways that guide NSCs homing to gliomas are still unknown. Microglia and astrocytes secrete a variety of angiogenic and inflammatory agents that play a role in NSC homing [35]. These homing properties can also be triggered by hypoxia via the secretion of a key element called the Transcription Factor Hypoxia-Inducible Factor-1α (HIF-1α). HIF-1α can promote the upregulation of chemoattractant substances, including various chemokines and other molecules acting as growth factors like insulin-like growth factor 1 (IGF1), stromal cell-derived factor 1 (SDF-1), vascular endothelial growth factor (VEGF), monocyte chemotactic protein 1 (MCP1), and fibroblast growth factor 2 (FGF2) [36]. The down-regulation of HIF-1α in glioblastoma cells leads to the decline of SDF-1 and the expression of VEGF with NSC tumor tropism suppression [37]. Finally, as conventional cancer therapies, namely radiation and chemotherapy, are associated with the hypoxia-induced upregulation of chemokines by malignant cells, the use of these therapies concomitantly with NSC delivery may enhance the chemotactic pathways and signals that potentiate stem cell migration and thus allow improved overall therapeutic efficacy [38] (Figure 2).
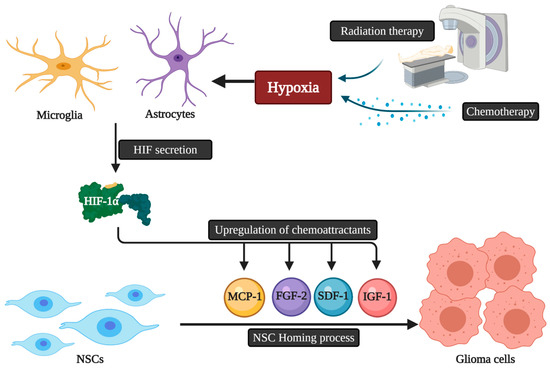
Figure 2. The possible relation of chemoradiotherapy and HIF-1α secretion with NSCs homing to glioblastoma. NSCs: Neural stem cells; HIF: Hypoxia-inducible factor; IGF1: Insulin-like growth factor 1; SDF-1: Stromal cell-derived factor 1; VEGF: Vascular endothelial growth factor; MCP1: Monocyte chemotactic protein 1; FGF2: Fibroblast growth factor 2.
3. Neuroprotective and Neurotrophic Functions of Neural Stem Cells Therapy
Endogenous subventricular stem cells have the ability to divide and to migrate to the injured site in stroke and other CNS injuries. They can also undergo a process of differentiation resulting in mature cells that can participate in the recovery [39][40][41][42]. The application of NSCs is a promising research avenue due to their potential in improving the outcome of CNS injury and neurodegeneration [43]. The multipotency and self-regenerative attributes of NSCs are crucial for nervous tissue repair [44]. NSCs are thus considered as an ideal source to continuously produce glial cells and neurons to repair neural networks in the damaged nervous system [45]. NSCs can enhance the recovery from brain injury through their migration and cell replacement properties, in addition to the enhancement of nutritional and trophic supplementation effects using paracrine processes [46][47]. They can also control inflammation in the brain and provide some neuroprotective activities [48][49][50]. Moreover, NSC therapies can also positively influence intracranial blood perfusion via promoting angiogenesis as they can increase angiogenic factors expression in the brain [51][52]. In addition to their numerous advantages, in vivo studies have shown that intravenously administered NSCs can cross the blood–brain barrier [53][54] and exert their activities without producing toxicity in the normal components of the brain [55]. Furthermore, the ability of NSCs to cross the blood–brain barrier has been closely linked to the expression of certain cell surface adhesion molecules such as CD44, VLA-4 [56], as well as the inflammatory state of the CNS. In an in vivo study by Pluchio et al., tagged NSCs injected intravenously were detected in the CNS in mice pre-treated with lipopolysaccharide or tumor necrosis factor and interleukin 1β—inflammatory mediators used to mimic an inflammatory-like state [56]. More specifically, in an in vivo model of gliomas, the expression of VEGF, HGF, and zonulin—factors that increase the permeability of the blood–brain barrier—induced transmigration of the NSCs to the CNS after being injected into the systemic circulation [57]. In addition, data from recent reports showed that the systemic stem cells’ administration efficiency was much higher in animals with neurodegeneration than wild-type animals [56][58]. The in vivo-tracking of NSCs homing to glioblastoma using immuno-histochemical studies revealed that the systemically administrated progenitor cells can cross the barrier and localize in glioblastoma foci [54][59]. The quantitative optical analysis showed that, when the intravenous route is used, about 1.4% of NSCs co-localized with the tumor while the intraventricular delivery resulted in the localization of more than 4% of NSCs [54]. Politi et al. monitored the accumulation of intravenously administered NPCs in a model of autoimmune encephalomyelitis using a human magnetic resonance scanner. They could detect the transplanted cells in about 80% of the brain lesions 24 h after the injection. The continued assessment showed the presence of NPCs 20 days after the injection. The neuropathological study of the brains showed that the transplanted stem cells were exclusively in inflammatory regions of neurodegeneration and not in normal tissue, suggesting their potential role in the reversal of the inflammatory process [60]. Finally, the implementation of exogenous human NSCs into the dentate gyrus can also activate the production of endogenous NSCs [58].
4. Effects of Neural Stem Cells in Glioma
The subcutaneous injection of normal NSCs and human glioma (U251) cell lines in nude mice led to the promotion of the animals’ survival [59]. This observation was concomitant with a decline in mutant p53 production and phosphorylation of protein kinase B (AKT) and extracellular-regulated kinase (ERK1/2). A significant increase in an important apoptotic molecule called caspase-3 was also noted, suggesting that normal NSCs may exert direct effects against malignant glioma [59]. In another report, cultures containing U87 stem-like cells in contact with an NSC-conditioned medium showed low viability and multiplication of U87 cells without significant modulation of their astrocytic differentiation capacity. Moreover, the invasive and migratory functions of U87 stem-like cells were also reduced [60].
It was also established that endogenous normal stem cells belonging to the subventricular zone can also target glioma-proliferating cells and attenuate the mass growth with a potential impact on survival [61]. Vitamin K-dependent factor protein S, released by the tumoral environment, can trigger this specific tropism via the modulation of the tyrosine kinase receptor (Tyro3) action [62].
This entry is adapted from the peer-reviewed paper 10.3390/ijms22052258
References
- Couturier, C.P.; Ayyadhury, S.; Le, P.U.; Nadaf, J.; Monlong, J.; Riva, G.; Allache, R.; Baig, S.; Yan, X.; Bourgey, M.; et al. Single-cell RNA-seq reveals that glioblastoma recapitulates a normal neurodevelopmental hierarchy. Nat. Commun. 2020, 1, 11–19.
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro. Oncol. 2019, 21, V1–V100.
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820.
- National Guideline Alliance (UK). Brain Tumours (Primary) and Brain Metastases in Adults; National Institute for Health and Care Excellence: London, UK, 2018.
- Sepúlveda-Sánchez, J.M.; Langa, J.M.; Arráez, M.; Fuster, J.; Laín, A.H.; Reynés, G.; González, V.R.; Vicente, E.; Denis, M.V. Gallego SEOM clinical guideline of diagnosis and management of low-grade glioma (2017). Clin. Transl. Oncol. 2018, 20, 3–15.
- Stupp, R.; Brada, M.; van den Bent, M.J.; Tonn, J.C.; Pentheroudakis, G. High-grade glioma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014, 25, 93–101.
- Shah, K. Stem cell-based therapies for tumors in the brain: Are we there yet? Neuro. Oncol. 2016, 18, 1066–1078.
- Taylor, O.G.; Brzozowski, J.S.; Skelding, K.A. Glioblastoma multiforme: An overview of emerging therapeutic targets. Front. Oncol. 2019, 9, 1–11.
- Jiang, Y.; Jahagirdar, B.N.; Reinhardt, R.L.; Schwartz, R.E.; Keene, C.D.; Ortiz-Gonzalez, X.R.; Reyes, M.; Lenvik, T.; Lund, T.; Blackstad, M.; et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002, 418, 41–49.
- Zomer, H.D.; Vidane, A.S.; Gonçalves, N.N.; Ambrósio, C.E. Mesenchymal and induced pluripotent stem cells: General insights and clinical perspectives. Stem Cells Cloning Adv. Appl. 2015, 8, 125–134.
- Gage, F.H. Mammalian Neural Stem Cells. Science 2000, 287, 1433–1438.
- Carbajal, K.S.; Schaumburg, C.; Strieter, R.; Kane, J.; Lane, T.E. Migration of engrafted neural stem cells is mediated by CXCL12 signaling through CXCR4 in a viral model of multiple sclerosis. Proc. Natl. Acad. Sci. USA 2010, 107, 11068–11073.
- Sasportas, L.S.; Kasmieh, R.; Wakimoto, H.; Hingtgen, S.; van de Water, J.A.J.M.; Mohapatra, G.; Figueiredo, J.L.; Martuza, R.L.; Weissleder, R.; Shah, K. Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy. Proc. Natl. Acad. Sci. USA 2009, 106, 4822–4827.
- Namba, H.; Kawaji, H.; Yamasaki, T. Use of genetically engineered stem cells for Glioma therapy (Review). Oncol. Lett. 2016, 11, 9–15.
- Spaeth, E.; Klopp, A.; Dembinski, J.; Andreeff, M.; Marini, F. Inflammation and tumor microenvironments: Defining the migratory itinerary of mesenchymal stem cells. Gene Ther. 2008, 15, 730–738.
- Kim, D.S.; Kim, J.H.; Lee, J.K.; Choi, S.J.; Kim, J.S.; Jeun, S.S.; Oh, W.; Yang, Y.S.; Chang, J.W. Overexpression of CXC chemokine receptors is required for the superior glioma-tracking property of umbilical cord blood-derived mesenchymal stem cells. Stem Cells Dev. 2009, 18, 511–520.
- Egea, V.; Von Baumgarten, L.; Schichor, C.; Berninger, B.; Popp, T.; Neth, P.; Goldbrunner, R.; Kienast, Y.; Winkler, F.; Jochum, M.; et al. TNF-α respecifies human mesenchymal stem cells to a neural fate and promotes migration toward experimental glioma. Cell Death Differ. 2011, 18, 853–863.
- Menon, L.G.; Picinich, S.; Koneru, R.; Gao, H.; Lin, S.Y.; Koneru, M.; Mayer-Kuckuk, P.; Glod, J.; Banerjee, D. Differential Gene Expression Associated with Migration of Mesenchymal Stem Cells to Conditioned Medium from Tumor Cells or Bone Marrow Cells. Stem Cells 2007, 25, 520–528.
- Young, J.S.; Morshed, R.A.; Kim, J.W.; Balyasnikova, I.V.; Ahmed, A.U.; Lesniak, M.S. Advances in stem cells, induced pluripotent stem cells, and engineered cells: Delivery vehicles for anti-glioma therapy. Expert Opin. Drug Deliv. 2014, 11, 1733–1746.
- Jeung, H.A.; Soo, Y.L.; Jeong, Y.J.; Kyung, G.C.; Kim, S.U.; Myung, A.L. Identification of gliotropic factors that induce human stem cell migration to malignant tumor. J. Proteome Res. 2009, 8, 2873–2881.
- Ziu, M.; Schmidt, N.O.; Cargioli, T.G.; Aboody, K.S.; Black, P.M.L.; Carroll, R.S. Glioma-produced extracellular matrix influences brain tumor tropism of human neural stem cells. J. Neurooncol. 2006, 79, 125–133.
- Pincus, D.W.; Keyoung, H.M.; Harrison-Restelli, C.; Goodman, R.R.; Fraser, R.A.R.; Edgar, M.; Sakakibara, S.; Okano, H.; Nedergaard, M.; Goldman, S.A. Fibroblast growth factor-2/brain-derived neurotrophic factor—associated maturation of new neurons generated from adult human subependymal cells. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 1998, 43, 576–585.
- Roy, N.S.; Wang, S.; Jiang, L.; Kang, J.; Benraiss, A.; Harrison-Restelli, C.; Fraser, R.A.R.; Couldwell, W.T.; Kawaguchi, A.; Okano, H. In vitro neurogenesis by progenitor cells isolated from the adult human hippocampus. Nat. Med. 2000, 6, 271–277.
- Ahmed, A.U.; Alexiades, N.G.; Lesniak, M.S. The use of neural stem cells in cancer gene therapy: Predicting the path to the clinic. Curr. Opin. Mol. Ther. 2010, 12, 546–552.
- Kim, S.-K.; Kim, S.U.; Park, I.H.; Bang, J.H.; Aboody, K.S.; Wang, K.-C.; Cho, B.-K.; Kim, M.; Menon, L.G.; Black, P.M. Human neural stem cells target experimental intracranial medulloblastoma and deliver a therapeutic gene leading to tumor regression. Clin. Cancer Res. 2006, 12, 5550–5556.
- Ahmed, A.U.; Thaci, B.; Alexiades, N.G.; Han, Y.; Qian, S.; Liu, F.; Balyasnikova, I.V.; Ulasov, I.Y.; Aboody, K.S.; Lesniak, M.S. Neural stem cell-based cell carriers enhance therapeutic efficacy of an oncolytic adenovirus in an orthotopic mouse model of human glioblastoma. Mol. Ther. 2011, 19, 1714–1726.
- Metz, M.Z.; Gutova, M.; Lacey, S.F.; Abramyants, Y.; Vo, T.; Gilchrist, M.; Tirughana, R.; Ghoda, L.Y.; Barish, M.E.; Brown, C.E. Neural stem cell-mediated delivery of irinotecan-activating carboxylesterases to glioma: Implications for clinical use. Stem Cells Transl. Med. 2013, 2, 983–992.
- Mooney, R.; Weng, Y.; Tirughana-Sambandan, R.; Valenzuela, V.; Aramburo, S.; Garcia, E.; Li, Z.; Gutova, M.; Annala, A.J.; Berlin, J.M. Neural stem cells improve intracranial nanoparticle retention and tumor-selective distribution. Futur. Oncol. 2014, 10, 401–415.
- Aboody, K.S.; Brown, A.; Rainov, N.G.; Bower, K.A.; Liu, S.; Yang, W.; Small, J.E.; Herrlinger, U.; Ourednik, V.; Black, P.M. Neural stem cells display extensive tropism for pathology in adult brain: Evidence from intracranial gliomas. Proc. Natl. Acad. Sci. USA 2000, 97, 12846–12851.
- Aboody, K.S.; Bush, R.A.; Garcia, E.; Metz, M.Z.; Najbauer, J.; Justus, K.A.; Phelps, D.A.; Remack, J.S.; Yoon, K.J.; Gillespie, S.; et al. Development of a tumor-selective approach to treat metastatic cancer. PLoS ONE 2006, 1, e23.
- Joo, K.M.; Park, I.H.; Shin, J.Y.; Jin, J.; Kang, B.G.; Kim, M.H.; Lee, S.J.; Jo, M.; Kim, S.U.; Nam, D.-H. Human neural stem cells can target and deliver therapeutic genes to breast cancer brain metastases. Mol. Ther. 2009, 17, 570–575.
- Kim, J.-H.; Lee, J.-E.; Kim, S.U.; Cho, K.-G. Stereological analysis on migration of human neural stem cells in the brain of rats bearing glioma. Neurosurgery 2010, 66, 333–342.
- Barish, M.E.; Herrmann, K.; Tang, Y.; Argalian Herculian, S.; Metz, M.; Aramburo, S.; Tirughana, R.; Gutova, M.; Annala, A.; Moats, R.A.; et al. Human Neural Stem Cell Biodistribution and Predicted Tumor Coverage by a Diffusible Therapeutic in a Mouse Glioma Model. Stem Cells Transl. Med. 2017, 6, 1522–1532.
- Carey-Ewend, A.G.; Hagler, S.B.; Bomba, H.N.; Goetz, M.J.; Bago, J.R.; Hingtgen, S.D. Developing Bio-Inspired 3D Models of Brain Cancer to Evaluate Tumor-Homing Neural Stem Cell Therapy. Tissue Eng. 2020, 22, 417–429.
- Müller, F.-J.; Snyder, E.Y.; Loring, J.F. Gene therapy: Can neural stem cells deliver? Nat. Rev. Neurosci. 2006, 7, 75–84.
- Park, K.I.; Hack, M.A.; Ourednik, J.; Yandava, B.; Flax, J.D.; Stieg, P.E.; Gullans, S.; Jensen, F.E.; Sidman, R.L.; Ourednik, V.; et al. Acute injury directs the migration, proliferation, and differentiation of solid organ stem cells: Evidence from the effect of hypoxia-ischemia in the CNS on clonal “reporter” neural stem cells. Exp. Neurol. 2006, 199, 156–178.
- Zhao, D.; Najbauer, J.; Garcia, E.; Metz, M.Z.; Gutova, M.; Glackin, C.A.; Kim, S.U.; Aboody, K.S. Neural stem cell tropism to glioma: Critical role of tumor hypoxia. Mol. Cancer Res. 2008, 6, 1819–1829.
- Kim, S.M.; Oh, J.H.; Park, S.A.; Ryu, C.H.; Lim, J.Y.; Kim, D.-S.; Chang, J.W.; Oh, W.; Jeun, S.-S. Irradiation enhances the tumor tropism and therapeutic potential of tumor necrosis factor-related apoptosis-inducing ligand-secreting human umbilical cord blood-derived mesenchymal stem cells in glioma therapy. Stem Cells 2010, 28, 2217–2228.
- Arvidsson, A.; Collin, T.; Kirik, D.; Kokaia, Z.; Lindvall, O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 2002, 8, 963–970.
- Thored, P.; Arvidsson, A.; Cacci, E.; Ahlenius, H.; Kallur, T.; Darsalia, V.; Ekdahl, C.T.; Kokaia, Z.; Lindvall, O. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells 2006, 24, 739–747.
- Yamashita, T.; Ninomiya, M.; Acosta, P.H.; García-Verdugo, J.M.; Sunabori, T.; Sakaguchi, M.; Adachi, K.; Kojima, T.; Hirota, Y.; Kawase, T.; et al. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J. Neurosci. 2006, 26, 6627–6636.
- Teng, Y.D.; Liao, W.-L.; Choi, H.; Konya, D.; Sabharwal, S.; Langer, R.; Sidman, R.L.; Snyder, E.Y.; Frontera, W.R. Physical activity-mediated functional recovery after spinal cord injury: Potential roles of neural stem cells. Regen. Med. 2006, 1, 763–776.
- Jiao, Y.; Liu, Y.-W.; Chen, W.-G.; Liu, J. Neuroregeneration and functional recovery after stroke: Advancing neural stem cell therapy toward clinical application. Neural Regen. Res. 2021, 16, 80–92.
- Ross, H.H.; Ambrosio, F.; Trumbower, R.D.; Reier, P.J.; Behrman, A.L.; Wolf, S.L. Neural Stem Cell Therapy and Rehabilitation in the Central Nervous System: Emerging Partnerships. Phys. Ther. 2016, 96, 734–742.
- Annese, V.; Navarro-Guerrero, E.; Rodríguez-Prieto, I.; Pardal, R. Physiological plasticity of neural-crest-derived stem cells in the adult mammalian carotid body. Cell Rep. 2017, 19, 471–478.
- Zhang, Z.G.; Buller, B.; Chopp, M. Exosomes—beyond stem cells for restorative therapy in stroke and neurological injury. Nat. Rev. Neurol. 2019, 15, 193–203.
- Baker, E.W.; Kinder, H.A.; West, F.D. Neural stem cell therapy for stroke: A multimechanistic approach to restoring neurological function. Brain Behav. 2019, 9, e01214.
- Taupin, P. Adult neurogenesis, neuroinflammation and therapeutic potential of adult neural stem cells. Int. J. Med. Sci. 2008, 5, 127.
- Reekmans, K.P.; Praet, J.; De Vocht, N.; Tambuyzer, B.R.; Bergwerf, I.; Daans, J.; Baekelandt, V.; Vanhoutte, G.; Goossens, H.; Jorens, P.G. Clinical potential of intravenous neural stem cell delivery for treatment of neuroinflammatory disease in mice? Cell Transplant. 2011, 20, 851–870.
- Rong, Y.; Liu, W.; Wang, J.; Fan, J.; Luo, Y.; Li, L.; Kong, F.; Chen, J.; Tang, P.; Cai, W. Neural stem cell-derived small extracellular vesicles attenuate apoptosis and neuroinflammation after traumatic spinal cord injury by activating autophagy. Cell Death Dis. 2019, 10, 1–18.
- Lee, H.J.; Kim, K.S.; Park, I.H.; Kim, S.U. Human Neural Stem Cells Over-Expressing VEGF Provide Neuroprotection, Angiogenesis and Functional Recovery in Mouse Stroke Model. PLoS ONE 2007, 2, e156.
- Ryu, S.; Lee, S.-H.; Kim, S.U.; Yoon, B.-W. Human neural stem cells promote proliferation of endogenous neural stem cells and enhance angiogenesis in ischemic rat brain. Neural Regen. Res. 2016, 11, 298–304.
- Aboody, K.S.; Najbauer, J.; Metz, M.Z.; D’Apuzzo, M.; Gutova, M.; Annala, A.J.; Synold, T.W.; Couture, L.A.; Blanchard, S.; Moats, R.A.; et al. Neural stem cell-mediated enzyme/prodrug therapy for glioma: Preclinical studies. Sci. Transl. Med. 2013, 5, 184ra59.
- Tang, Y.; Shah, K.; Messerli, S.M.; Snyder, E.; Breakefield, X.; Weissleder, R. In vivo tracking of neural progenitor cell migration to glioblastomas. Hum. Gene Ther. 2003, 14, 1247–1254.
- Einstein, O.; Ben-Hur, T. The changing face of neural stem cell therapy in neurologic diseases. Arch. Neurol. 2008, 65, 452–456.
- Pluchino, S.; Quattrini, A.; Brambilla, E.; Gritti, A.; Salani, G.; Dina, G.; Galli, R.; Del Carro, U.; Amadio, S.; Bergami, A.; et al. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature 2003, 422, 688–694.
- Díaz-Coránguez, M.; Segovia, J.; López-Ornelas, A.; Puerta-Guardo, H.; Ludert, J.; Chávez, B.; Meraz-Cruz, N.; González-Mariscal, L. Transmigration of Neural Stem Cells across the Blood Brain Barrier Induced by Glioma Cells. PLoS ONE 2013, 8, e60665.
- Park, D.-H.; Eve, D.J.; Sanberg, P.R.; Musso, J., 3rd; Bachstetter, A.D.; Wolfson, A.; Schlunk, A.; Baradez, M.-O.; Sinden, J.D.; Gemma, C. Increased neuronal proliferation in the dentate gyrus of aged rats following neural stem cell implantation. Stem Cells Dev. 2010, 19, 175–180.
- An, J.; Yan, H.; Li, X.; Tan, R.; Chen, X.; Zhang, Z.; Liu, Y.; Zhang, P.; Lu, H.; Liu, Y. The inhibiting effect of neural stem cells on proliferation and invasion of glioma cells. Oncotarget 2017, 8, 76949–76960.
- Li, X.; Tan, R.; Hu, X.; Jiao, Q.; Rahman, M.S.; Chen, X.; Zhang, P.; An, J.; Lu, H.; Liu, Y. Neural stem cell-derived factors inhibit the growth and invasion of U87 stem-like cells in vitro. J. Cell. Biochem. 2019, 120, 5472–5479.
- Glass, R.; Synowitz, M.; Kronenberg, G.; Walzlein, J.; Markovic, D.S.; Wang, L.; Gast, D.; Kempermann, G.; Kettenmann, H. Glioblastoma-Induced Attraction of Endogenous Neural Precursor Cells Is Associated with Improved Survival. J. Neurosci. 2005, 25, 2637–2646.
- Ginisty, A.; Oliver, L.; Arnault, P.; Vallette, F.; Benzakour, O.; Coronas, V. The vitamin K-dependent factor, protein S, regulates brain neural stem cell migration and phagocytic activities towards glioma cells. Eur. J. Pharmacol. 2019, 855, 30–39.
