Necrotizing enterocolitis (NEC) is a devastating disease predominately found in premature infants that is associated with significant morbidity and mortality. Despite decades of research, medical management with broad spectrum antibiotics and bowel rest has remained relatively unchanged, with no significant improvement in patient outcomes. The etiology of NEC is multi-factorial; however, gastrointestinal dysbiosis plays a prominent role in a neonate’s vulnerability to and development of NEC. Probiotics have recently emerged as a new avenue for NEC therapy. However, current delivery methods are associated with potential limitations, including the need for at least daily administration in order to obtain any improvement in outcomes. We present a novel formulation of enterally delivered probiotics that addresses the current limitations. A single enteral dose of Lactobacillus reuteri delivered in a biofilm formulation increases probiotic survival in acidic gastric conditions, increases probiotic adherence to gastrointestinal epithelial cells, and reduces the incidence, severity, and neurocognitive sequelae of NEC in experimental models.
- necrotizing enterocolitis
- Lactobacillus reuteri
- probiotics
- dextranomer microspheres
1. Introduction
Necrotizing enterocolitis (NEC) is a disease that has been a major source of morbidity and mortality for premature neonates for decades. Affecting 10% of infants with birth weight < 1500 g, NEC is a neonatal intestinal disease that is manifested by excessive inflammation that may progress to tissue destruction, bacterial translocation, and sepsis. The disease carries a mortality rate as high as 20–30% [1][2]. Despite decades of research and an estimated annual cost to the health care system of nearly USD 1 billion, NEC remains the number one cause of death from gastrointestinal disease in premature infants [2]. Thus far, treatment and attempts at prevention of NEC have remained subpar, with surviving infants often being left with debilitating morbidities including short-gut syndrome, cholestatic liver disease, and poor growth and neurodevelopmental outcomes [3][4].
The etiology of NEC is multi-factorial with prematurity, low birth weight, administration of enteral feeds, and antibiotic exposure associated with development of the disease [2][5]. Bacterial colonization of the gastrointestinal tract is essential to healthy gut development, with strong evidence indicating that gut dysbiosis plays a prominent role in patient vulnerability and development of NEC [6][7][8][9][10]. Large proportions of beneficial health-promoting bacteria, including Lactobacillus and Bifidobacteria species, are present in healthy full-term breast-fed infants [11][12][13]. Additionally, breast milk contains significant amounts of undigestible oligosaccharides that play a role as prebiotics, nurturing and promoting the growth of the favorable gut microorganisms necessary for bacterial-epithelial cross talk, which is crucial for nascent gut and immune system development [11][12]. Conversely, premature infants have reduced microbiome diversity and stability, with smaller proportions of beneficial bacteria including Lactobacillus and Bifidobacterium species, and increased levels of bacteria that can become pathogenic including Gammaproteobacteria (i.e., Escherichia coli, Klebsiella pneumoniae), which is evident in infants that develop NEC [10][14][15][16][17][18][19][20].
To counter the altered intestinal microbiome and to reduce the pathogenic bacterial colonization frequently seen in premature infants, administration of probiotics, or live microorganisms that confer a health benefit on the host, emerged as a means of NEC prevention in the late 1990s [21][22]. Since then, numerous trials evaluating the efficacy of probiotics in preventing NEC have been conducted, with some demonstrating favorable results [23][24]. Oral administration of Lactobacillus and Bifidobacterium was shown to prevent NEC in very low birth weight infants [25][26], and when administered in combination with breast milk, there was greater reduction in the incidence of NEC compared to infants receiving breast milk alone [6][27]. Furthermore, using animal models of experimental NEC, probiotics have been shown to inhibit inflammation, reduce apoptosis, inhibit Toll-like receptor 4 (TLR4) activation, and protect against intestinal mucosal barrier breakdown [28][29][30][31][32].
However, there are significant concerns and limitations regarding the current method of probiotic administration. An acidic gastric environment, interactions with bile acids, pressure from the host immune system, and competition with commensal and pathogenic bacteria can rapidly render probiotic bacteria ineffective, with a crippled capacity to adhere to and colonize the gut [33][34]. Due to the inability to be retained within the host, oral administration is required daily, if not multiple times per day, to witness even a modest beneficial effect that is effectively lost upon the cessation of probiotic administration [35]. Additionally, repeated administration of oral probiotic bacteria to premature infants with compromised gut barrier function can be problematic, given the risk of inducing bacteremia or sepsis from the probiotic administered [36][37][38][39].
To overcome these concerns and limitations, we introduced a novel probiotic delivery system that delivers beneficial health-promoting Lactobacillus reuteri (ATCC 23272) in a biofilm state rather than in a free-living planktonic state [40][41]. Probiotics delivered as a biofilm, i.e., an adhered or aggregated community of bacteria that produce a self-forming protective matrix of DNA, proteins, lipids, and oligosaccharides, are more resistant to harsh environmental conditions such as acidic gastric pH, laminar/turbulent fluid forces, anti-microbial agents, and host immune defenses compared to free-living planktonic bacteria [42][43]. The use of probiotics in their biofilm state has been investigated and utilized in a few conditions, including antagonizing pathogenic infections in implants and incorporation into anti-neoplastic strategies as immunoregulators [44][45][46][47]. However, the delivery of probiotics in a biofilm state is a new and innovative strategy in the management and prevention of NEC.
2. Production of L. reuteri Biofilm by Adherence to Dextranomer Microspheres
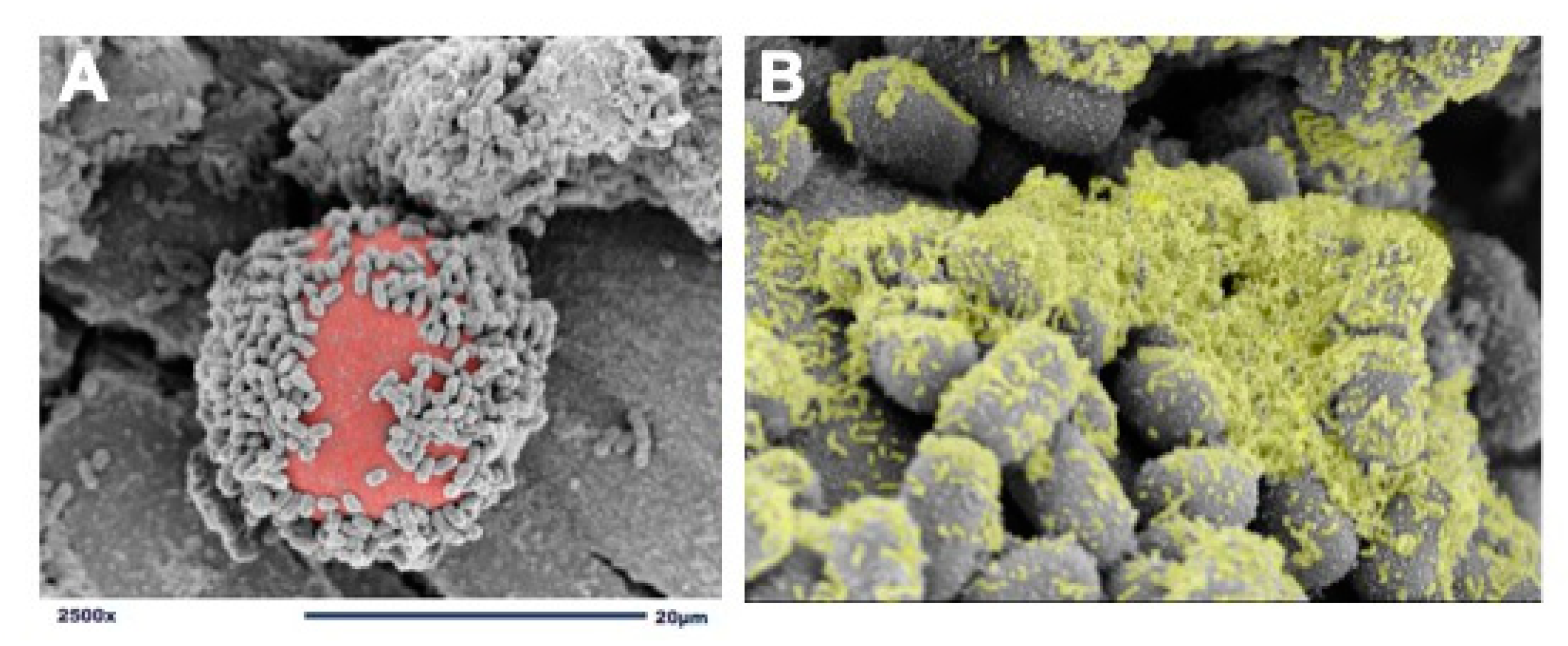
We use dextranomer microspheres (DMs) as a surface for L. reuteri attachment and biofilm formation [40]. DMs are porous, semi-permeable, biocompatible, biodegradable, non-immunogenic, non-allergic, Generally Recognized As Safe (GRAS) microspheres composed of cross-linked dextran. DMs are currently being used in several Food and Drug Administration (FDA)-approved medical products and are accepted as safe for human administration [48][49][50]. In order to create a probiotic biofilm, cultures of L. reuteri are introduced to DMs and undergo a brief incubation period to allow for adherence and biofilm formation (Figure 1). Importantly, known pathogens including Escherichia coli, Salmonella typhimurium, and Clostridioides difficile do not detectibly bind to DMs, thereby not providing pathobionts with a scaffold to adhere and grow [40]. Additionally, because DMs are porous, they can be preloaded with nutritious prebiotic substances that contribute to probiotic growth and promote further biofilm production. For example, disaccharides, that under regular circumstances would be promptly diluted, metabolized, and absorbed within the proximal gastrointestinal tract, will remain undiluted within the DMs and gradually diffuse out to provide their beneficial prebiotic contents at high concentrations discriminatively to the adhered probiotics. DMs are used to take advantage of L. reuteri’s GTF native ability to bind to cross-linked dextran. The GTF-dependent selective binding of L. reuteri to DMs results in a biofilm state with: (1) enhanced binding of L. reuteri to intestinal epithelial cells, (2) protection against low gastric pH, and (3) access to high concentrations of beneficial luminal substances to L. reuteri in order to augment its probiotic effects.
Figure 1. Adherence of L. reuteri to dextranomer microspheres. (A) scanning electron microscopy (SEM) image demonstrating the adherence of L. reuteri to the surface of a biocompatible dextranomer microsphere (DM; red); (B) magnified SEM image demonstrating the production of biofilm (green) by L. reuteri adhered to a sucrose-loaded DM.
3. Conclusions
Necrotizing enterocolitis continues to be a major source of morbidity and mortality for premature infants. Despite years of research and advancements in critical care, improvement in the outcomes of infants suffering from NEC are subtle at best. Probiotics have shown promise as a potential treatment to reduce the incidence and severity of NEC; however, current delivery methods present legitimate concerns. Our proposed method of delivering a single enteral dose of the probiotic Lactobacillus reuteri in a biofilm formulation alleviates most of these concerns. Investigations using our experimental animal model have demonstrated the ability of our L. reuteri biofilm formulation to significantly reduce the incidence and severity of NEC, decrease NEC-related mortality, stabilize the intestinal mucosal barrier, and down-regulate the production of proinflammatory cytokines. Given the effects of NEC and of gut microbes on infant neurodevelopment, future studies will determine whether our enhanced probiotic formulation will help prevent the deleterious effects of NEC on neurocognitive development.
Since probiotics offer a potential benefit in other infectious or inflammatory conditions, additional investigation is underway for the use of our enhanced probiotic formulation in the treatment and management of several other gastrointestinal diseases, including Clostridioides difficile colitis [51] and inflammatory bowel disease.
This entry is adapted from the peer-reviewed paper 10.3390/nu13030918
References
- Henry, M.C.W.; Moss, R.L. Necrotizing Enterocolitis. Annu. Rev. Med. 2009, 60, 111–124.
- Neu, J.; Walker, W.A. Necrotizing Enterocolitis. N. Eng. J. Med. 2011, 364, 255–264.
- Petty, J.K.; Ziegler, M.M. Operative strategies for necrotizing enterocolitis: The prevention and treatment of short-bowel syndrome. Semin. Pediatr. Surg. 2005, 14, 191–198.
- Ganguli, K.; Walker, W.A. Probiotics in the Prevention of Necrotizing Enterocolitis. J. Clin. Gastroenterol. 2011, 45, S133–S138.
- Hackam, D.J.; Good, M.; Sodhi, C.P. Mechanisms of gut barrier failure in the pathogenesis of necrotizing enterocolitis: Toll-like receptors throw the switch. Semin. Pediatr. Surg. 2013, 22, 76–82.
- Grölund, M.-M.; Lehtonen, O.-P.; Eerola, E.; Kero, P. Fecal Microflora in Healthy Infants Born by Different Methods of Delivery: Permanent Changes in Intestinal Flora After Cesarean Delivery. J. Pediatr. Gastroenterol. Nutr. 1999, 28, 19–25.
- Harmsen, H.J.M.; Wildeboer–Veloo, A.C.M.; Raangs, G.C.; Wagendorp, A.A.; Klijn, N.; Bindels, J.G.; Welling, G.W. Analysis of Intestinal Flora Development in Breast-Fed and Formula-Fed Infants by Using Molecular Identification and Detection Methods. J. Pediatr. Gastroenterol. Nutr. 2000, 30, 61–67.
- Mai, V.; Young, C.M.; Ukhanova, M.; Wang, X.; Sun, Y.; Casella, G.; Theriaque, D.; Li, N.; Sharma, R.; Hudak, M.; et al. Fecal Microbiota in Premature Infants Prior to Necrotizing Enterocolitis. PLoS ONE 2011, 6, e20647.
- Morrow, A.L.; Lagomarcino, A.J.; Schibler, K.R.; Taft, D.H.; Yu, Z.; Wang, B.; Altaye, M.; Wagner, M.; Gevers, D.; Ward, D.V.; et al. Early microbial and metabolomic signatures predict later onset of necrotizing enterocolitis in preterm infants. Microbiome 2013, 1, 1–16.
- Warner, B.B.; Deych, E.; Zhou, Y.; Hall-Moore, C.; Weinstock, G.M.; Sodergren, E.; Shaikh, N.; Hoffmann, J.A.; Linneman, L.A.; Hamvas, A.; et al. Gut bacteria dysbiosis and necrotising enterocolitis in very low birthweight infants: A prospective case-control study. Lancet 2016, 387, 1928–1946.
- Weng, M.; Walker, W.A. The role of gut microbiota in programming the immune phenotype. J. Dev. Orig. Health Dis. 2013, 4, 203–214.
- Guarner, F.; Khan, A.G.; Garisch, J.; Eliakim, R.; Gangl, A.; Thomson, A.; Krabshuis, J.; Lemair, T.; Kaufmann, P.; De Paula, J.A.; et al. World Gastroenterology Organisation Global Guidelines. J. Clin. Gastroenterol. 2012, 46, 468–481.
- Kleessen, B.; Bunke, H.; Tovar, K.; Noack, J.; Sawatzki, G. Influence of two infant formulas and human milk on the development of the faecal flora in newborn infants. Acta Paediatr. 1995, 84, 1347–1356.
- Mshvildadze, M.; Neu, J.; Mai, V. Intestinal microbiota development in the premature neonate: Establishment of a lasting commensal relationship? Nutr. Rev. 2008, 66, 658–663.
- Groer, M.W.; A Luciano, A.; Dishaw, L.J.; Ashmeade, T.L.; Miller, E.; A Gilbert, J. Development of the preterm infant gut microbiome: A research priority. Microbiome 2014, 2, 38.
- Berrington, J.E.; Stewart, C.J.; Embleton, N.D.; Cummings, S.P. Gut microbiota in preterm infants: Assessment and relevance to health and disease. Arch. Dis. Child. Fetal Neonatal Ed. 2013, 98, F286–F290.
- Cassir, N.; Simeoni, U.; La Scola, B. Gut microbiota and the pathogenesis of necrotizing enterocolitis in preterm neonates. Future Microbiol. 2016, 11, 273–292.
- Grishin, A.; Papillon, S.; Bell, B.; Wang, J.; Ford, H.R. The role of the intestinal microbiota in the pathogenesis of necrotizing enterocolitis. Semin. Pediatr. Surg. 2013, 22, 69–75.
- Pammi, M.; Cope, J.; Tarr, P.I.; Warner, B.B.; Morrow, A.L.; Mai, V.; Gregory, K.E.; Kroll, J.S.; McMurtry, V.; Ferris, M.J.; et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: A systematic review and meta-analysis. Microbiome 2017, 5, 1–15.
- Wang, Y.; Hoenig, J.D.; Malin, K.J.; Qamar, S.; Petrof, E.O.; Sun, J.; Antonopoulos, D.A.; Chang, E.B.; Claud, E.C. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J. 2009, 3, 944–954.
- Hoyos, A.B. Reduced incidence of necrotizing enterocolitis associated with enteral administration of Lactobacillus acidophilus and Bifidobacterium infantis to neonates in an intensive care unit. Int. J. Infect. Dis. 1999, 3, 197–202.
- Reid, G.; Jass, J.; Sebulsky, M.T.; McCormick, J.K. Potential Uses of Probiotics in Clinical Practice. Clin. Microbiol. Rev. 2003, 16, 658–672.
- Wang, Q.; Dong, J.; Zhu, Y. Probiotic supplement reduces risk of necrotizing enterocolitis and mortality in preterm very low-birth-weight infants: An updated meta-analysis of 20 randomized, controlled trials. J. Pediatr. Surg. 2012, 47, 241–248.
- AlFaleh, K.; Anabrees, J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst. Rev. 2014, CD005496.
- Bin-Nun, A.; Bromiker, R.; Wilschanski, M.; Kaplan, M.; Rudensky, B.; Hammerman, C. Oral Probiotics Prevent Necrotizing Enterocolitis in Very Low Birth Weight Neonates. J. Pediatr. 2005, 147, 192–196.
- Braga, T.D.; Da Silva, G.A.P.; De Lira, P.I.C.; Lima, M.D.C. Efficacy of Bifidobacterium breve and Lactobacillus casei oral supplementation on necrotizing enterocolitis in very-low-birth-weight preterm infants: A double-blind, randomized, controlled trial. Am. J. Clin. Nutr. 2010, 93, 81–86.
- Lin, H.-C.; Hsu, C.-H.; Chen, H.-L.; Chung, M.-Y.; Hsu, J.-F.; Lien, R.-I.; Tsao, L.-Y.; Chen, C.-H.; Su, B.-H. Oral Probiotics Prevent Necrotizing Enterocolitis in Very Low Birth Weight Preterm Infants: A Multicenter, Randomized, Controlled Trial. Pediatrics 2008, 122, 693–700.
- Good, M.; Sodhi, C.P.; Ozolek, J.A.; Buck, R.H.; Goehring, K.C.; Thomas, D.L.; Vikram, A.; Bibby, K.; Morowitz, M.J.; Firek, B.; et al. Lactobacillus rhamnosus HN001 decreases the severity of necrotizing enterocolitis in neonatal mice and preterm piglets: Evidence in mice for a role of TLR9. Am. J. Physiol. Liver Physiol. 2014, 306, G1021–G1032.
- Underwood, M.A.; Arriola, J.; Gerber, C.W.; Kaveti, A.; Kalanetra, K.M.; Kananurak, A.; Bevins, C.L.; Mills, D.A.; Dvorak, B. Bifidobacterium longum subsp. infantis in experimental necrotizing enterocolitis: Alterations in inflammation, innate immune response, and the microbiota. Pediatr. Res. 2014, 76, 326–333.
- Weng, M.; Ganguli, K.; Zhu, W.; Shi, H.N.; Walker, W.A. Conditioned medium from Bifidobacteria infantis protects against Cronobacter sakazakii-induced intestinal inflammation in newborn mice. Am. J. Physiol. Liver Physiol. 2014, 306, G779–G787.
- Bergmann, K.R.; Liu, S.X.; Tian, R.; Kushnir, A.; Turner, J.R.; Li, H.-L.; Chou, P.M.; Weber, C.R.; De Plaen, I.G. Bifidobacteria Stabilize Claudins at Tight Junctions and Prevent Intestinal Barrier Dysfunction in Mouse Necrotizing Enterocolitis. Am. J. Pathol. 2013, 182, 1595–1606.
- Arciero, J.; Ermentrout, G.B.; Siggers, R.; Afrazi, A.; Hackam, D.; Vodovotz, Y.; Rubin, J. Modeling the interactions of bacteria and Toll-like receptor-mediated inflammation in necrotizing enterocolitis. J. Theor. Biol. 2013, 321, 83–99.
- Ding, W.; Shah, N. Acid, Bile, and Heat Tolerance of Free and Microencapsulated Probiotic Bacteria. J. Food Sci. 2007, 72, M446–M450.
- Alander, M.; Satokari, R.; Korpela, R.; Saxelin, M.; Vilpponen-Salmela, T.; Mattila-Sandholm, T.; Von Wright, A. Persistence of Colonization of Human Colonic Mucosa by a Probiotic Strain, Lactobacillus rhamnosus GG, after Oral Consumption. Appl. Environ. Microbiol. 1999, 65, 351–354.
- Mackos, A.R.; Galley, J.D.; Eubank, T.D.; Easterling, R.S.; Parry, N.M.; Fox, J.G.; Lyte, M.; Bailey, M.T. Social stress-enhanced severity of Citrobacter rodentium-induced colitis is CCL2-dependent and attenuated by probiotic Lactobacillus reuteri. Mucosal Immunol. 2016, 9, 515–526.
- De Groote, M.A.; Frank, D.N.; Dowell, E.; Glode, M.P.; Pace, N.R. Lactobacillus rhamnosus gg bacteremia associated with probiotic use in a child with short gut syndrome. Pediatr. Infect. Dis. J. 2005, 24, 278–280.
- Kunz, A.N.; Noel, J.M.; Fairchok, M.P. Two Cases of Lactobacillus Bacteremia During Probiotic Treatment of Short Gut Syndrome. J. Pediatr. Gastroenterol. Nutr. 2004, 38, 457–458.
- Land, M.H.; Rouster-Stevens, K.; Woods, C.R.; Cannon, M.L.; Cnota, J.; Shetty, A.K. Lactobacillus sepsis associated with probiotic therapy. Pediatrics 2005, 115, 178–181.
- Salminen, M.K.; Tynkkynen, S.; Rautelin, H.; Saxelin, M.; Vaara, M.; Ruutu, P.; Sarna, S.; Valtonen, V.; Järvinen, A. Lactobacillus Bacteremia during a Rapid Increase in Probiotic Use of Lactobacillus rhamnosus GG in Finland. Clin. Infect. Dis. 2002, 35, 1155–1160.
- Navarro, J.B.; Mashburn-Warren, L.; Bakaletz, L.O.; Bailey, M.T.; Goodman, S.D. Enhanced Probiotic Potential of Lactobacillus reuteri When Delivered as a Biofilm on Dextranomer Microspheres That Contain Beneficial Cargo. Front. Microbiol. 2017, 8, 489.
- Olson, J.K.; Rager, T.M.; Navarro, J.B.; Mashburn-Warren, L.; Goodman, S.D.; Besner, G.E. Harvesting the benefits of biofilms: A novel probiotic delivery system for the prevention of necrotizing enterocolitis. J. Pediatr. Surg. 2016, 51, 936–941.
- Salas-Jara, M.J.; Ilabaca, A.; Vega, M.; García, A. Biofilm Forming Lactobacillus: New Challenges for the Development of Probiotics. Microorganisms 2016, 4, 35.
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the Natural environment to infectious diseases. Nat. Rev. Genet. 2004, 2, 95–108.
- Han, C.; Song, J.; Hu, J.; Fu, H.; Feng, Y.; Mu, R.; Xing, Z.; Wang, Z.; Wang, L.; Zhang, J.; et al. Smectite promotes probiotic biofilm formation in the gut for cancer immunotherapy. Cell Rep. 2021, 34, 108706.
- Alfarrayeh, I.; Fekete, C.; Gazdag, Z.; Papp, G. Propolis ethanolic extract has double-face in vitro effect on the planktonic growth and biofilm formation of some commercial probiotics. Saudi J. Biol. Sci. 2021, 28, 1033–1039.
- Tan, L.; Fu, J.; Feng, F.; Liu, X.; Cui, Z.; Li, B.; Han, Y.; Zheng, Y.; Yeung, K.W.K.; Li, Z.; et al. Engineered probiotics biofilm enhances osseointegration via immunoregulation and anti-infection. Sci. Adv. 2020, 6, eaba5723.
- Wu, D.; Li, X.; Yu, Y.; Gong, B.; Zhou, X. Heparin stimulates biofilm formation of Escherichia coli strain Nissle 1917. Biotechnol. Lett. 2021, 43, 235–246.
- Stenberg, A.; Lackgren, G. A New Bioimplant for the Endoscopic Treatment of Vesicoureteral Reflux: Experimental and Short-term Clinical Results. J. Urol. 1995, 154, 800–803.
- Jacobsson, S.; Jonsson, L.; Rank, F.; Rothman, U. Studies on Healing of Debrisan-Treated Wounds. Scand. J. Plast. Reconstr. Surg. 1976, 10, 97–101.
- Hoy, S.M. Dextranomer in Stabilized Sodium Hyaluronate (Solesta®). Drugs 2012, 72, 1671–1678.
- Shelby, R.D.; Janzow, G.E.; Mashburn-Warren, L.; Galley, J.; Tengberg, N.; Navarro, J.; Conces, M.; Bailey, M.T.; Goodman, S.D.; Besner, G.E. A novel probiotic therapeutic in a murine model of Clostridioides difficile colitis. Gut Microbes 2020, 12.