In recent years, titanium dioxide (TiO2) has increasingly been used as an inorganic ultraviolet (UV) filter for sun protection. However, nano-TiO2 may also pose risks to the health of humans and the environment. Thus, to adequately assess its potential adverse effects, a comprehensive understanding of the behaviour and fate of TiO2 in different environments is crucial. Advances in analytical and modelling methods continue to improve researchers’ ability to quantify and determine the state of nano-TiO2 in various environments. However, due to the complexity of environmental and nanoparticle factors and their interplay, this remains a challenging and poorly resolved feat.
- nano-TiO2
- inorganic UV filter
- sunscreen
- surface coating deterioration
- SP-ICP-MS
- fate and transport modelling
- risk assessment
- Bayesian network
- aquatic pollution
1. Introduction
Skin cancer (melanoma and non-melanoma) is one of the most common cancer types worldwide [1][2]. To reduce the risks of it, public awareness campaigns that promote the importance of the use of broad-spectrum (280–400 nm) ultraviolet radiation (UVR) protection have increased over the last few decades. These campaigns have been encouraging the use of sunscreen as an important protection measure. As a result, the use of sunscreen globally has increased significantly. For example, due to the Australian campaign ‘SunSmart’, sunscreen use has risen by 25% over the past 30 years [3].
However, with the increased application of sunscreen, there are concerns regarding the inevitable release and environmental consequences of sunscreen residues. These concerns are further cemented because the full extent of potential environmental consequences has not yet been clarified. Based on the risks to human and environmental health, some governments have started introducing national bans on certain organic ultraviolet (UV) filters [4]. Subsequently, inorganic UV filters such as titanium dioxide (TiO2) or zinc oxide (ZnO) gained popularity as the ‘reef-safe’ and ‘eco-friendly’ alternatives. However, there is a continued debate on whether inorganic UV filters, especially in nanoparticle (NP) form, are safe to use or whether they pose a risk to humans and the environment, particularly when used for longer periods.
Sobek et al. [5] classified TiO2 as toxic to aquatic life with long-lasting effects based on the regulation on classification, labelling, and packaging (CLP) of substances and mixtures. Furthermore, the International Agency for Research on Cancer [6] has categorised TiO2 as possibly carcinogenic (Group 2B).
Besides being potentially cytotoxic and genotoxic [7][8], TiO2 is a known photocatalyst and is capable of generating reactive oxygen species (ROS) such as hydrogen peroxide (H2O2), hydroxyl radicals (OH·), or singlet oxygen (1O2). This is another drawback of TiO2, as ROS can induce oxidative stress and catalyse DNA damage [9][10]. To reduce the catalytic activity, recent studies have been focusing on reducing the ROS production by either doping with other elements or by coating the synthesised NP [11]. However, previous studies have shown that these coatings can (partially) deteriorate when subject to some exposure conditions [12][13], affecting both the degree of toxicity and the fate of the environmental.
Even though the number of (eco-)toxicity studies has substantially increased over the last few decades, experimental studies that conduct long-term investigations are still limited [14] and rarely consider real environmental conditions as an exposure scenario [15]. Furthermore, Minetto et al. [15] reported that, as studies do not follow a standardised experimental procedure, results are rarely comparable and reproducible. Study outcomes of the same core material may also be inconsistent due to the effect of different particle properties, such as particle size (PS), shape, surface coating on the (eco-)toxicity, and the different NP behaviour in various environmental or laboratory settings [16][17]. A recent study published in 2021 reports that solar radiation significantly increases the ecotoxicity of nano-TiO2 in aquatic environments, particularly if organic UV filters are simultaneously present [18]. Consequently, eco-toxicologists need a better understanding of the particle behaviour to interpret the results appropriately and to develop innovative toxicity studies.
In addition to the common eco-toxicological dose descriptors, more behavioural toxicological endpoints are needed to identify potential risks; for example, the exposure to TiO2 caused substantial disruptions in the swimming behaviour of clownfish, whereas their mortality rate only slightly increased [19]. Another aspect that is understudied is the effect of chronic exposure to by-products of aged nano-TiO2 in aquatic organisms. For instance, Fouqueray et al. [20] observed a decrease in growth and reproduction of Daphnia Magna that was fed nano-TiO2 residue-contaminated algae over 21 days. Such observations also raise concerns regarding the potential for trophic transfer and bioaccumulation in the food chain.
Based on a literature search, algae were the most vulnerable group of aquatic organisms when exposed to nano-TiO2 with a no observed effect concentration (NOEC) of <0.2 mg TiO2/L [21]. The NOEC can be converted to the predicted no-effect concentration (PNEC) by dividing the NOEC by an assessment factor (AF). Estimates of the PNEC are essential for carrying out an environmental risk assessment (ERA). Due to the limited number of long-term studies, applying an AF of 100–1000 is considered conservative and reasonable [22]. Based on these estimates, the PNEC value is 0.2–2 µg/L, which is consistent with the findings of previous studies, which have produced values such as 1 µg/L [23], 16 µg/L [24], and 20 µg/L [25].
Besides the PNEC value, a precise determination of the predicted environmental concentration (PEC) of nano-TiO2 is essential for an accurate ERA. The PEC can be derived from both analytical and modelling studies; however, neither approach has, so far, been able to resolve the variability of PEC in different environments in detail [26]. This can partly be attributed to challenges associated with appropriate and robust analytical methods but also to the complexity and breadth of this emergent field. As analytical techniques are not able to explain all real-scenario observations, modelling approaches are considered a critical and complementary tool to fill the knowledge gaps [27]. Understanding the behaviour of nano-TiO2 in diverse environments is important for building a robust model. However, this can rarely be done through analytical studies, and, therefore, it necessitates hybrid approaches to overcome these challenges.
2. Distribution and Fate of Nano-TiO2 in the Environment
Owing to its unique properties, nano-TiO2 is applied widely, including to cosmetics (59%), paint and coatings (13%), electronics (7%), cleaning agents (6%), filters (6%), plastics (4%), and wastewater (WW) disinfectants (<1%) [28]. It is estimated that sunscreens make up about 25% of the nano-TiO2 market share [29]. Consequently, sunscreens are likely to be one of the significant sources of nano-TiO2 released into the environment.
2.1. Exposure Pathways to the Environment
To evaluate the environmental risk of nano-TiO2 in sunscreen, it is helpful to determine the potential input and distribution channels. Figure 1 illustrates the exposure pathways of nano-TiO2 from sunscreens to the environment. The release of nano-TiO2 into aqueous environments is likely to occur once users enter the water after applying sunscreen. Depending on the viscosity of the formulation, 10−40% of NPs are released from the initially applied sunscreen [30].
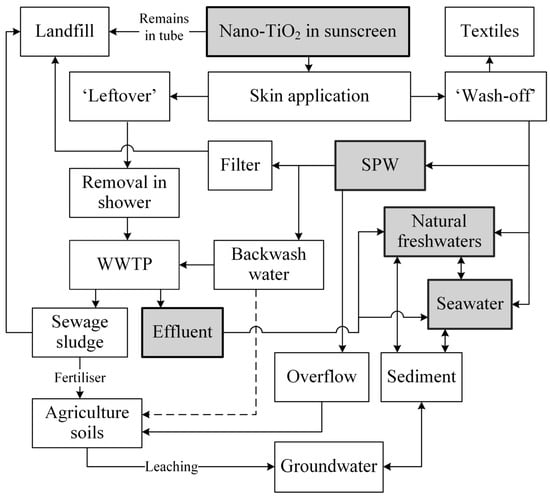
The remaining nano-TiO2 residues enter the WW stream after being washed off in the shower. In the WW treatment plants (WWTPs), it is estimated that, on average, approximately 34% and 87% of nano-TiO2 is removed from the primary and secondary settling tanks, respectively [31]. However, since sewage sludge is often used as soil fertiliser, nano-TiO2 could contaminate agricultural soils and may even leach into the groundwater, consequently re-entering the aqueous environment [32][33]. The leaching behaviour greatly depends on the nature of the soil and has not been studied adequately [14][34]. In one study, Dulger et al. [35] investigated the leaching potential of nano-TiO2 in landfills and concluded that about 81–97% of the introduced nano-TiO2 is retained on the solid surface. However, to date, the extent to which nano-TiO2 leaches from sewage sludge remains unclear.
Another exposure pathway that is only occasionally considered in the literature is the washing off of sunscreen during activities in swimming pool water (SPW). However, this should be considered as part of realistic and regionalised calculations as the number of privately owned swimming pools continues to increase steadily. For example, in Australia, residential pool ownership has risen from 12% to 13% over the last three years [36], and it is expected that sunscreen is used as most of these swimming pools are outdoors. Therefore, nano-TiO2 released from sunscreen is likely to be found in (1) diverse aqueous media (e.g., SPW, natural waters); (2) WWTPs; (3) sewage sludge; (4) agriculture soils; and (5) landfill. This review paper primarily focuses on the fate and behaviour of nano-TiO2 in the first of these environments, the aqueous media.
2.2. Behaviour and Fate of Nano-TiO2
Understanding the particle behaviour and fate of nano-TiO2 helps to predict its concentration and state in the environment, which then determine the risk and impact on the environment. The following three sections discuss the influence of selected nano-TiO2 properties and environmental conditions on the particle behaviour and fate and conclude with an overview of experimental research findings.
2.2.1. The Pivotal Role of Nano-TiO2 Characteristics
Commercially available nano-TiO2 suitable for incorporating into sunscreen formulas varies in its characteristics such as PS, shape, crystalline phase, core composition, and surface properties. These physicochemical properties can significantly affect the behaviour, fate, and (eco)toxicity of nano-TiO2 as discussed below.
Due to manufacturing processes, the PS of TiO2 can vary in precision and accuracy, and thus, is usually high in polydispersity that may extend beyond the nanometre scale. However, the PS for obtaining a transparent sunscreen with adequate UV attenuation typically ranges from 10−40 nm [37][38]. An increase in PS shifts the UV attenuation capability of TiO2 to longer wavelengths, and, hence, influences the range of UV protection [39]. Therefore, to maintain the desired UV attenuation, the tendency of smaller particles to form aggregates and agglomerates should be prevented [40]. However, smaller particles exhibit higher toxicity [41] and are even able to penetrate human skin and enter the bloodstream [42]. Similarly, Pelclova et al. [43] detected nano-TiO2 (<14 nm) in biological fluids such as plasma and urine due to the absorption of nano-TiO2 through the human skin. Moreover, the photocatalytic activity of TiO2 is size-dependent as a result of charge-carrier dynamics, light absorption and scattering efficiency, as well as the specific surface area [44].
The particle shape of nano-TiO2 found in sunscreens varies from spherical, elongated (rod-shaped, needle-shaped) to ellipsoidal forms [45][46]. The NP shape also affects the toxicity, including by influencing the rate of particle uptake by primary human immune cells [47]. However, to date, studies reporting correlations between particle shape and environmental or human health risks are limited [48][49].
TiO2 can exist in three crystalline forms: anatase, rutile, and brookite [32]. However, only pure forms of rutile or anatase or a mixture of both are incorporated in sunscreens [46][50]. Among other characteristics, the crystal structures determine the potential toxicity of TiO2. For example, anatase and anatase mixtures are under suspicion of causing oxidative stress or cytotoxicity [51][52]. Despite these concerns, many sunscreens often still contain anatase [46].
The core composition of nano-TiO2 can be altered by metal doping with, for example, manganese (Mg), vanadium (V), or iron (Fe) [32]. Variations in the core composition can change the overall physicochemical properties, and, thus, impact the holistic behaviour and fate of the NPs in the environment [53][54]. The aim of structural modifications is primarily to lower or quench the generation of ROS, which reduces the phototoxicity of nano-TiO2 [55][56].
The surface chemistry is a key factor that influences nano-TiO2 characteristics such as photocatalytic activity or dispersibility [57]. As smaller particles tend to aggregate readily [40], surface coatings are frequently applied to sustain particle stability in the sunscreen [58]. The formulation type of a sunscreen determines what surface treatment to use to promote dispersibility and particle stabilisation. Oil-in-water (O/W) types allow for the incorporation and stabilisation of nano-TiO2 as is, whereas water-in-oil (W/O) types usually require organic coatings that change the hydrophilic character of the surface to a lipophilic one [59]. The photocatalytic effect has also been suppressed by applying inorganic layers such as aluminium oxide Al2O3, aluminium hydroxide Al(OH)3, (hydrated) silica SiO2, or a combination of the above [60][61] to passivate the TiO2 surface. Additionally, they can be combined with organic surface treatments to improve the dispersibility in W/O types [62].
However, surface treatments can wear out over time and are susceptible to alteration or degradation when exposed to diverse media. Therefore, previous studies have attempted to examine the influence of extrinsic factors. For example, if hydrophobic organic or polymeric coatings such as polydimethylsiloxane (PDMS) are exposed to pH neutral ultrapure water, over time (hours to days), the PDMS layer completely dissolves and the particle surface becomes hydrophilic [63]. This promotes the dispersibility of nano-TiO2 in aqueous environments and the formation of stable colloids. Similarly, Auffan et al. [13] observed a complete dissolution of the PDMS layer and a partial deterioration of the Al(OH)3 layer in contact with water (pH = 5). On the contrary, Wu et al. [62] found that layers of a structurally related polymer (hydrogen dimethicone) do not dissolve in water and remain hydrophobic even after sonification. However, if 1% (v/v) organic solvent such as ethanol (EtOH) was added, the organic coating dissolved.
Nevertheless, the Al(OH)3 layer seems to retain its protective properties when in contact with deionised (DI) water, as the coating still inhibits the generation of ROS [13]. However, if exposed to SPW or seawater, a significant redistribution of the Al(OH)3 coating occurs [12][64]. Herein, it was found that especially chlorine (HOCl/OCl−) was responsible for diminishing the coating’s integrity [65]. The redistribution of the Al(OH)3 coating also resulted in enhanced photocatalytic activity compared to unaged nano-TiO2 [12]. According to Al-Abed et al. [12], the layer thickness is critical for the prevention of photocatalytic activities. Moreover, previous studies have indicated that UVR could also affect the integrity of the Al(OH)3 layer [62]. The damage or loss of protective surface layers not only influences transport or fate processes but also restores the photoactivity of nano-TiO2 [66] and is therefore undesirable.
To overcome these shortcomings and to ensure the sunscreen endures the harsh environmental conditions, new surface treatments (Table 1) have been developed in recent years. Table 1 summarises recent findings that primarily refer to novel organic and TiO2 hybrid nanostructures or the incorporation of TiO2 into inorganic auxiliary structures. Table 1 only consists of surface treatments that would seem viable for commercial use. We defined a surface treatment as viable if it had a realistic number of manufacturing process steps.
| Surface Treatment | Benefits | Reference | |
|---|---|---|---|
 R: Chemically coated lignosulfonate |
Lignin/TiO2 nanocomposites Previous study with similar approach: Encapsulation of TiO2 by different types of lignin [67] |
Good dispersibility; high sun protection factor (SPF) | [68] |
 CNC: Cellulose nanocrystals |
Organic/TiO2 hybrid nanostructures TiO2 NPs grafted onto cellulose nanocrystals (CNC) |
High SPF; in o/w: replaces surfactant in formulation | [69] |
 TA: Tannic acid p-MS: porous polymer microspheres |
Tannin/TiO2 multilayers on p-MS Deposition on porous polymethylmethacrylate (PMMA) microspheres Layer-by-layer assembly of TiO2 and TA via ligand-to-metal complexation |
Reduced ROS; high SPF | [70] |
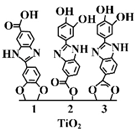 |
Surface functionalisation with Oxisol Oxisol: Polyphenol dihydroxyphenyl benzimidazole carboxylic acid Addition mechanism of Oxisol to TiO2 Steric stabilisation likely |
Reduced ROS; reduced photocatalytic activity; high SPF | [71] |
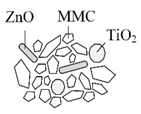 MMC: Mesoporous magnesium carbonate cluster |
MMC-TiO2-ZnO Incorporation of TiO2 and ZnO into an amorphous MMC structure (+ dimethicone layer) |
Comparable SPF; no photocatalytic activity | [72] |
 |
TiO2 multifunctional coating Dense and homogeneous encapsulation |
Improved esthetic feel; reduced photocatalytic activity; high SPF | [73] |
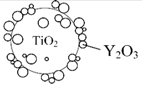 Y2O3: Yttrium oxide |
Y2O3/TiO2 nanocomposites Deposition of Y2O3 onto TiO2 Uniform Y2O3 coating |
Enhanced UV attenuation; reduced photocatalytic activity; biocompatible | [74] |
 CeO2: Cerium dioxide |
CeO2/TiO2 nanocomposites CeO2 nanodot encrusted TiO2 NPs |
Reduced photocatalytic activity; biocompatible | [75] |
Importantly, even though new surface treatments may improve the performance of TiO2-based UV filters, there still remain uncertainties around their ecotoxicity and fate. Given that the surface coatings are largely responsible for the fate, exposure and hazard of nano-TiO2, often independently of the TiO2 core [57], it is critical that new coatings and formulations are assessed for their (eco)toxicity before or as they become commercialised and mass-produced.
2.2.2. The Influence of Environmental Conditions
Assessing the influence of environmental factors such as pH, ionic strength (IS), and composition and occurrence of natural organic matter (NOM) is essential when evaluating the behaviour of nano-TiO2 in real environments. The impact of these factors on the colloidal stability can often be explained by the Derjaguin-Landau-Verwey-Overbeek (DLVO) theory [76][77] that considers the combined effects of van der Waals (VDW) attraction and electrical double layer (EDL) repulsion. However, in natural environmental systems, nano-TiO2 is likely to adsorb water molecules and macromolecules, such as NOM at the interface, which results in further interparticle forces (e.g., hydration or steric interactions). Therefore, the assessment of colloidal stability should also include non-DLVO forces. Based on experimental results (Table 2), three key factors were identified that substantially affect the behaviour of nano-TiO2.
Firstly, the interplay between the pH of the media and the isoelectric point (IEP) of TiO2 significantly influences the colloidal stability of nano-TiO2. That is, if the pH is close to the IEP, the surface charge changes sign with only small pH variations, considerably reducing the colloidal stability due to the annihilation of electrostatic repulsion [63]. Conversely, the NPs are considered stable if the zeta potential exceeds ±30 mV at the given pH of the environment [78].
Recent research has established that the IEPs of TiO2 NPs extracted from commercial sunscreens were all below 4.6 [45]. As the pH in natural waters is between 6–9 [79], the surface charge of TiO2 NP is expected to be negative. This promotes the stabilisation of nano-TiO2 due to EDL repulsion but only if no further environmental factors affect the colloidal stability.
The IEP of TiO2 differs depending on its crystalline structure (e.g., 3.5 for anatase and 6.5 for rutile) [80] and can change as a result of the application of additional coatings. For example, the IEP decreases if coated with SiO2 (IEP of 2.0) or increases if coated with Al2O3 or Al(OH)3 (IEP of 9 or 6.8, respectively) [80].These alterations can affect the fate and behaviour of nano-TiO2 in the environment. However, as the colloidal stability not only depends on the IEP as a function of the pH but also on the general chemistry of the environment, it is assumed that the primary aim of novel coatings is to reduce the photocatalytic activity rather than to influence the IEP of nano-TiO2.
Nevertheless, information on both the pH and IEP in combination with other influencing factors can aid in describing and predicting the fate and behaviour of nano-TiO2. For example, Englehart et al. [81] observed that a polymer sunscreen additive decreased the initial IEP of TiO2 from 6.3 to less than 5, which entailed a diminished capacity of water-saturated porous media (e.g., sand) to retain nano-TiO2 at environmentally relevant pH values.
Secondly, the IS and the type of ion also affect the colloidal stability of nano-TiO2 in diverse environments. Zhang et al. [82] investigated the impact of ions such as Na+, Ca2+, Cl−, and PO43− at a high and low IS on the stability of nano-TiO2. They observed that at a low IS the surface complexation with PO43− contributed to an elevated negative surface charge, thus promoting the NP stability due to the electrostatic repulsion. However, with an increasing IS, the negative surface charge of nano-TiO2 could no longer increase as the number of complexation sites on the particle surface was limited and already used up with PO43−. Consequently, at higher IS, the high compression of the EDL can overcome the limited electrostatic repulsion, leading to a destabilisation of the nano-TiO2. Furthermore, they observed that Na+ and Ca2+ were equally able to destabilise nano-TiO2 at the same IS, while some cations, especially divalent cations like Ca2+, were able to induce bridging of two negatively charged surfaces [83] and promote the formation of aggregates.
Thirdly, the type and concentration of NOM significantly affect the colloidal stability of nano-TiO2 as the adsorption of polymers at the interface can, for instance, lead to attractive (polymer bridging) or repulsive steric interactions. In the literature, researchers investigating the influence of different types of NOM on colloidal stability have primarily focused on fulvic (FA) and humic acids (HA). Even though both acids can adsorb onto the NP surface, the adsorption percentage of HA has been shown to be twice as much in water compared to FA due to its greater hydrophobicity [83]. Therefore, HA hindered the particle aggregation more effectively than FA. However, Luo et al.’s study observed that this only applies to media with an IS lower than the critical coagulation concentration (CCC) of nano-TiO2. In the case of IS > CCC, the presence of HA promotes the nano-TiO2 aggregation even more. As reported by Slomberg et al. [84], nano-TiO2 prefers to interact with anionic NOM with high or medium molecular weights.
The assessment of the impact of NOM should always consider the whole environmental matrix, as this significantly influences the experimental outcome. At high IS, nano-TiO2 can aggregate due to reduced repulsive forces as a result of the EDL compression [85]. However, this aggregation behaviour can be modified or reduced in the presence of NOM due to electrostatic and steric forces [83]. For example, Labille et al. [86] studied the heteroaggregation potential of TiO2 and smectite clay considering the influence of pH, IS, and NOM. Regardless of whether the pH is above or below the IEP of the original TiO2 NPs, the adsorption of NOM on the surface of clay and TiO2 supports the colloidal stability even at a high IS (0.1 M NaCl). However, Luo et al. [83] observed the destabilisation of nano-TiO2 in the presence of NOM at pH 8 > IEP and an even lower IS (>0.005 M CaCl2), which is likely explained by the ability of Ca2+ ions to link two negatively charged surfaces—in this case, NOM and nano-TiO2—and thus enhance aggregation.
2.2.3. Experimentally Observed Behaviour and Fate Processes
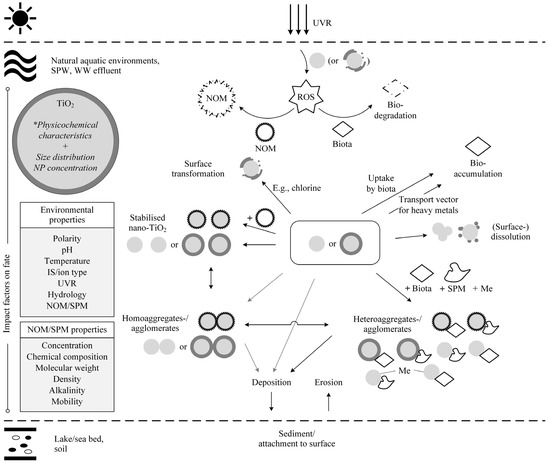
Having considered the key influential factors separately, this section focuses on the experimentally observed behaviour and fate processes that were described in the context of specific environments. Overall, the environmental processes (Figure 2) that dominantly affected the behaviour and fate of nano-TiO2 were (1) transport processes (sedimentation, resuspension); (2) transformation processes (surface transformation, photolysis, homo-/heteroaggregation and/or -agglomeration); and (3) bioaccumulation (transport vector for heavy metals). Table 2 summarises the experimental findings from the past decade that help to understand the fate and behaviour of nano-TiO2 in various matrices.
| Matrix | Exposure | Predominant Processes | Implication | Reference |
|---|---|---|---|---|
| Sunscreen | Oxybenzone, avobenzone, octyl methoxycinnamate | Photodegradation of organic UV filters | Joint toxicity | [87][88] |
| Acrylates/C10-30 alkyl acrylate cross-polymer (stabilising agent) | Adsorption of the polymer, decrease IEP from 6.3 to <5 | Electrostatic NP stabilization, enhanced NP mobility at environmentally relevant pH values (6–9), reduced filtration efficiency | [81] | |
| EtOH, UVR | Partial degradation of the hydrogen dimethicone coating due to the dissolving ability of EtOH | Photodegradation of the organic and subjacent layer: Al(OH)3 | [62] | |
| Hydroxy acids (Salicylic/citric acid) | Biomolecular chelation of Ti4+ | Induced solubilization by chelation | [8] | |
| Additional NPs in formulation | Higher phototoxicity | [14] | ||
| SPW | Calcium sulphate dihydrate (CaSO4 • 2 H2O) |
Adsorption of Ca2+ increased zeta potential | Reduced colloidal stability | [65] |
| Chlorine | Surface transformation, redistribution of the Al(OH)3 protective layer | Reduced agglomeration, formation of porous aggregates, depletion of Al(OH)3 coating from the nano-TiO2 surface | [65] | |
| Surface transformation, redistribution of the Al(OH)3 protective layer | Depletion of Al(OH)3 coating from the nano-TiO2 surface, photocatalytic formation of hydroxyl radicals (•OH) | [12][64] | ||
| Potassium cetyl phosphate (C16H34KO4P, PCP) and chlorine | Degradation of PCP releases PO43− ions, subsequent formation of AlPO4 precipitates | Acceleration of protective coating depletion | [65] | |
| Change of physicochemical properties, e.g., smaller particles, more spherical | [64] | |||
| Dissolution, dominance of dissolved Ti species | [89] | |||
| Natural aquatic environment | UVR, seawater | Photocatalytic reactions | Formation of hydrogen peroxide (H2O2) | [90] |
| Shear forces (induced by light, sonication) | Partial disagglomeration | Enhanced NP mobility | [91] | |
| Higher ambient temperature | Hydrodynamic diameter increases | Enhanced NP sedimentation | [92] | |
| UVR + dissolved organic matter (DOM) (fresh-/seawater) |
Photocatalytic halogenation of DOM | Formation of organobromine/-iodine compounds | [93] | |
| Natural clay colloids (SPM) (1) pH < IEP (2) pH > IEP |
(1) Heteroaggregation (attractive electrostatic forces) (2) Weak affinity between NPs—SPM (both negatively charged) |
Enhanced (1) NP aggregation (2) NP stabilisation |
[86] | |
| HA (NOM) (pH < IEP) | Adsorption of macromolecules (1) at a low HA conc.: NP surface charge neutralisation; (2) at a high HA conc.: NP surface charge inversion |
Enhanced (1) NP aggregation (2) NP stabilisation |
[94] | |
| HA (NOM) (pH ≈ IEP) | Adsorption of macromolecules | Induced partial disaggregation | [94] | |
| HA, FA (NOM) (pH > IEP) |
(1) IS < CCC: Adsorption of macromolecules (electrosteric repulsion) (2) IS > CCC: EDL compression + NOM entanglement |
Enhanced (1) NP stabilisation (2) NP aggregation |
[83] | |
| NOM (various) | Adsorption of macromolecules (1) low NOM conc.: interparticle bridging; (2) high NOM conc.: repulsive steric interactions |
(1) Flocculation (dependent on NOM type, sometimes salt addition required) (2) steric NP stabilisation |
[63] | |
| NOM | Adsorption of macromolecules | Photosensitiser AND ROS quencher (dominant) | [85] | |
| NaCl (Seawater) + Algae | Adsorption NPs—microalgae (note: NPs—NPs) |
Intensified heteroagglomeration with increase in IS (note: homo-agglomeration is not affected by IS) |
[95] | |
| NaCl (Seawater) (IS > CCC) |
Aggregation | Induced NP aggregation | [96] | |
| UVR, seawater | Release of dissolved trace metals and inorganic nutrients | Greater bioavailability, bioaccumulation | [97] | |
| Siderophore-producing organisms | Biomolecular chelation of Ti4+ | Bioaccumulation, induced NP solubility | [8] | |
| Presence of Cu | Transport vector for heavy metals, Cu2+ absorption on TiO2 surface | Bioaccumulation of Cu | [98] | |
| Presence of Cd, As | Transport vector for heavy metals, Cd2+, AsO43- absorption on TiO2 surface | Bioaccumulation of Cd, As | [99] | |
| Aged TiO2 under UVR (in presence of Cd2+) | Production of singlet oxygen (1O2) | Higher sensitivity of cells to Cd | [100] |
In sunscreens, nano-TiO2 can negatively affect other sunscreen constituents such as organic UV filters. That is, the toxicity of the sunscreen can increase due to the TiO2-induced photodegradation of oxybenzone [87], avobenzone, and octyl methoxycinnamate [88], which are commonly used organic UV filters. Furthermore, the behaviour and (eco-)toxicity of nano-TiO2 in the environment can also be affected by other sunscreen components. For example, the use of the C10-C30 alkyl acrylate cross-polymer can increase the mobility and, thus, the concentration of nano-TiO2 in the environment [81]. Moreover, EtOH is commonly used in sunscreens to improve the compatibility and ductility of the formulation. However, EtOH dissolves the organic coating of nano-TiO2, leaving the NPs rather unstable and vulnerable to subsequent photolysis of superjacent layers [62].
Research on SPW has primarily focused on the impact of pool water ingredients on the integrity of nano-TiO2 coatings. For example, Virkutyte et al. [65] ascertained that chlorine degrades potassium cetyl phosphate (PCP), a conventional emulsifying agent in O/W formulations, thus releasing PO43– ions. This can accelerate the deterioration of the Al(OH)3 layer by promoting the formation of AlPO4 precipitates. Similarly, many studies have demonstrated that exposure to SPW worsened the protective Al(OH)3 coating on the nano-TiO2, thereby increasing the photocatalytic activity and toxicity of nano-TiO2 [12][65]. Besides changes in the surface chemistry, particles can also become more spherical and smaller when in contact with SPW [64]. This is in line with the finding that the dissolved form of Ti dominates in SPW [89], which suggests that the smaller, spherical forms represent a progressed state of TiO2 transformation. Jeon et al. [30] found that TiO2 is likely to accumulate in SPW even after a multi-stage treatment (filtration, UV irradiation, heating, and chlorination) process.
Even though more studies have examined the fate and behaviour of nano-TiO2 in natural aquatic environments compared to synthetic aqueous environments, the evaluation of synergistic interactions between multiple environmental influencing factors in both milieus is insufficient. Only a handful of researchers have studied various interacting factors in natural aquatic environments at once, and their results contradict. For example, Li et al. [101] assessed the effect of numerous natural water properties on the NP stability and found that the IS, especially Ca2+ and Mg2+, and the pH were the most important governing factors. In contrast, Slomberg et al. [84] observed that the nano-TiO2 stability in natural and synthetic lake water could not be compared even if the water has the same pH and ionic composition. This observation supports the general advice to, if possible, collect field samples instead of conducting laboratory studies as only field studies reflect the actual chemistry of the environment. In the case of Slomberg et al. [84], this refers to various combinations of NOM, suspended particulate matter (SPM), and micro-organisms that seem to have significantly affected the particle stability.
Besides the influence of NOM on the aggregation and agglomeration behaviour, NOM can also affect the photocatalytic behaviour of nano-TiO2 by either quenching or sensitising the generation of ROS [85]. Furthermore, a recent study has reported that nano-TiO2 can initiate the halogenation of dissolved organic matter (DOM) in fresh water and seawater, which results in the formation of organobromine and iodine compounds [93]. Previous studies have also revealed that TiO2 can increase the bioaccumulation of other environmental toxicants by effectively operating as a transport vector for heavy metals such as cadmium (Cd) or arsenic (As) [99]. Therefore, adverse effects indirectly arising from the presence of nano-TiO2 in the environment should also be considered in ecotoxicity studies.
In summary, the prediction of the behaviour and fate of nano-TiO2 based on identified predominant transport and transformation processes is still inadequate. Figure 2 schematically depicts the environmental processes that have been identified in aqueous environments. Due to the variety of interactions that occur in a single matrix, it is not yet possible to determine a distinct behaviour and fate of nano-TiO2 in one matrix, not to mention to transfer and apply the outcome to another environmental matrix. Consequently, to provide a full picture, particle and environmental characteristics should be examined comprehensively. However, this requires advanced analytical approaches that allow the characterisation of nano-TiO2 in complex matrices.
This entry is adapted from the peer-reviewed paper 10.3390/w13050734
References
- The Skin Cancer Foundation. Skin Cancer Facts & Statistics. 2020. Available online: (accessed on 5 July 2019).
- WHO. Cancer. 2018. Available online: (accessed on 12 October 2020).
- Tabbakh, T.; Volkov, A.; Wakefield, M.; Dobbinson, S. Implementation of the SunSmart program and population sun protection behaviour in Melbourne, Australia: Results from cross-sectional summer surveys from 1987 to 2017. PLoS Med. 2019, 16, e1002932.
- Adler, B.L.; DeLeo, V.A. Sunscreen safety: A Review of recent studies on humans and the environment. Curr. Dermatol. Rep. 2020, 9, 1–9.
- Sobek, A.; Bejgarn, S.; Rudén, C.; Molander, L.; Breitholtz, M. In the shadow of the cosmetic directive—Inconsistencies in EU environmental hazard classification requirements for UV-filters. Sci. Total. Environ. 2013, 461–462, 706–711.
- IARC. CAS No. 13463-67-7 Titanium Dioxide. 12/12/2019 ed.; International Agency for Research on Cancer: Lyon, France, 2010; Volume 93.
- Zhang, X.; Li, W.; Yang, Z. Toxicology of nanosized titanium dioxide: An update. Arch. Toxicol. 2015, 89, 2207–2217.
- Sharma, S.; Sharma, R.K.; Gaur, K.; Torres, J.F.C.; Loza-Rosas, S.A.; Torres, A.; Saxena, M.; Julin, M.; Tinoco, A.D. Fueling a hot debate on the application of TiO2 nanoparticles in sunscreen. Materials 2019, 12, 2317.
- Fenoglio, I.; Ponti, J.; Alloa, E.; Ghiazza, M.; Corazzari, I.; Capomaccio, R.; Rembges, D.; Oliaro-Bosso, S.; Rossi, F. Singlet oxygen plays a key role in the toxicity and DNA damage caused by nanometric TiO2 in human keratinocytes. Nanoscale 2013, 5, 6567–6576.
- Mu, Q.; Jiang, G.; Chen, L.; Zhou, H.; Fourches, D.; Tropsha, A.; Yan, B. Chemical basis of interactions between engineered nanoparticles and biological systems. Chem. Rev. 2014, 114, 7740–7781.
- Egambaram, O.P.; Pillai, S.K.; Ray, S.S. Materials science challenges in skin UV protection: A review. Photochem. Photobiol. 2020, 96, 779–797.
- Al-Abed, S.R.; Virkutyte, J.; Ortenzio, J.N.R.; McCarrick, R.M.; Degn, L.L.; Zucker, R.; Coates, N.H.; Childs, K.; Ma, H.; Diamond, S.; et al. Environmental aging alters Al(OH)3 coating of TiO2 nanoparticles enhancing their photocatalytic and phototoxic activities. Environ. Sci. Nano 2016, 3, 593–601.
- Auffan, M.; Pedeutour, M.; Rose, J.; Masion, A.; Ziarelli, F.; Borschneck, D.; Chanéac, C.; Botta, C.; Chaurand, P.; Labille, J.; et al. Structural degradation at the surface of a TiO2-based nanomaterial used in cosmetics. Environ. Sci. Technol. 2010, 44, 2689–2694.
- Donia, D.T.; Carbone, M. Fate of the nanoparticles in environmental cycles. Int. J. Environ. Sci. Technol. 2018, 16, 583–600.
- Minetto, D.; Libralato, G.; Ghirardini, A.V. Ecotoxicity of engineered TiO2 nanoparticles to saltwater organisms: An overview. Environ. Int. 2014, 66, 18–27.
- Picado, A.; Paixão, S.M.; Moita, L.; Silva, L.; Diniz, M.S.; Lourenço, J.; Peres, I.; Castro, L.; Correia, J.B.; Pereira, J.; et al. A multi-integrated approach on toxicity effects of engineered TiO2 nanoparticles. Front. Environ. Sci. Eng. 2015, 9, 793–803.
- Scown, T.M.; Van Aerle, R.; Tyler, C.R. Review: Do engineered nanoparticles pose a significant threat to the aquatic environment? Crit. Rev. Toxicol. 2010, 40, 653–670.
- De La Vega, A.C.S.; Cruz-Alcalde, A.; Mazón, C.S.; Martí, C.B.; Diaz-Cruz, M.S. Nano-TiO2 phototoxicity in fresh and seawater: Daphnia magna and Artemia sp. as proxies. Water 2020, 13, 55.
- Barone, A.N.; Hayes, C.E.; Kerr, J.J.; Lee, R.C.; Flaherty, D.B. Acute toxicity testing of TiO2-based vs. oxybenzone-based sunscreens on clownfish (Amphiprion ocellaris). Environ. Sci. Pollut. Res. 2019, 26, 14513–14520.
- Fouqueray, M.; Dufils, B.; Vollat, B.; Chaurand, P.; Botta, C.; Abacci, K.; Labille, J.; Rose, J.; Garric, J. Effects of aged TiO2 nanomaterial from sunscreen on Daphnia magna exposed by dietary route. Environ. Pollut. 2012, 163, 55–61.
- Fu, L.; Hamzeh, M.; Dodard, S.; Zhao, Y.H.; Sunahara, G.I. Effects of TiO2 nanoparticles on ROS production and growth inhibition using freshwater green algae pre-exposed to UV irradiation. Environ. Toxicol. Pharmacol. 2015, 39, 1074–1080.
- ChemSafetyPro. How to Calculate Predicted No-Effect Concentration (PNEC). Available online: (accessed on 16 July 2020).
- Musee, N. Simulated environmental risk estimation of engineered nanomaterials: A case of cosmetics in Johannesburg City. Hum. Exp. Toxicol. 2011, 30, 1181–1195.
- Coll, C.; Notter, D.; Gottschalk, F.; Sun, T.; Som, C.; Nowack, B. Probabilistic environmental risk assessment of five nanomaterials (nano-TiO2, nano-Ag, nano-ZnO, CNT, and fullerenes). Nanotoxicology 2015, 10, 436–444.
- Slijkerman, D.; Onderzoeksformatie, I.; Keur, M. Sunscreen Ecoproducts: Product Claims, Potential Effects and Environmental Risks of Applied UV Filters; Wageningen University and Research: Den Helder, The Netherlands, 2018.
- Lead, J.R.; Batley, G.E.; Alvarez, P.J.J.; Croteau, M.-N.; Handy, R.D.; McLaughlin, M.J.; Judy, J.D.; Schirmer, K. Nanomaterials in the environment: Behavior, fate, bioavailability, and effects-An updated review. Environ. Toxicol. Chem. 2018, 37, 2029–2063.
- Baalousha, M.; Cornelis, G.; Kuhlbusch, T.A.J.; Lynch, I.; Nickel, C.; Peijnenburg, W.; Brink, N.W.V.D. Modeling nanomaterial fate and uptake in the environment: Current knowledge and future trends. Environ. Sci. Nano 2016, 3, 323–345.
- Sun, T.Y.; Gottschalk, F.; Hungerbühler, K.; Nowack, B. Comprehensive probabilistic modelling of environmental emissions of engineered nanomaterials. Environ. Pollut. 2014, 185, 69–76.
- Number of Products, vs. Titanium Dioxide; Nanodatabase: DTU Environment, the Danish Ecological Council and Danish Consumer Council, 2020.
- Jeon, S.-K.; Kim, E.-J.; Lee, J.; Lee, S. Potential risks of TiO2 and ZnO nanoparticles released from sunscreens into outdoor swimming pools. J. Hazard. Mater. 2016, 317, 312–318.
- Choi, S.; Johnston, M.; Wang, G.-S.; Huang, C. A seasonal observation on the distribution of engineered nanoparticles in municipal wastewater treatment systems exemplified by TiO2 and ZnO. Sci. Total. Environ. 2018, 625, 1321–1329.
- EPA, U. Nanomaterial Case Studies: Nanoscale Titanium Dioxide in Water Treatment and in Topical Sunscreen (Final); US Environmental Protection Agency Research Triangle Park: Washington, DC, USA, 2010.
- Wiechers, J.W.; Musee, N. Engineered inorganic nanoparticles and cosmetics: Facts, issues, knowledge gaps and challenges. J. Biomed. Nanotechnol. 2010, 6, 408–431.
- Cornelis, G.; Hund-Rinke, K.; Kuhlbusch, T.; Van den Brink, N.; Nickel, C. Fate and bioavailability of engineered nanoparticles in soils: A review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2720–2764.
- Dulger, M.; Sakallioglu, T.; Temizel, I.; Demirel, B.; Copty, N.; Onay, T.; Uyguner-Demirel, C.; Karanfil, T. Leaching potential of nano-scale titanium dioxide in fresh municipal solid waste. Chemosphere 2016, 144, 1567–1572.
- Morgan, R.; Swimming Pool Ownership Increases in Australia. Roy Morgan Research: 2018. Available online: (accessed on 27 May 2020).
- Nischwitz, V.; Goenaga-Infante, H. Improved sample preparation and quality control for the characterisation of titanium dioxide nanoparticles in sunscreens using flow field flow fractionation on-line with inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 2012, 27, 1084–1092.
- Dan, Y.; Shi, H.; Stephan, C.; Liang, X. Rapid analysis of titanium dioxide nanoparticles in sunscreens using single particle inductively coupled plasma-mass spectrometry. Microchem. J. 2015, 122, 119–126.
- Popov, A.P.; Lademann, J.; Priezzhev, A.V.; Myllylä, R.A. Effect of size of TiO2 nanoparticles embedded into stratum corneum on ultraviolet-A and ultraviolet-B sun-blocking properties of the skin. J. Biomed. Opt. 2005, 10, 064037.
- Walter, D. Primary Particles—Agglomerates—Aggregates. In Nanomaterials; Wiley: Bonn, Germany, 2013; pp. 9–24.
- Lin, X.; Li, J.; Ma, S.; Liu, G.; Yang, K.; Tong, M.; Lin, D. Toxicity of TiO2 nanoparticles to escherichia coli: Effects of particle size, crystal phase and water chemistry. PLoS ONE 2014, 9, e110247.
- Solaiman, S.M.; Algie, J.; Bakand, S.; Sluyter, R.; Sencadas, V.; Lerch, M.; Huang, X.-F.; Konstantinov, K.; Barker, P.J. Nano-sunscreens—A double-edged sword in protecting consumers from harm: Viewing Australian regulatory policies through the lenses of the European Union. Crit. Rev. Toxicol. 2019, 49, 122–139.
- Pelclova, D.; Navratil, T.; Kacerova, T.; Zamostna, B.; Fenclova, Z.; Vlckova, S.; Kacer, P. NanoTiO2 sunscreen does not prevent systemic oxidative stress caused by UV radiation and a minor amount of NanoTiO2 is absorbed in humans. Nanomater. 2019, 9, 888.
- Almquist, C.B.; Biswas, P. Role of synthesis method and particle size of nanostructured TiO2 on its photoactivity. J. Catal. 2002, 212, 145–156.
- Philippe, A.; Košík, J.; Welle, A.; Guigner, J.-M.; Clemens, O.; Schaumann, G.E. Extraction and characterization methods for titanium dioxide nanoparticles from commercialized sunscreens. Environ. Sci. Nano 2017, 5, 191–202.
- Bairi, V.G.; Lim, J.-H.; Fong, A.; Linder, S.W. Size characterization of metal oxide nanoparticles in commercial sunscreen products. J. Nanoparticle Res. 2017, 19, 256.
- Robertson, T.A.; Sanchez, W.Y.; Roberts, M.S. Are commercially available nanoparticles safe when applied to the skin? J. Biomed. Nanotechnol. 2010, 6, 452–468.
- Catalano, R.; Labille, J.; Gaglio, D.; Alijagic, A.; Napodano, E.; Slomberg, D.; Campos, A.; Pinsino, A. Safety evaluation of TiO2 nanoparticle-based sunscreen UV filters on the development and the immunological state of the sea urchin Paracentrotus Lividus. Nanomaterials 2020, 10, 2102.
- Hsiao, I.-L.; Huang, Y.-J. Effects of various physicochemical characteristics on the toxicities of ZnO and TiO2 nanoparticles toward human lung epithelial cells. Sci. Total. Environ. 2011, 409, 1219–1228.
- Lu, P.; Fang, S.; Cheng, W.; Huang, S.; Cheng, H. Characterization of titanium dioxide and zinc oxide nanoparticles in sunscreen powder by comparing different measurement methods. J. Food Drug Anal. 2018, 26, 1192–1200.
- He, X.; Hwang, H.-M. Engineered TiO2 Nanoparticles: Their Fate and Effects in Natural Aquatic Environments; Nova Science Publishers Inc.: Jackson, MS, USA, 2014; pp. 1–20.
- Johnston, H.J.; Hutchison, G.R.; Christensen, F.M.; Peters, S.; Hankin, S.; Stone, V. Identification of the mechanisms that drive the toxicity of TiO2 particulates: The contribution of physicochemical characteristics. Part. Fibre Toxicol. 2009, 6, 33.
- Corinaldesi, C.; Marcellini, F.; Nepote, E.; Damiani, E.; Danovaro, R. Impact of inorganic UV filters contained in sunscreen products on tropical stony corals (Acropora spp.). Sci. Total Environ. 2018, 637–638, 1279–1285.
- Borm, P.J.; Robbins, D.; Haubold, S.; Kuhlbusch, T.; Fissan, H.; Donaldson, K.; Schins, R.; Stone, V.; Kreyling, W.; Lademann, J.; et al. The potential risks of nanomaterials: A review carried out for ECETOC. Part. Fibre Toxicol. 2006, 3, 11.
- Wakefield, G.; Lipscomb, S.; Holland, E.; Knowland, J. The effects of manganese doping on UVA absorption and free radical generation of micronised titanium dioxide and its consequences for the photostability of UVA absorbing organic sunscreen components. Photochem. Photobiol. Sci. 2004, 3, 648–652.
- Park, B.; Martin, A.P.; Harris, C.; Guest, R.; Whittingham, A.; Jenkinson, P. Preliminary in vitro investigation of the potential health effects of Optisol™, a nanoparticulate manganese modified titanium dioxide UV-filter used in certain sunscreen products. Nanotoxicology 2009, 3, 73–90.
- Labille, J.; Catalano, R.; Slomberg, D.; Motellier, S.; Pinsino, A.; Hennebert, P.; Santaella, C.; Bartolomei, V. Assessing sunscreen lifecycle to minimize environmental risk posed by nanoparticulate UV-filters—A review for safer-by-design products. Front. Environ. Sci. 2020, 8.
- Jacobs, J.F.; Van De Poel, I.; Osseweijer, P. Sunscreens with titanium dioxide (TiO2) nano-particles: A societal experiment. NanoEthics 2010, 4, 103–113.
- Osterwalder, U.; Sohn, M.; Herzog, B. Global state of sunscreens. Photodermatol. Photoimmunol. Photomed. 2014, 30, 62–80.
- Rossano, M.; Hucher, N.; Picard, C.; Colletta, D.; Le Foll, F.; Grisel, M. Effects of aging on structure and stability of TiO2 nanoparticle-containing oil-in-water emulsions. Int. J. Pharm. 2014, 461, 89–96.
- Dréno, B.; Alexis, A.; Chuberre, B.; Marinovich, M. Safety of titanium dioxide nanoparticles in cosmetics. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 34–46.
- Wu, W.; Xiang, Q.; Wu, Z.; Shan, G.; Zhu, L. Depletion of double-layer coated nano-TiO2 and generation of reactive oxygen species in the presence of ethanol under simulated solar irradiation. NanoImpact 2018, 11, 164–169.
- Labille, J.; Feng, J.; Botta, C.; Borschneck, D.; Sammut, M.; Cabie, M.; Auffan, M.; Rose, J.; Bottero, J.-Y. Aging of TiO2 nanocomposites used in sunscreen. Dispersion and fate of the degradation products in aqueous environment. Environ. Pollut. 2010, 158, 3482–3489.
- Virkutyte, J.; Al-Abed, S.R. Statistical evaluation of potential damage to the Al(OH)3 layer on nTiO2 particles in the presence of swimming pool and seawater. J. Nanoparticle Res. 2012, 14, 787.
- Virkutyte, J.; Al-Abed, S.R.; Dionysiou, D.D. Depletion of the protective aluminum hydroxide coating in TiO2-based sunscreens by swimming pool water ingredients. Chem. Eng. J. 2012, 191, 95–103.
- Wallis, L.K.; Diamond, S.A.; Ma, H.; Hoff, D.J.; Al-Abed, S.R.; Li, S. Chronic TiO2 nanoparticle exposure to a benthic organism, Hyalella azteca: Impact of solar UV radiation and material surface coatings on toxicity. Sci. Total. Environ. 2014, 499, 356–362.
- Morsella, M.; D’Alessandro, N.; Lanterna, A.E.; Scaiano, J.C. Improving the sunscreen properties of TiO2 through an understanding of its catalytic properties. ACS Omega 2016, 1, 464–469.
- Yu, J.; Li, L.; Qian, Y.; Lou, H.; Yang, D.; Qiu, X. Facile and green preparation of high UV-blocking lignin/titanium dioxide nanocomposites for developing natural sunscreens. Ind. Eng. Chem. Res. 2018, 57, 15740–15748.
- Shandilya, N.; Capron, I. Safer-by-design hybrid nanostructures: An alternative to conventional titanium dioxide UV filters in skin care products. RSC Adv. 2017, 7, 20430–20439.
- Son, H.Y.; Koo, B.I.; Lee, J.B.; Kim, K.R.; Kim, W.; Jang, J.; Yoon, M.S.; Cho, J.-W.; Nam, Y.S. Tannin-titanium oxide multilayer as a photochemically suppressed ultraviolet filter. ACS Appl. Mater. Interfaces 2018, 10, 27344–27354.
- Battistin, M.; Dissette, V.; Bonetto, A.; Durini, E.; Manfredini, S.; Marcomini, A.; Casagrande, E.; Brunetta, A.; Ziosi, P.; Molesini, S.; et al. A new approach to UV protection by direct surface functionalization of TiO2 with the antioxidant polyphenol dihydroxyphenyl benzimidazole carboxylic acid. Nanomater. 2020, 10, 231.
- Åhlén, M.; Cheung, O.; Strømme, M. Amorphous mesoporous magnesium carbonate as a functional support for UV-blocking semiconductor nanoparticles for cosmetic applications. ACS Omega 2019, 4, 4429–4436.
- Bernstein, E.F.; Sarkas, H.W.; Ba, P.B.; Bouche, D. Beyond sun protection factor: An approach to environmental protection with novel mineral coatings in a vehicle containing a blend of skincare ingredients. J. Cosmet. Dermatol. 2020, 19, 407–415.
- Borrás, M.C.; Sluyter, R.; Barker, P.J.; Konstantinov, K.; Bakand, S. Y2O3 decorated TiO2 nanoparticles: Enhanced UV attenuation and suppressed photocatalytic activity with promise for cosmetic and sunscreen applications. J. Photochem. Photobiol. B: Biol. 2020, 207, 111883.
- Morlando, A.; Borrás, M.C.; Rehman, Y.; Bakand, S.; Barker, P.; Sluyter, R.; Konstantinov, K. Development of CeO2 nanodot encrusted TiO2 nanoparticles with reduced photocatalytic activity and increased biocompatibility towards a human keratinocyte cell line. J. Mater. Chem. B 2020, 8, 4016–4028.
- Derjaguin, B.; Landau, L. Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes. Prog. Surf. Sci. 1993, 43, 30–59.
- Overbeek, T.; Verwey, E. Theory of the Stability of Lyophobic Colloids: The Interaction of Sol Particles Having An Electric Double Layer; Elsevier: Amsterdam, The Netherlands, 1948.
- Beck, R.; Guterres, S.; Pohlmann, A. Nanocosmetics and Nanomedicines; Springer: Berlin/Heidelberg, Germany, 2011.
- Stumm, W.; Morgan, J.J.; ProQuest, E. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters, 3rd ed.; Wiley: New York, NY, USA, 1996.
- Nobbmann, U. Nanomaterial Isoelectric Points IEPs; Malvern Panalytical Ltd: Malvern, UK, 2017.
- Englehart, J.; Lyon, B.A.; Abriola, L.M.; Becker, M.D.; Wang, Y.; Pennell, K.D. Influence of a polymer sunscreen additive on the transport and retention of titanium dioxide nanoparticles in water-saturated porous media. Environ. Sci. Nano 2015, 3, 157–168.
- Zhang, C.; Lohwacharin, J.; Takizawa, S. Properties of residual titanium dioxide nanoparticles after extended periods of mixing and settling in synthetic and natural waters. Sci. Rep. 2017, 7, 9943.
- Luo, M.; Huang, Y.; Zhu, M.; Tang, Y.-N.; Ren, T.; Ren, J.; Wang, H.; Li, F. Properties of different natural organic matter influence the adsorption and aggregation behavior of TiO2 nanoparticles. J. Saudi Chem. Soc. 2018, 22, 146–154.
- Slomberg, D.L.; Ollivier, P.; Miche, H.; Angeletti, B.; Bruchet, A.; Philibert, M.; Brant, J.; Labille, J. Nanoparticle stability in lake water shaped by natural organic matter properties and presence of particulate matter. Sci. Total. Environ. 2019, 656, 338–346.
- Li, S.; Ma, H.; Wallis, L.K.; Etterson, M.A.; Riley, B.; Hoff, D.J.; Diamond, S.A. Impact of natural organic matter on particle behavior and phototoxicity of titanium dioxide nanoparticles. Sci. Total. Environ. 2016, 542, 324–333.
- Labille, J.; Harns, C.; Bottero, J.-Y.; Brant, J. Heteroaggregation of titanium dioxide nanoparticles with natural clay colloids. Environ. Sci. Technol. 2015, 49, 6608–6616.
- De La Vega, A.C.S.; Molins-Delgado, D.; Barceló, D.; Díaz-Cruz, M.S. Nanosized titanium dioxide UV filter increases mixture toxicity when combined with parabens. Ecotoxicol. Environ. Saf. 2019, 184, 109565.
- Kim, E.; Kim, M.; Im, N.; Park, S. Photolysis of the organic UV filter, avobenzone, combined with octyl methoxycinnamate by nano-TiO2 composites. J. Photochem. Photobiol. B: Biol. 2015, 149, 196–203.
- Holbrook, R.D.; Motabar, D.; Quiñones, O.; Stanford, B.; Vanderford, B.; Moss, D. Titanium distribution in swimming pool water is dominated by dissolved species. Environ. Pollut. 2013, 181, 68–74.
- Sánchez-Quiles, D.; Tovar-Sánchez, A. Sunscreens as a source of hydrogen peroxide production in coastal waters. Environ. Sci. Technol. 2014, 48, 9037–9042.
- Zhou, D.; Bennett, S.W.; Keller, A.A. Increased mobility of metal oxide nanoparticles due to photo and thermal induced disagglomeration. PLoS ONE 2012, 7, e37363.
- Lv, X.; Tao, J.; Chen, B.; Zhu, X. Roles of temperature and flow velocity on the mobility of nano-sized titanium dioxide in natural waters. Sci. Total. Environ. 2016, 565, 849–856.
- Hao, Z.; Yin, Y.; Wang, J.; Cao, D.; Liu, J. Formation of organobromine and organoiodine compounds by engineered TiO2 nanoparticle-induced photohalogenation of dissolved organic matter in environmental waters. Sci. Total. Environ. 2018, 631–632, 158–168.
- Loosli, F.; Le Coustumer, P.; Stoll, S. TiO2 nanoparticles aggregation and disaggregation in presence of alginate and Suwannee River humic acids. pH and concentration effects on nanoparticle stability. Water Res. 2013, 47, 6052–6063.
- Sendra, M.; Yeste, M.; Gatica, J.; Moreno-Garrido, I.; Blasco, J. Homoagglomeration and heteroagglomeration of TiO2, in nanoparticle and bulk form, onto freshwater and marine microalgae. Sci. Total. Environ. 2017, 592, 403–411.
- Botta, C.; Labille, J.; Auffan, M.; Borschneck, D.; Miche, H.; Cabié, M.; Masion, A.; Rose, J.; Bottero, J.-Y. TiO2-based nanoparticles released in water from commercialized sunscreens in a life-cycle perspective: Structures and quantities. Environ. Pollut. 2011, 159, 1543–1550.
- Rodríguez-Romero, A.; Ruiz-Gutiérrez, G.; Viguri, J.R.; Tovar-Sánchez, A. Sunscreens as a new source of metals and nutrients to coastal waters. Environ. Sci. Technol. 2019, 53, 10177–10187.
- Fan, W.; Cui, M.; Liu, H.; Wang, C.; Shi, Z.; Tan, C.; Yang, X. Nano-TiO2 enhances the toxicity of copper in natural water to daphnia magna. Environ. Pollut. 2011, 159, 729–734.
- Skocaj, M.; Filipic, M.; Petkovic, J.; Novak, S. Titanium dioxide in our everyday life; is it safe? Radiol. Oncol. 2011, 45, 227–247.
- Santaella, C.; Allainmat, B.; Simonet, F.; Chanéac, C.; Labille, J.; Auffan, M.; Rose, J.; Achouak, W. Aged TiO2-based nanocomposite used in sunscreens produces singlet oxygen under long-wave UV and sensitizesescherichia colito cadmium. Environ. Sci. Technol. 2014, 48, 5245–5253.
- Li, L.; Sillanpää, M.; Risto, M. Influences of water properties on the aggregation and deposition of engineered titanium dioxide nanoparticles in natural waters. Environ. Pollut. 2016, 219, 132–138.
