The global warming and the dangerous climate change arising from the massive emission of CO2 from the burning of fossil fuels have motivated the search for alternative clean and sustainable energy sources. However, the industrial development and population necessities make the decoupling of economic growth from fossil fuels unimaginable and, consequently, the capture and conversion of CO2 to fuels seems to be, nowadays, one of the most promising and attractive solutions in a world with high energy demand. In this respect, the electrochemical CO2 conversion using renewable electricity provides a promising solution. However, faradaic efficiency of common electro-catalysts is low, and therefore, the design of highly selective, energy-efficient, and cost-effective electrocatalysts is critical. Carbon-based materials present some advantages such as relatively low cost and renewability, excellent electrical conductivity, and tunable textural and chemical surface, which show them as competitive materials for the electro-reduction of CO2
- carbon dioxide
- electro-reduction
- carbon-based materials
- value-added products
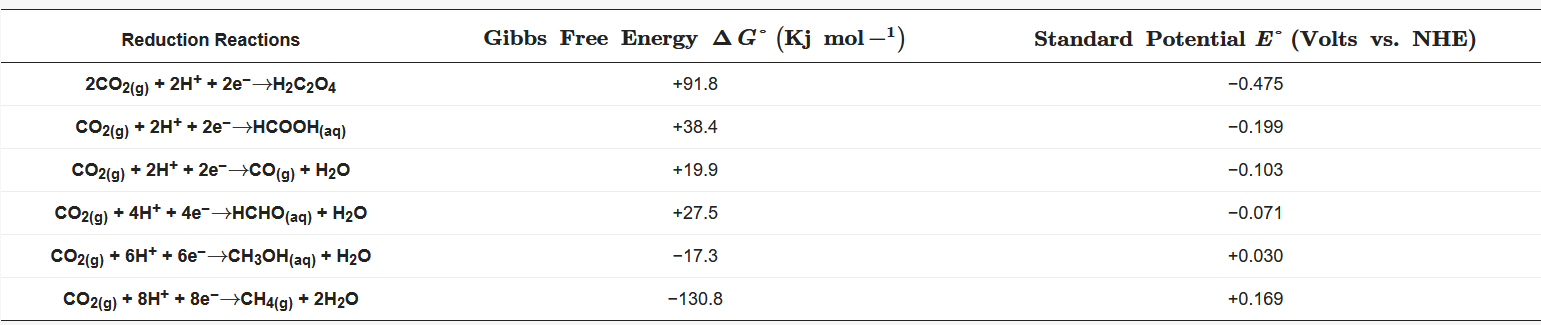
The energy supply currently depends mostly on fossil fuels, causing a continuous accumulation and, therefore, an excess of CO2 in the atmosphere, bringning negative effects on the environment. The population and live standards growth make nor imaginable the decoupling of energy supply from fossil fuels. Faced with this situation, different altenatives have been proposed to mitagate the enviromental impact and dependence on nonrenewable energy sources. The conversion of CO2 into value-added products by chemical reactions seems to be the most promising and attractive solution since, together with the reduction of the atmospheric CO2 levels, CO2 is efficiently recycled stablishing an ideal zero-emission carbon balance. CO2 can be converted to added-value products by photochemical [1] [2] [3] [4], thermochemical [5] [6] [7] [8], radiochemical [9] [10], biochemical [11] [12] [13] [14], and electrochemical strategies [15] [16] [17] [18]. However, the most interesting alternative is the capture and use of CO2 as raw material to produce various products ( Table 1) through its electrochemical reduction since this is a flexible and controllable process with mild and safe operating conditions and low equipment cost, which also allows coupling environmentally friendly non-fossil energy from renewable sources. Taking into account these advantages, many efforts have been made worldwide in the development and improvement of the technology available for CO2 electro-conversion.
Table 1. Equilibrium potential and Gibbs free energy for CO2 reduction reactions.
However, despite the fact that electro-reduction of CO2 (CO2RR) is thermodynamically viable, its transformation presents very slow reaction kinetics and usually requires significant energy expenditure [19] due to the high stability and inertness of the CO2 molecule [20]. Therefore, an extensive research has been developed by the overall scientific community focused on the electrocatalyst design, since the efficiency and selectivity of the reduction reaction is strongly dependent on the electrode nature, properties, and configuration [21]. An ideal catalyst for CO2 electroreduction requires: (i) Being able to mediate the transfer of electrons coupled to protons, (ii) having a low over potential for the activation of the CO2 molecule, (iii) exhibiting a selectivity preferably towards a target product, and (iv) preserving structural integrity during prolonged operation.
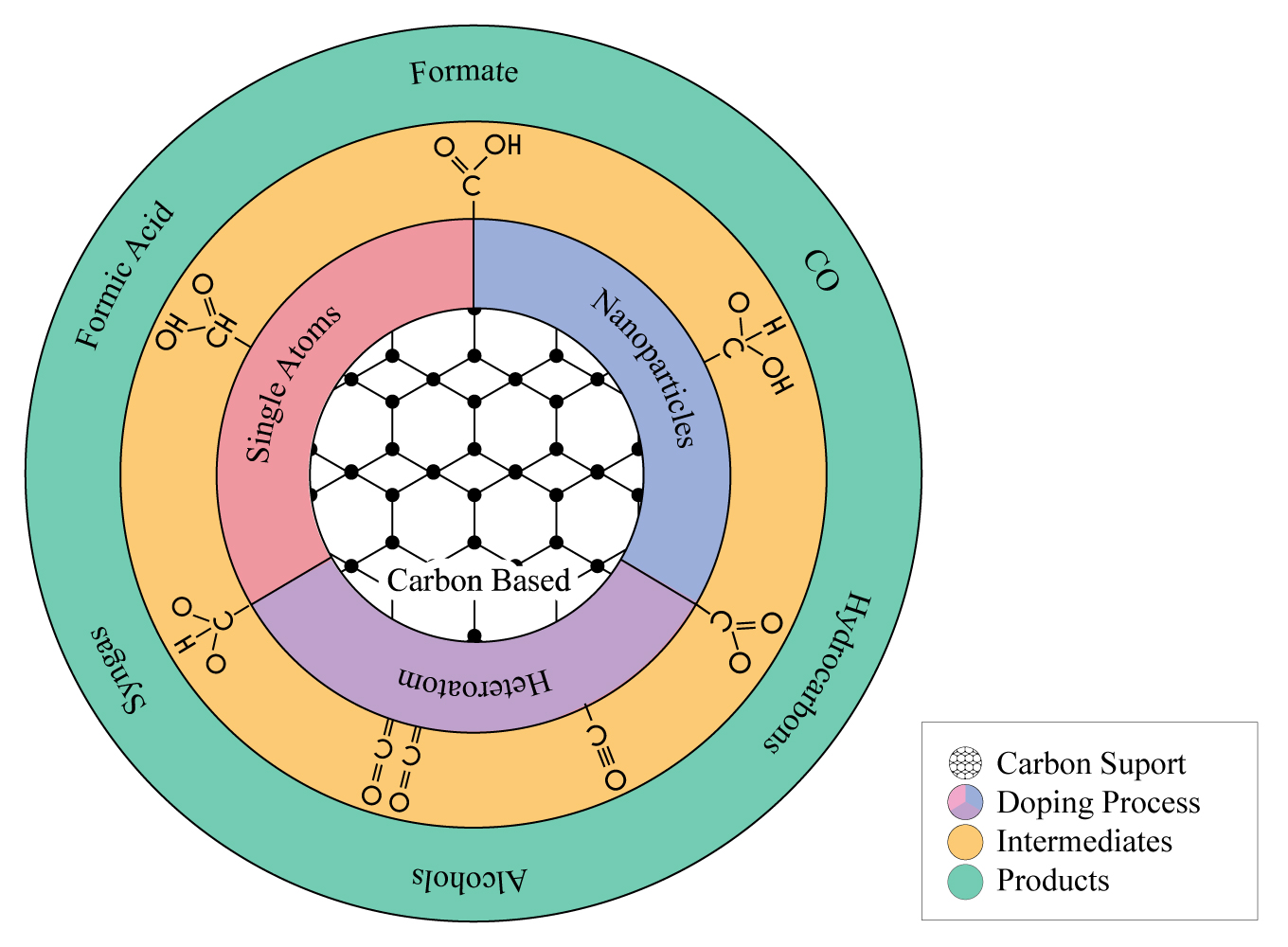
Lately, carbon-based catalysts have attracted much attention due to their relatively low cost and renewability, good chemical stability, excellent electrical conductivity, tunable textural and chemical surface, and large surface area, containing micropores, mesopores, and macropores that favor adsorption, access, and diffusion of molecules to the internal active sites of the material [22]. Due to these particular characteristics, carbon-based materials have been extensively used as electrocatalysts for CO2 reduction either as supports to disperse different metallic particles with several sizes (single-atoms, dual-atoms, nanoparticles) or as direct catalyst by functionalization with heteroatoms to prepare economical and sustainable metal-free electro-catalysts [ 23 ] . ( Figura 1 ).
Figure 1. Value-added products using carbon- based catalysts
This entry is adapted from the peer-reviewed paper 10.3390/catal11030351
References
- Kaneco, S.; Kurimoto, H.; Shimizu, Y.; Ohta, K.; Mizuno, T. Photocatalytic reduction of CO2 using TiO2 powders in super-critical fluid CO2. Energy 1999, 24, 21–30, doi:10.1016/S0360-5442(98)00070-X.
- Mori, K.; Yamashita, H.; Anpo, M. Photocatalytic reduction of CO2 with H2O on various titanium oxide photocatalysts. RSC Adv. 2012, 2, 3165–3172, doi:10.1039/C2RA01332K.
- Xie, S.; Zhang, Q.; Liu, G.; Wang, Y. Photocatalytic and photoelectrocatalytic reduction of CO2 using heterogeneous catalysts with controlled nanostructures. Chem. Commun. 2016, 52, 35–59, doi:10.1039/C5CC07613G.
- Wang, C.; Sun, Z.; Zheng, Y.; Hu, Y.H. Recent progress in visible light photocatalytic conversion of carbon dioxide. J. Mater. Chem. A 2019, 7, 865–887, doi:10.1039/C8TA09865D.
- Chueh, W.C.; Haile, S.M. Ceria as a Thermochemical Reaction Medium for Selectively Generating Syngas or Methane from H2O and CO2. ChemSusChem 2009, 2, 735–739, doi:10.1002/cssc.200900138.
- Frey, M.; Édouard, D.; Roger, A.-C. Optimization of structured cellular foam-based catalysts for low-temperature carbon dioxide methanation in a platelet milli-reactor. C. R. Chim. 2015, 18, 283–292, doi:10.1016/j.crci.2015.01.002.
- Roy, S.; Cherevotan, A.; Peter, S.C. Thermochemical CO2 Hydrogenation to Single Carbon Products: Scientific and Techno-logical Challenges. ACS Energy Lett. 2018, 3, 1938–1966, doi:10.1021/acsenergylett.8b00740.
- Ali, N.; Bilal, M.; Nazir, M.S.; Khan, A.; Ali, F.; Iqbal, H.M.N. Thermochemical and electrochemical aspects of carbon dioxide methanation: A sustainable approach to generate fuel via waste to energy theme. Sci. Total Environ. 2020, 712, 136482, doi:10.1016/j.scitotenv.2019.136482.
- Grodkowski, J.; Neta, P. Copper-Catalyzed Radiolytic Reduction of CO2 to CO in Aqueous Solutions. J. Phys. Chem. B 2001, 105, 4967–4972, doi:10.1021/jp004567d.
- Grodkowski, J.; Neta, P. Copper-Catalyzed Radiolytic Reduction of CO2 to CO in Aqueous Solutions. J. Phys. Chem. B 2001, 105, 4967–4972, doi:10.1021/jp004567d.
- Ramirez-Corredores, M.M.; Gadikota, G.; Huang, E.E.; Gaffney, A.M. Radiation-Induced Chemistry of Carbon Dioxide: A Pathway to Close the Carbon Loop for a Circular Economy. Front. Energy Res. 2020, 8, 108, doi:10.3389/fenrg.2020.00108.
- Machado, I.M.P.; Atsumi, S. Cyanobacterial biofuel production. J. Biotechnol. 2012, 162, 50–56, doi:10.1016/j.jbiotec.2012.03.005.
- Rabinovitch-Deere, C.A.; Oliver, J.W.K.; Rodriguez, G.M.; Atsumi, S. Synthetic Biology and Metabolic Engineering Ap-proaches To Produce Biofuels. Chem. Rev. 2013, 113, 4611–4632, doi:10.1021/cr300361t
- Rittmann, S.; Seifert, A.; Herwig, C. Essential prerequisites for successful bioprocess development of biological CH4 pro-duction from CO2 and H2. Crit. Rev. Biotechnol. 2015, 35, 141–151, doi:10.3109/07388551.2013.820685.
- Scibioh, M.A.; Viswanathan, B. Chapter 6—Biochemical Reduction of CO2. In Carbon Dioxide to Chemicals and Fuels; Scibioh, M.A., Viswanathan, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 255–306, ISBN 978-0-444-63996-7.
- Tuci, G.; Filippi, J.; Rossin, A.; Luconi, L.; Pham-Huu, C.; Yakhvarov, D.; Vizza, F.; Giambastiani, G. CO2 electrochemical reduction by exohedral N-pyridine decorated metal-free carbon nanotubes. Energies 2020, 13, 2703, doi:10.3390/en13112703.
- Jia, C.; Dastafkan, K.; Ren, W.; Yang, W.; Zhao, C. Carbon-based catalysts for electrochemical CO2 reduction. Sustain. Energy Fuels 2019, 3, 2890–2906, doi:10.1039/c9se00527g.
- Irabien, A.; Alvarez-Guerra, M.; Albo, J.; Dominguez-Ramos, A. Electrochemical Conversion of CO2 to Value-Added Products; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128131602.
- Mondal, B.; Song, J.; Neese, F.; Ye, S. Bio-inspired mechanistic insights into CO2 reduction. Curr. Opin. Chem. Biol. 2015, 25, 103–109, doi:10.1016/j.cbpa.2014.12.022.
- Mikkelsen, M.; Jørgensen, M.; Krebs, F.C. The teraton challenge. A review of fixation and transformation of carbon dioxide. Energy Environ. Sci. 2010, 3, 43–81, doi:10.1039/B912904A.
- Sharma, P.P.; Wu, J.; Yadav, R.M.; Liu, M.; Wright, C.J.; Tiwary, C.S.; Yakobson, B.I.; Lou, J.; Ajayan, P.M.; Zhou, X.-D. Ni-trogen-Doped Carbon Nanotube Arrays for High-Efficiency Electrochemical Reduction of CO2: On the Understanding of Defects, Defect Density, and Selectivity. Angew. Chem. 2015, 127, 13905–13909, doi:10.1002/ange.201506062
- Fernandes, D.M.; Peixoto, A.F.; Freire, C. Nitrogen-doped metal-free carbon catalysts for (electro)chemical CO2 conversion and valorisation. Dalt. Trans. 2019, 48, 13508–13528, doi:10.1039/c9dt01691k.
- Navalon, S.; Dhakshinamoorthy, A.; Alvaro, M.; Garcia, H. Carbocatalysis by Graphene-Based Materials. Chem. Rev. 2014, 114, 6179–6212, doi:10.1021/cr4007347.