Nanotechnology has been fostering excellent prospects in the development of enzymatic biosensors, since enzyme immobilization onto conductive nanostructures can improve characteristics that are crucial in biosensor transduction, such as surface-to-volume ratio, signal response, selectivity, sensitivity, conductivity, and biocatalytic activity, among others.
- biosensors
- nanomaterials
- enzyme immobilization
1. Nanomaterial-Based Biosensors
| Nature | Nanomaterial | Applications | Reference |
|---|---|---|---|
| Organic | Carbon nanotubes | Biomedical | [137] |
| Hybrid | GR-MWNTs/AuNP (1) | Biomedical | [138] |
| Hybrid | Au@PDMPAHCl (2) | Biomedical | [139] |
| Inorganic | Fe3O4 | Biomedical | [140] |
| Inorganic | Fe3O4-silica NPs (3) | Biomedical | [141] |
| Hybrid | CS/AuNPs-GNs (4) | Food and environmental | [142] |
| Inorganic | Ceria nanospheres | Food | [143] |
| Hybrid | MNP-PAMAM-PtNP/rGO-CMC (5) | Food | [144] |
| Organic | MnO2 modified MWCNTs * | Biomedical | [145] |
| Organic | Tobacco mosaic virus | Biomedical | [146] |
| Hybrid | Carbon ceramic | Biomedical | [147] |
| Organic | poly(l-aspartic acid)/MWCNT * | Food | [148] |
| Hybrid | Chi-Gr cry/PB/SPCE (6) | Uric acid detection | [149] |
| Hybrid | Titanim dioxide hybrid | Biomedical | [150] |
| Inorganic | Semiconductorquantum dots | Biomedical | [151] |
| Organic | Carbon black | Biomedical and environmental | [152] |
| Hybrid | Electrospun nanofibers | Biomedical | [153] |
(1) Gold nanoparticles prepared at graphene and multi-walled carbon nanotubes; (2) Core–shell gold nanoparticles stabilized with poly(3-dimethylammonium-1-propyne hydrochloride); (3) Magnetic nanoparticles–silica core shell; (4) Chitosan/gold nanoparticle−graphene nanosheets; (5) Poly(dopamine)-modified magnetic nanoparticles coated with four-generation ethylenediamine and core polyamidoamine G-4 dendrimers, all decorated with platinum nanoparticles on the surface of glassy carbon electrodes coated with graphene oxide and carboxymethylcellulose; (6) Porous cryogel platform of graphene-incorporated chitosan on top of a Prussian blue layer electrodeposited on a screen-printed carbon electrode. * Multi-walled carbon nanotubes (MWCNT).
The standard structure of biosensors is a classic system containing three fundamental elements: a bioreceptor, which is responsible for the selectivity of the device, a transducer that translates the physical or chemical change, leading to analyte recognition, and a signal processing unit [154]. The projection stage of the interaction between the biological and the transducer systems is fundamental for method design and possible test protocols for the nanosensor. In this phase, chemistry and computational biochemistry are essential elements to enable greater security to the method and generate considerable savings in reagents, human resources, and time.
Among medical applications, nanobiosensors are viable tools for detecting viruses or bacteria, which can cause potentially deadly diseases. Joshi et al. +developed a biocompatible, economically competitive, reduced graphene oxide (rGO) film. The film was obtained from shellac using a heat treatment (TrGO). After analysis, its relevant structural, chemical, and electrical properties were compared to similar films. After the heat treatment, the rGO (TrGO) film showed good crystallinity, low foil resistance, and high carbon content. From the TrGO, electrochemical immunosensors were produced without labels for the quantitative detection of the H1N1 influenza virus employing electrochemical impedance spectroscopy. These nanosensors exhibited high stability and reproducibility. The detection limits were of 26 and 33 plaque-forming units, respectively, in phosphate-buffered saline and diluted saliva. These low-cost TrGO-based sensors showed great potential as biosensors based on reliable and robust nanomaterials for general clinical applications [155].
Cancer diagnosis has become increasingly precise and selective, but errors are still common. Especially in this specific field, such errors must be minimized and procedures must reach high levels of accuracy. One of the most lethal types of cancer is ovary cancer, which is commonly diagnosed by biomarkers such as CA125, Mucin 1, HE4, and others, which may be present in the bloodstream [156]. However, there is a latent need for less expensive, more straightforward, and portable diagnostic tools for the timely diagnosis of ovarian as well as other types of cancer [157,158].
A possible solution is the employment of nanomaterial-based biosensors as tumor markers. In one of their pieces of research, Raghav et al. [159] presented an impedimetric immunosensor for free detection of the CA-125 marker. The CA125 immunosensor was produced using an electrode printed on a screen modified with Au–Ag NPs and functionalized with amine, which confers a larger surface area, besides providing the immobilization of antibodies in the correct orientation, that is, the formation of covalent amide bonds across the region antibody Fc. The functionalized immunosensor exhibited a linear response of up to 1–150 IU/mL (r2 = 0.994). The dynamic range of 1.0–1000 IU/mL reported in the literature makes it suitable for detecting CA125 without the need for any sample pretreatment, such as dilution, separation, or other adjacent processes. It is noteworthy that no significant interference was noticed from the chemical reagents or the serum proteins present in the blood. Considerable research is still being carried out to study the parameters that govern this linear response in more depth and whether they are dependent on nanoparticle size or on other particular properties of the nanomaterials used [159].
From this perspective, developing new sensing tools to identify tumor cells have evolved significantly due to the pressing need for less invasive and more accurate methods. These biosensors are mainly used to detect specific tumor biomarkers for different types of cancer. The literature already has a vast library of biomarkers but some methods are insufficiently selective. A classic example of a biomarker is the human mucin one protein (MUC1), which is the most common biomarker for monitoring metastatic breast tumors. In the study by Paimard et al. [160], the authors reported on an impedimetric assay for MUC1 identification using a gold nanocomposite with a nanofiber core shell on a multi-walled nanotube (MWCNT) that had been covalently modified with the MUC1 binding aptamer. MUC1 was detected by changing the surface resistance of the synthesized electrode. This nanobiosensor exhibited a high detection limit (LD) of 2.7 nM, good stability, and selectivity in the narrow region of 5–115 nM of MUC1. The assay was successfully applied to determine MUC1 in enriched serum samples and yielded satisfactory recoveries [160]. Despite the fact that this work is relatively recent, the laboratory tests were considered significant and indicated the possibility of their use on clinical trials from the first stage of the disease.
In the food industry, nanosensors are promising alternatives to solve several common problems in the sector [161]. The development of “smart tags” based on nanomaterials can evaluate product quality. These labels can interact with gases, microorganisms, and other by-products generated during food decomposition or adverse reactions with the packaging material. Many labels use a color change in the indicators present in the sensors as a means to alert consumers about the quality of a product [162,163]. This is in line with the concept of smart packaging (SP), which encompasses any type of container that provides specific functionality beyond their basic function of being a physical barrier between the food product and the surrounding environment [164]. Many packages are currently formulated with nanomaterials that provide improvements to their physical, chemical, and biochemical properties, imparting a longer shelf life for different products.
Practical applications in this area include the work of Faalnouri et al. [162], who developed surface plasma resonance (SPR) nanosensors to detect amoxicillin in milk samples using a molecular printing technique. Laboratory tests have shown that this nanosensor has a low detection limit and a high sensitivity and selectivity for identifying amoxicillin. As this study is very recent, the scientific community awaits for further tests before the product can be commercialized [162]. In addition, Xiang et al. [165] proposed the synthesis of a new nanosensor functionalized with black phosphorene (BP) in two-dimensional layers (2D). The tool was used to detect ochratoxin A (OTA) in grape juice and red wine samples. OTA is a toxic metabolite secreted by species of Aspergillus and Penicillium fungi, and it can cause severe nephrotoxic, immunotoxic, and carcinogenic effects [166]. In the literature, short reports on the electrochemical detection of this metabolite using a chemically modified electrode can be found. The BP-modified nanosensor exhibited an excellent linear electrochemical response to OTA in the concentration range of 0.3–10 μg/mL, with a detection limit of 0.18 μg/mL under ideal conditions. It was concluded that this electrochemical nanosensor showed good stability, superior anti-fouling property, and excellent sensitivity for OTA detection [165].
In summary, nanomaterials in the preparation and functionalization of biosensors have been proved to be a promising opportunity for solving several problems in the food sector, in agriculture, in applied medicine, in the enzyme preparation industry, among others, all of which are discussed in the next sections [130,167,168,169,170].
2. Enzymes
It has been observed that nanomaterial-based biosensors have elevated sensitivity and specificity [171]. These characteristics can be improved both by improving their conductivity and via the creation of a layer of nanomaterial on the surface of the transducer, onto where a wide variety of compounds can be immobilized, including biological materials [172,173]. It is important to highlight that materials with a high specific surface, such as NP, can increase the number of bioreceptor units within a reduced volume while still acting as a transduction element [152]. However, the variation in size, shape, and composition of nanoparticles, along with the general instability of their suspensions, can influence reaction performances and response times, potentially causing low reproducibility and negatively affecting their commercial interest [173].
Enzymatic biosensors, on the other hand, show less stability, lower signal intensity, higher cost, and in some cases, they require association with a mediating system [174]. However, they can be highly sensitive and selective [171]. Biomolecules can be immobilized and combined with other materials by surface modification through recombination or the introduction of binders [171]. Enzymatic biosensors are easy to use, sensible to very low concentrations, highly precise, and even when associated with NPS, they show great potential for miniaturization and real-time diagnostic capability, apart from requiring minimal sample preparation and promoting high yields [175].
Enzymes are biocatalysts that facilitate a plethora of reactions in biological systems, apart from being essential entities for sustaining life in several living organisms [176,177,178]. They are synthesized in animals, vegetables, fungi, and microorganisms [179,180], and their structure is composed of linear chains of amino acids that fold into complex, highly accurate tertiary structures with hydrophobic nuclei surrounded by hydrophilic layers [181,182]. The complexity of their three-dimensional structures provides the chemical environment necessary to catalyze a particular reaction mechanism, and they also present a defined region within their structure, called the active site, where catalysis takes place [183,184].
Several chemical-based transformation processes still employed in various industrial sectors show many disadvantages, from both a commercial and an environmental perspective, such as low yields, very high temperature, pressure, and acidity or alkalinity requirements, and high costs [185]. The environmental and economic impact imparted by using enzymes is greatly reduced by their potential of creation of more active variants than those found in nature [186]. Enzyme-assisted catalytic processes are highly efficient and advantageous due to the possibility of operation under mild conditions of reaction, high selectivity and specificity, lower environmental and physiological toxicity, and reduced costs and waste generation, all of which leads to more optimized production routes [187,188,189,190,191,192,193].
It is important to highlight that enzymes, when used in their free form, present limitations relating to their stability, efficiency, and specificity. Most of them are soluble in water, which makes it difficult to recover and reuse them [194]. Despite its excellent performance potential, industrial applications were made impossible due to these undesirable characteristics [195,196]. In this scenario, immobilization techniques stand out as alternatives to overcome these limitations, since they can offer better stability, increased activity and selectivity, excellent resistance, improvements in product separation and purification, and the possibility of enzyme reuse, rendering processes increasingly efficient [192,193,194,195,196,197,198,199,200,201,202,203,204,205,206].
Enzyme Immobilization
Enzymatic immobilization is based on the confinement of enzyme molecules on the surface of a reliable support that is different from that in which the substrate or products are present [202,203,204,205,206,207,208,209]. In contrast with their solubilized form, immobilized enzymes provide a large enzyme to substrate ratio, efficient digestion, and secure handling, in addition to showing more significant activity and the possibility of reuse for several cycles [197,210,211,212,213]. The stability of free enzymes is mainly dictated by its intrinsic structure, while the stability of their immobilized counterparts is highly dependent on several other factors, as shown in Figure 4. These factors are responsible for the stability of immobilized enzymes under different temperatures and storage conditions. The experimental variables can be expected to increase or decrease during the immobilization process [214,215,216,217].

Figure 4. Determining factors for the stability of immobilized enzymes.
According to the modes of interaction between enzymes and supports, immobilization methods can be classified into physical or chemical methods, as shown in the scheme in Figure 5 [180,218]. Physical methods show weaker monovalent interactions, such as hydrogen bonds, hydrophobic interactions, van der Waals forces, affinity or ionic bonding of enzymes to the support, or the mechanical containment of enzymes within the support [219,220,221,222]. In chemical methods, the formation of covalent bonds occurs from ether, thioether, amide, or carbamate bonds between the enzyme and the support material [223]. Each immobilization technique is applicable to a specific process, and choices are usually made based on the costs and sensitivity required [200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224].
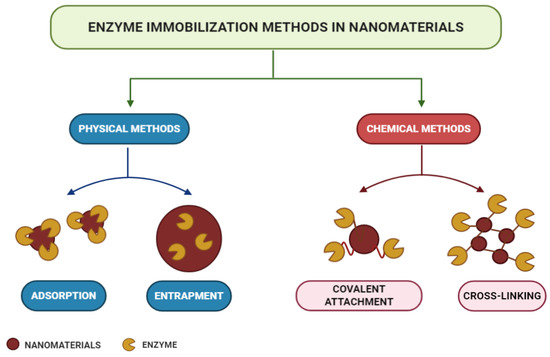
Figure 5. Enzyme immobilization methods.
Adsorption is a simple but efficient method of enzymatic immobilization [225]. Adsorption can be of physical, ionic, or affinity nature, with physical adsorption being the most commonly used method [180]. The latter is probably the fastest, most straightforward, and economical technique [226]. It is a reversible method in which enzymes are physically bound to the support material. It involves weak intermolecular interactions, such as Van der Waals forces, electrostatic forces, hydrophobic interactions, and hydrogen bonds [227,228]. With this technique, immobilized enzymes can be easily removed from the support, allowing its reuse in subsequent immobilization cycles [229]. In the study carried out by Lin et al. [230], FeO@C nanoparticles functionalized with amine were used as magnetic carriers for laccase immobilization by adsorption. As a result, the operational, pH, and storage stability of the immobilized laccase were significantly improved, and after 10 consecutive operations, it maintained its residual activity above 60%.
The trapping method is based on the incorporation of the enzyme into a polymeric network by covalent or non-covalent bonds that enable the passage of the substrate while retaining the enzyme [231,232]. In this method, enzymes are not bound to the support matrix, unlike in other methods [233]. Different strategies can be used for entrapment, such as the inclusion of the enzymes within a highly cross-linked polymeric matrix, their dissolution in a non-aqueous phase, or their separation through semipermeable microcapsules [207]. The entrapment to charged polymeric membranes appears as an alternative to the functionalization method, since this allows the immobilization of enzymes at high concentrations [229,230,231,232,233,234,235]. The technique presents several advantages, as it does not require many steps or extensive purification stages, apart from being fast and the reaction being able to run under moderate conditions [236,237]. However, difficulties regarding diffusion exist in the transport of substrate and product, when these have high molecular weight [238]. In the study by Xu et al. [239], an Aspergillus niger lipase was doubly immobilized via encapsulation in SiO2 nanoparticles in sol–gel powder prepared with tetramethoxysilane (TMOS) and methyltreimetotoxysilane (MTMS) catalyzed polymerization. The results indicated that under ideal conditions, the immobilized lipase retained 92% of its protein load and 94% of its total enzymatic activity, in addition to showing higher thermal and pH stability than its free form, confirming their great potential for industrial applications.
Enzyme crosslinking involves a bifunctional agent, usually glutaraldehyde [240], in the preparation of immobilized enzymes without the need for a carrier [241]. The advantages of this approach are high enzyme activity and low production costs due to the exclusion of additional expensive carriers [211]. The cross-linked enzyme aggregate (CLEA) method is an independent immobilization technique, which enables the production of recyclable and stable biocatalysts with high activity retention [242,243]. They can be applied to immobilize almost all enzymes and present many positive economic advantages and environmental benefits in industrial biocatalysis [237,244]. They are produced by the crosslinking of enzymatic aggregates resulting from the mixing of an aqueous protein solution with organic solvents, polymers, or anionic salts, or by crosslinking the bifunctional reagent, which generates a three-dimensional polymeric matrix [228,245]. Doraiswamy, Sarathi, and Pennathur [246] carried out a study using nanoparticles of magnetite (MGNP-CLEAs) and graphene oxide (GO-CLEAs), cross-linked with glutaraldehyde, as supports for the immobilization of the enzyme Staphylococcus spp through the CLEA method. As a result, MGNP-CLEAs have been shown to have better stability over a wide temperature and pH range, together with an increase in their reusability and storage stability.
Covalent bonding occurs through functional groups of enzymes that are not essential for its catalytic activity [247]. Nucleophilic functional groups of amino acid side chains, such as hydroxyl, amino, carboxylic, and thiol, are usually involved in the formation of these bonds [248]. Immobilization by covalent bonding is advantageous in that it forms strong bonds between the enzyme and the support, preventing enzyme leaching [249]. However, the amount of materials available commercially for covalent immobilization is low compared to immobilization by adsorption [250]. With the use of this technique, there is a slow release of enzymes and an improvement in its storage capacity and shelf life, so it is favorable for continuous applications at full scales [251]. Osuna et al. [252] studied the immobilization of Aspergillus niger lipases by covalent bonding on magnetic nanoparticles coated with chitosan (CMNP) and obtained by co-precipitation. The results showed high storage stability for 50 days in immobilized derivatives that maintained their initial activity above 80% after 15 hydrolytic cycles.
The characteristics of the support used for enzyme immobilization are fundamental factors to determine their performance [190,253]. An adequate support must present physical resistance to pressure and hydrophilicity, be readily available, and of low cost [254].
(References would be added automatically after the entry is online)
This entry is adapted from the peer-reviewed paper 10.3390/electrochem2010012
