Two-photon imaging (2PI) is a fluorescence-based laser scanning microscopy technique commonly used in studies across various fields of research, including neurobiology, embryology, and tissue engineering. In principle, it involves two infrared photons simultaneously exciting a single fluorophore in a sample, thereby causing it to emit light in a specific wavelength region, also called fluorescence emission spectrum. This fluorescence is normally detected in a wavelength region close to the maximum of this spectrum, allowing the sample to be identified based on its specific fluorescent characteristics.
- two-photon
- 2-photon
- imaging
- microscopy
- epilepsy
- seizures
- neurovascular
1. Introduction
Compared to confocal single-photon microscopy, the main advantages of 2PI include reduced damage of tissues, allowing intravital studies, greater penetration depth of up to 2 mm in the brain, and ex vivo deep tissue imaging. Simultaneously, it maintains the typical characteristics of confocal microscopy, such as optical slicing capabilities with the possibility to perform 3-dimensional (3D) structural visualization and quantification, and its high subcellular XY resolution of around 400 nanometers [1][2][3]. We also note that, while many clinical techniques, such as ultrasound, CT, PET, and MRI, can penetrate much deeper in tissues, their resolution is up to a factor 1000 worse and cannot image at a subcellular level. At the moment, 2PI is mostly applied in biological research, but its potential is strongly underestimated and undervalued. Its possibilities in 3D visualization and quantification deserve more attention. There are developments in the direction of medical applications and endoscopy.
2. Two-Photon Imaging and Epilepsy
There is an enormous need to further understand the pathophysiology of epilepsy. Being one of the most common neurological disorders, epilepsy accounts for the highest disability-adjusted life years [4], affecting around sixty million people worldwide [5], of whom 30–40% are drug-resistant [6]. The societal burden of chronic epilepsy is massive and encompasses around 80% of total epilepsy-related costs [4].
In recent years, epilepsy research has made a shift toward a vascular-centered concept of epileptogenesis [7][8][9][10]. This has resulted in the identification of an important role of neurovascular alterations [11] and increased blood–brain barrier permeability in the pathophysiology of epilepsy [12][13][14]. Indeed, recent reports have emphasized similarities between alterations in epilepsy and microvascular dysfunction [15][16][17]. Additionally, oxidative stress has been implicated in epilepsy—as this process has been shown to induce structural cell damage and to facilitate the formation of lipofuscin, which itself also induces cellular damage [18][19][20]. Nevertheless, further research into the different aspects of the pathophysiology of epilepsy is needed to connect these areas.
In this regard, the application of 2PI in epilepsy research entails high potential to improve our understanding of seizure-induced anatomical and pathophysiological changes across different scales, ranging from neuronal structures to neuronal microcircuits and the neurovasculature [21][22]. Moreover, advances in two-photon laser scanning have enabled in vivo imaging of the brain while preserving the natural neuronal environment [23]. Consequently, pathological activity in these in vivo models may represent epileptic conditions more accurately [24]. Furthermore, two-photon uncaging is a powerful technique that is of significance in epilepsy research. It essentially involves the use of the two-photon excitation laser to photolyse or activate certain biological compounds such as neurotransmitters while also allowing the visualization of the immediate effects of this photochemical excitation without any other interference. In addition, fluorescence lifetime imaging, a technique based on differences in the decay rates (on the nanoseconds time-scale) of fluorophores in samples rather than intensity [25] can be advantageous when studying specific types of environments and the effects thereof on fluorophore. Lifetime imaging can be used with two-photon excitation (2P-FLIM) as well as with confocal microscopy and multiphoton tomography. When probing molecular environments wherein intensity-based measurements alone are often insufficient, fluorescence lifetime-based measurements may be capable of yielding additional data [25]. Finally, the development of a wide range of fluorescent markers has made it possible to study various aspects of the pathophysiology of epilepsy, such as neurotransmitter levels [26][27], metabolism [27][28], pathological neuronal activity [26], and hypersynchronous network firing [29].
Previous studies that employed 2PI to evaluate the mechanisms associated with epileptic seizures have indeed contributed significantly to our understanding of this complex disorder.
Oxidative Stress
Oxidative stress plays an important pathophysiological role in intracellular mechanisms in all of the aforementioned cells and processes [30][31][32]. Most of the involved proteins and molecules in oxidative stress are difficult to assess due to their short lifespan and rapid reactivity with redox state regulating components [33]. Hence, a robust analysis of the role of oxidative stress in epilepsy pathomechanisms using 2PI requires assessment of non-degradable fluorescent or autofluorescent composites. In this regard, lipofuscin forms an interesting end-product of oxidative stress as it is a nondegradable substance with strong and specific autofluorescent properties [34][35]. It is most commonly found in the cytoplasm of cells with a low rate of mitosis, such as neurons [36]. Its aggregation is associated with the pathophysiology of epilepsy as well as with neurodegenerative disorders [20][37]. We recently associated lipofuscin with the pathophysiology of epilepsy using 2PI in vascular and tissue samples of epilepsy patients that were harvested following surgical resection of epileptic brain regions [19]. We found that lipofuscin is present in the brain’s pial arterial wall and neocortical parenchyma in young epilepsy patients and age-matched controls. In the three-dimensional reconstruction of the 2PI image stack, the particles appeared to be mainly located within the adventitia and to a lesser extent in the tunica media (shown in Figure 1) [19]. Further quantitative analysis of lipofuscin particles suggested that progressive accumulation of lipofuscin in the vascular wall may be related to the pathophysiology of epilepsy.
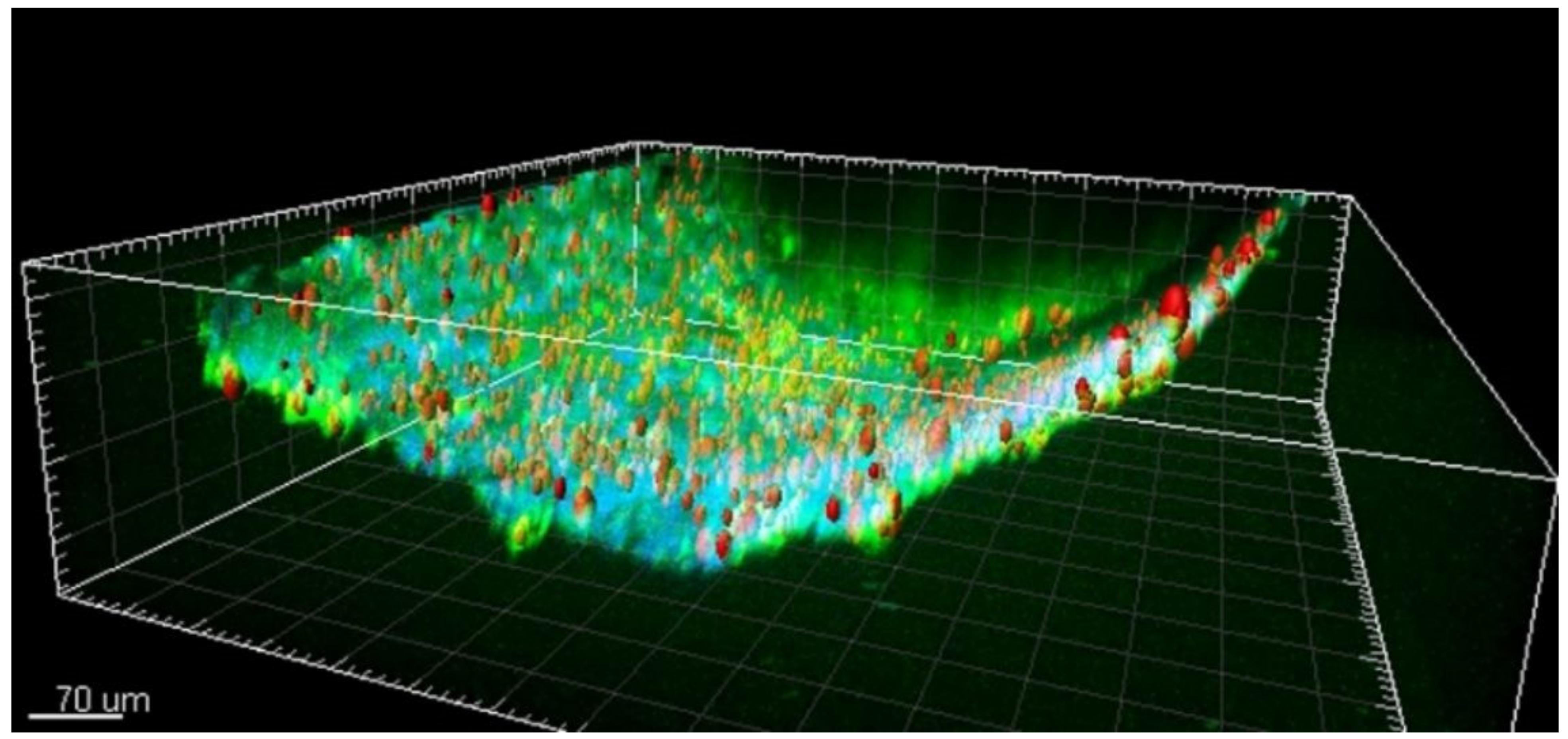
Figure 1. A representative two-photon 3D reconstruction image of lipofuscin autofluorescent particles. Scalebar: 70 μm. Adapted from “Shedding light on human cerebral lipofuscin: An explorative study on identification and quantification” (Hakvoort et al., Journal of Comparative Neurology, 2020 [19]). A license to reuse this figure from its original source was acquired through RightsLink. (Reprinted with permission from ref. [19]. Copyright 2020 IEEE).
Previous studies also reported on lipofuscin aggregation in association with human epilepsy. Liu et al. [38] described excessive lipofuscin accumulation within dysmorphic neurons in young adult patients with focal frontal lobe epilepsy. Furthermore, lipofuscin has been found in mesial temporal lobe structures of epilepsy patients. Here, oxidative damage and neuronal degeneration induced by seizures seemed to contribute to the formation of lipofuscin in the hippocampus of kainate-treated mouse epilepsy models [39]. In patients with temporal lobe epilepsy, the hippocampal antioxidative system was upregulated [40]. This finding was most pronounced in patients with hippocampal sclerosis [40]. Another article reported on the identification of lipofuscin granules within the entorhinal regions and amygdala of epileptic tissue [41].
These findings provide a basis for understanding the potential involvement of oxidative alterations in the pathophysiology of epilepsy. Analysis of lipofuscin could be a new method to detect and quantify oxidative stress. Using 2PI, lipofuscin is recognized relatively easily based on its specific autofluorescent and lifetime characteristics [19]. Furthermore, the finding of lipofuscin accumulation within the vascular wall in addition to lipofuscin-accumulation in post-mitotic cells such as neurons warrants further assessment.
This entry is adapted from the peer-reviewed paper 10.3390/app11052404
References
- Helmchen, F.; Denk, W. Deep tissue two-photon microscopy. Nat. Methods 2005, 2, 932–940.
- Zipfel, W.R.; Williams, R.M.; Webb, W.W. Nonlinear magic: Multiphoton microscopy in the biosciences. Nat. Biotechnol. 2003, 21, 1369–1377.
- Douma, K.; Megens, R.T.; Reitsma, S.; Prinzen, L.; Slaaf, D.W.; van Zandvoort, M.A. Two-photon lifetime imaging of fluorescent probes in intact blood vessels: A window to sub-cellular structural information and binding status. Miscrosc. Res. Tech 2007, 70, 467–475.
- Beghi, E. Addressing the burden of epilepsy: Many unmet needs. Pharmacol. Res. 2016, 107, 79–84.
- Moshé, S.; Perucca, E.; Ryvlin, R.; Tomson, T. Epilepsy: New advances. Lancet 2015, 385, 884–898.
- Chen, Z.; Brodie, M.J.; Liew, D.; Kwan, P. Treatment Outcomes in Patients With Newly Diagnosed Epilepsy Treated With Established and New Antiepileptic Drugs. JAMA Neurol. 2018, 75, 279–286.
- Garbelli, R.; De Bock, F.; Medici, V. PDGFRβ(+) cells in human and experimental neuro-vascular dysplasia and seizures. Neuroscience 2015, 306, 18–27.
- Rigau, V.; Morin, M.; Rousset, M.C.; De Bock, F.; Lebrun, A.; Coubes, P.; Picot, M.C.; Baldy-Moulinier, M.; Bockaert, J.; Crespel, A.; et al. Angiogenesis is associated with blood-brain barrier permeability in temporal lobe epilepsy. Brain 2007, 130, 1942–1956.
- Kastanauskaite, A.; Alonso-Nanclares, L.; Blazquez-Llorca, L.; Pastor, J.; Sola, R.G.; Defelipe, J. Alterations of the microvascular network in sclerotic hippocampi from patients with epilepsy. J. Neuropathol. Exp. Neurol. 2009, 68, 939–950.
- Heinemann, U.; Kaufer, D.; Friedman, A. Blood-brain barrier dysfunction, TGFβ signaling, and astrocyte dysfunction in epilepsy. Glia 2012, 60, 1251–1257.
- Haeren, R.H.; Hartmans, S.A.; De Mey, J.; Hoogland, G.; Dings, J.T.; Schijns, O.E.; Van Kuijk, S.M.; Rijkers, K.; Schiffers, P.H.; van Overbeeke, J.J. Cerebral Artery Vasoconstriction is Endothelin-1 Dependent Requiring Neurogenic and Adrenergic Crosstalk. Curr. Neurovasc. Res. 2018, 14, 306–315.
- Van Vliet, E.A.; Auraujo, S.D.; Redeker, S.; Van Schaik, R.; Aronica, E.; Gorter, J.A. Blood-brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain 2006, 130, 521–534.
- Van Vliet, E.; Aronica, E.; Gorter, J. Blood–brain barrier dysfunction, seizures and epilepsy. Semin. Cell Dev. Biol. 2015, 38, 26–34.
- Van Vliet, E.A.; Otte, W.M.; Wadman, W.J.; Aronica, E.; Kooij, G.; De Vries, H.E.; Dijkhuizen, R.M.; Gorter, J.A. Blood-brain barrier leakage after status epilepticus in rapamycin-treated rats II: Potential mechanisms. Epilepsia 2015, 57, 70–78.
- Brigo, F.; Lattanzi, S. Poststroke seizures as stroke mimics: Clinical assessment and management. Epilepsy Behav. 2020, 104, 106297.
- Ndode-Ekane, X.E.; Hayward, N.M.; Gröhn, O.; Pitkänen, A. Vascular changes in epilepsy: Functional consequences and association with network plasticity in pilocarpine-induced experimental epilepsy. Neuroscience 2010, 116, 312–332.
- Haeren, R.H.; Rijkers, K.; Schijns, O.E.; Dings, J.T.; Hoogland, G.; van Zandvoort, M.A.; Vink, H.; van Overbeeke, J.J. In vivo assessment of the human cerebral microcirculation and its glycocalyx: A technical report. J. Neurosci. Methods 2018, 303, 114–125.
- Czerska, M.; Mikolajewska, K.; Zielinski, M.; Gromadzinska, J.; Wasowicz, W. Today’s oxidative stress markers. Med. Pr. 2015, 66, 393–405.
- Hakvoort, K.; Otto, L.; Haeren, R.H.; Hoogland, G.; Schijns, O.E.; Vink, H.; Klein, D.; van Zandvoort, M.A.; Rijkers, K. Shedding light on human cerebral lipofuscin: An explorative study on identification and quantification. J. Comp. Neurol. 2021, 529, 605–615.
- García, A.M.; Kun, A.; Calero, O.; Medina, M.; Calero, M. An overview of the role of lipofuscin in age-related neurodegeneration. Front. Neurosci. 2018, 12, 464.
- Douma, K.; Oostendorp, M.; Slaaf, D.W.; Post, M.J.; Backes, W.H.; van Zandvoort, M.A. Evaluation of magnetic resonance vessel size imaging by two-photon laser scanning microscopy. Magn. Reson. Med. 2010, 63, 930–939.
- Haeren, R.H.; Van de Ven, S.E.; van Zandvoort, M.A.; Vink, H.; van Overbeeke, J.J.; Hoogland, G.; Rijkers, K. Assessment and Imaging of the Cerebrovascular Glycocalyx. Curr. Neurovasc. Res. 2016, 13, 249–260.
- Hillman, E.M.C. Optical brain imaging in vivo: Techniques and applications from animal to man. J. Biomed. Opt. 2007, 12, 051402.
- Svoboda, K.; Yasuda, R. Principles of Two-Photon Excitation Microscopy and Its Applications to Neuroscience. Neuron 2006, 50, 823–839.
- Datta, R.; Heaster, T.M.; Sharick, J.T.; Gillette, A.A.; Skala, M.C. Fluorescence lifetime imaging microscopy: Fundamentals and advances in instrumentation, analysis, and applications. J. Biomed. Opt. 2020, 25, 1–43.
- Hoogland, G.; Hens, J.J.; De Wit, M.; van Veelen, C.W.; van Huffelen, A.C.; Gispen, W.H.; de Graan, P.N. Glutamate and gamma-aminobutyric acid content and release of synaptosomes from temporal lobe epilepsy patients. J. Neurosci. Res. 2000, 60, 686–695.
- Schijns, O.E.; Karaca, Ü.; Andrade, P.; De Nijs, L.; Küsters, B.; Peeters, A.; Dings, J.T.; Pannek, H.; Ebner, A.; Rijkers, K.; et al. Hippocampal GABA transporter distribution in patients with temporal lobe epilepsy and hippocampal sclerosis. J. Chem. Neuroanat. 2015, 68, 39–44.
- Upreti, C.; Otero, R.; Partida, C.; Skinner, F.; Thakker, R.; Pachecho, L.; Zhou, Z.; Maglakelidze, G.; Veliskova, J.; Romanovicz, D.; et al. Altered neurotransmitter release, vesicle recycling and presynaptic structure in the pilocarpine model of temporal lobe epilepsy. Brain 2012, 135, 869–885.
- Rossi, L.F.; Kullmann, D.M.; Wykes, R.C. The Enlightened Brain: Novel Imaging Methods Focus on Epileptic Networks at Multiple Scales. Front. Cell. Neurosci. 2018, 12, 82.
- Ogaki, A.; Ikegaya, Y.; Koyama, R. Vascular Abnormalities and the Role of Vascular Endothelial Growth Factor in the Epileptic Brain. Front. Pharmacol. 2020, 11, 1–8.
- Puttachary, S.; Sharma, S.; Stark, S.; Thippeswamy, T. Seizure-induced oxidative stress in temporal lobe epilepsy. Biomed. Res. Int. 2015, 2015, 1–20.
- Merelli, A.; Repetto, M.; Lazarowksi, A.; Auzmendi, J. Hypoxia, Oxidative Stress, and Inflammation: Three Faces of Neurodegenerative Diseases. J. Alzheimers Dis. 2020, 1–18.
- Katerji, M.; Filippova, M.; Duerksen-Hughes, P. Approaches and Methods to Measure Oxidative Stress in Clinical Samples: Research Applications in the Cancer Field. Oxid. Med. Cell. Longev. 2019, 2019, 1–29.
- Terman, A.; Brunk, U.T. Lipofuscin. Int. J. Biochem. Cell Biol. 2004, 36, 1400–1404.
- Tohma, H.; Hepworth, A.R.; Shavlakadze, T.; Grounds, M.D.; Arthur, P.G. Quantification of ceroid and lipofuscin in skeletal muscle. J. Histochem. Cytochm. 2011, 59, 769–779.
- Merlo, S.; Nakayama, A.B.S.; Brusco, J.; Rossi, M.A.; Carlotti, C.G.; Moreira, J.E. Lipofuscin Granules in the Epileptic Human Temporal Neocortex with Age. Ultrastruct. Pathol. 2015, 39, 378–384.
- Kohlschütter, A.; Schulz, A. CLN2 Disease (Classic Late Infantile Neuronal Ceroid Lipofuscinosis). Pediatr. Endocrinol. Rev. 2016, 13, 682–688.
- Liu, J.Y.; Reeves, C.; Diehl, B.; Coppola, A.; Al-Hajri, A.; Hoskote, C.; Al Maghairy, S.; Tachrount, M.; Groves, M.; Michalak, Z.; et al. Early Lipofuscin Accumulation in Frontal Lobe Epilepsy. Ann. Neurol. 2016, 80, 882–895.
- Kim, H.C.; Bing, G.; Jhoo, W.K.; Kim, W.K.; Shin, E.J.; Park, E.S.; Choi, Y.S.; Lee, D.W.; Shin, C.Y.; Ryu, J.R.; et al. Oxidative damage causes formation of lipofuscin-like substances in the hippocampus of the senescence-accelerated mouse after kainate treatment. Behav. Brain Res. 2002, 131, 211–220.
- Ristic, A.J.; Savić, D.; Sokić, D.; Pristov, J.; Nestorov, J.; Baščarević, V.; Raičević, S.; Savić, S.; Spasojević, I. Hippocampal antioxidative system in mesial temporal lobe epilepsy. Epilepsia 2015, 56, 789–799.
- Yilmazer-Hanke, D.M.; Wolf, H.K.; Schramm, J.; Elger, C.E.; Wiestler, O.D.; Blümcke, I. Subregional Pathology of the Amygdala Complex and Entorhinal Region in Surgical Specimens From Patients With Pharmacoresistant Temporal Lobe Epilepsy. J. Neuropathol. Exp. Neurol. 2000, 59, 907–920.
