Stomatal density, spacing, and patterning greatly influence the efficiency of gas exchange, photosynthesis, and water economy. They are regulated by a complex of extracellular and intracellular factors through the signaling pathways. After binding the extracellular epidermal patterning factor 1 (EPF1) and 2 (EPF2) as ligands, the receptor-ligand complexes activate by phosphorylation through the MAP-kinase cascades, regulating basic helix-loop-helix (bHLH) transcription factors SPEECHLESS (SPCH), MUTE, and FAMA.
- SPEECHLESS
- MUTE
- FAMA
- bHLH
- stomatal lineage
- stomatal development
1. Introduction
Arabidopsis thaliana (Arabidopsis) protodermal leaf cells differentiate into three main cell types: unicellular leaf hairs (trichomes), pavement cells, and pairs of stomatal GCs that facilitate transpiration and exchange of gases between plant and atmosphere [1,2,3]. Stomata development from leaf protodermal cells follows the single-cell spacing rule to ensure the separation of stomata with at least a single pavement cell [4,5]. Stomata formation comprises a series of steps called stomatal lineage [6]. Stomatal lineage cells may undergo normal stomatal development by following single-cell space rules, exit the lineage, be arrested at any developmental stage, or undergo apoptosis by activating programmed cell death [3,7,8]. The cell cycle is checked and regulated by cell cycle checkpoints, which are present between each phase of the cell cycle, and decide the possible developmental fate of a cell [7].
Stomatal lineage starts with stochastically selected leaf protodermal cell transition into the meristemoid mother cell (MMC). The MMC divides asymmetrically into a large daughter cell called a stomatal lineage ground cell (SLGC) and a meristemoid small daughter cell [5]. The SLGC in the daughter cells pair can differentiate into a pavement cell or reacquire MMC fate to undergo asymmetric spacing division to form satellite meristemoids [9]. Meristemoids can divide asymmetrically to amplify more SLGCs, transit into guard mother cells (GMC), or, rarely, exit the lineage [3]. The GMC will divide symmetrically into a pair of GCs with space between them to form a stoma, or its development can be arrested (Figure 1).
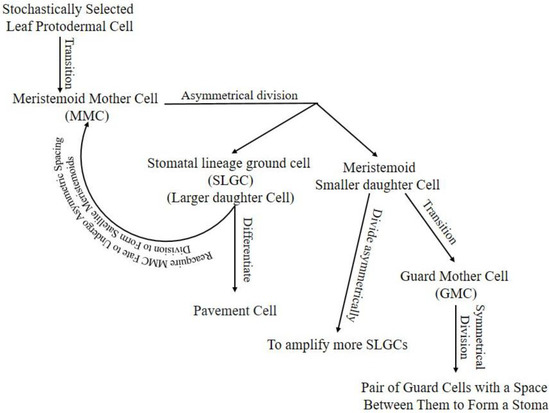
Figure 1. Stomatal lineage, starting from protodermal cell to pair of GCs enclosing stomata. Arrow lines indicate the progression of cells in the lineage. Hypothetically, the stomatal lineage ground cell (SLGC) will differentiate into a pavement cell if its neighboring cell is a meristemoid from another cell division. If the SLGC neighbor cell is an SLGC from another cell division, it will undergo a spacing division. Hypothetically, the meristemoid will progress into guard mother cells (GMC) if it is surrounded by SLGCs or pavement cells; otherwise, it will continue amplifying to attain enough SLGCs for a single-celled space rule. Cells may exit lineage in any stage due to unfavorable intrinsic or extrinsic conditions.
2. Role of SPEECHLESS (SPCH), MUTE, and FAMA in Stomata Development
Basic helix-loop-helix (bHLH) transcription factors (TFs) such as SPEECHLESS (SPCH), MUTE, and FAMA are critical for stomatal development. Mutations in any one of these TFs result in improperly developed stomata [6,10,11,12]. SPCH regulates the transition of protodermal cells into MMCs and asymmetric cell division subsequently. SPCH-defective mutants were unable to initiate stomatal lineage, with an entirely pavement-celled epidermis phenotype. The different fate decisions of the meristemoid cells (amplifying division, spacing division, or progression down the lineage) depends on the activity and available level of SPCH (Figure 2) [12,13]. MUTE regulates meristemoids’ transition into GMCs, confirmed by arrested lineage at the meristemoid stage in a MUTE-defective mutant. Overexpression of MUTE in the spch background partially rescued spch-phenotype, and in the wild-type, it exhibited a phenotype, an epidermis densely populated with stomata (Figure 2) [10,14]. These results demonstrate that MUTE has no role in SPCH-regulated asymmetric amplification and spacing cell division [14].
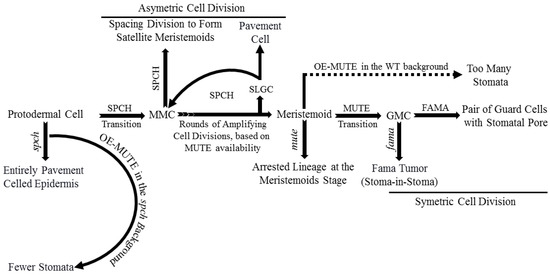
FAMA regulates symmetric cell division of each GMC into a pair of GCs with a space between them to form stomata and terminates lineage cell meristematic activity [11,15,16]. Defective FAMA exhibited uncontrolled symmetric cell division of GMCs with a FAMA-tumor phenotype. FAMA-RETROBLASTOMA-RELATED (RBR) interaction facilitates POLYCOMB REPRESSOR COMPLEX-mediated chromatin methylation to switch off SPCH and MUTE expression. Incorrect FAMA expression resulted in a stoma-in-stoma phenotype, where GCs were pushed into lineage by active SPCH (Figure 2) [11,15,16,17]. Furthermore, FAMA-activated genes such as POTASSIUM CHANNEL IN ARABIDOPSIS THALIANA 1 are required for GCs functioning and control of stomatal aperture [17].
3. Ligand, Receptor, MPK Cascade, and Associated Pathways in Stomatal Development
Peptide signaling, a well-known stomatal development regulator, is underpinned by EPIDERMAL PATTERNING FACTORS 1 (EPF1), EPF2, and EPIDERMAL PATTERNING FACTOR LIKE 9 (EPFL9, denoted as STOMAGEN, STOM) [18,19,20,21,22]. EPF1/2, as negative stomatal development regulators, bind/activate leucine-rich repeat receptor kinases (LRR-RKs). At the same time, STOM competes to bind the LRR-RKs with the EPF1/2 to positively regulate stomatal development [23,24,25]. EPF2 mainly regulates SPCH and the subsequent behavior of meristemoids, whereas EPF1 regulates the one-cell-spacing rule and participates in GMC’s autocrine regulation and inhibition of SLGCs from re-entering the stomatal lineage [26,27]. The ERECTA (ER) family, comprising ER, ERECTA-LIKE 1 (ERL1), and ERL2, are extensively studied LRR-RKs [28]. ERLI and ER play a role in the GMC development stage and facilitate the cell-specific MUTE activity, whereas ERL2 regulates SPCH, mainly as shown in Figure 3 [26,29]. The erl1 and erl2 single mutant and erl1 erl2 double mutant showed reduced SLGC, a less severe phenotype as compared to the er single mutant. The less severe phenotype in ERL mutants than ER mutants demonstrates the ER dominance during stomatal development [30]. Increased stomatal density and index in the er erl1 double mutant and elevated SLGCs in er mutant indicate that they are collectively regulating MUTE in the GMC stage. While slightly increased phenotype in the er erl2 double mutants compared to er, the single mutant suggests that erl2 is one of the main SPCH regulators during stomatal development [30].
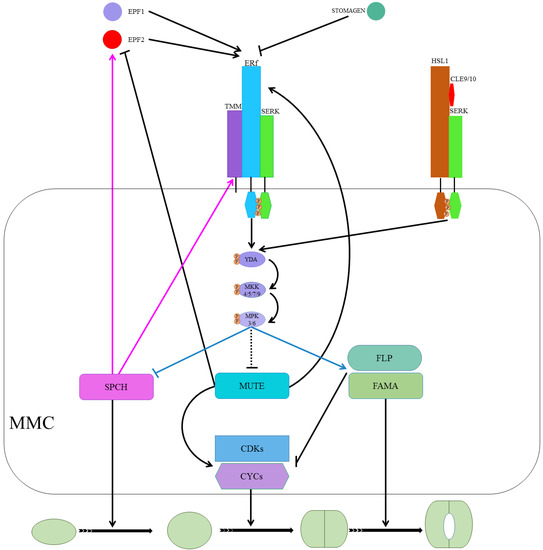
TOO MANY MOUTHS (TMM) binds to the ER family (ER and ERL1) to form active extracellular complexes to perceive EPF1 and EPF2 for regulating correct stomatal development [31,32]. TMM, which is mainly expressed in young GCs and stomatal precursor cells, is part of the autoregulatory mechanism and a direct target of SPCH transcription regulators [12,33]. SOMATIC EMBRYOGENESIS RECEPTOR KINASES (SERKs), an irrelative LRR-RK family, have been found required for coupling extracellular TMM/ER complexes to perceive peptide signaling for downstream intracellular signaling, as shown in Figure 3 [34]. There are five homologs (SERK1-5) in Arabidopsis, and single mutants of these genes did not alter stomatal development. However, serk1-1/serk2-1/SERK3-4 triple and serk1-1/serk2-1/SERK3-4/SERK3-5 quadruple mutants displayed clustered stomata phenotypes, and the phenotype for the latter was similar to er erl1 erl2 triple mutants [35].
Upon completion of the ER/SERK/TMM/EPF complex, the ER/SERK cytosolic kinase domain is phosphorylated to target mitogen-activated protein kinase kinase kinase (MPKKK), which is also known as YODA (YDA), a class MPK cascade [2,36,37,38]. YDA, in turn, phosphorylates mitogen-activated protein kinase kinases (MPKKs) that phosphorylate mitogen-activated protein kinase (MPKs) that directly phosphorylate the MPK cascade’s final target [3,37,38]. Only four (MPKK4/5/7/9) and three (MPK3/4/6), out of 20 in Arabidopsis, are involved in stomatal development [37,38,39]. MPK3/6 interacted with SPCH and MPK4 with MUTE, but no in vivo or phenotypic evidence for the latter has been reported previously [36,37,40]. The MPK3/6 was unable to interact with truncated SPCH without MAPK target domain (MPKTD) and exhibited clustered stomata, as shown in Figure 3 [41]. Furthermore, signaling peptides CLAVATA3/ESR-RELATED (CLE) family genes CLE9/10 regulate stomatal lineage development perceived by receptor kinase HAESA-LIKE 1 (HSL1) in a different pathway [42]. HSL1 recruits SERKs as co-receptors in the presence of CLE9/10 for different signaling modes during stomatal development, as shown in Figure 3 [42].
4. Feedback Regulation of SPCH, MUTE, and FAMA
SPCH, MUTE, and FAMA form a heterodimer with SCREAM 1 (SCRM1), SCRM2, and bHLH proteins [36,43,44]. INDUCER OF CBP EXPRESSION 1(ICE1)/SCRM2, as a scaffolding partner, facilitates MPK3/6-mediated phosphorylation of SPCH for its activity inhibition and consequent degradation [36,43,45]. MPK3/6-regulates phosphorylation and subsequent degradation of ICE1/SCRM2 during a process that plays a crucial role in stomatal cell fate specification [43]. Furthermore, FOUR LIPS (FLP) and AtMyb88 constrain GMC division, and an outnumbered GCs phenotype was observed in flp myb88-defective mutants [46]. SPCH/SCRM, which is suppressed by ER/SERK/TMM/EPF-induced phosphorylation of MPK cascade, induces expression of EPF2 and TMM in a negative feedback mechanism [12,33]. MUTE induces ERLI and suppresses EPF2, whereas ERL1, upon perceiving EPF1, regulates meristemoid transition into the GMC stage [20,26,32].
Additionally, cell cycle-related genes such as cyclins A and D (CYCA and CYCD, respectively) in association with CYCLIN-DEPENDENT KINASE A 1;1 and D1;1 (CDKA1;1 and CDKB1;1) play a key role in stomatal development [47,48]. CYCD4 participates in hypocotyl stomatal lineage divisions and CDKB1;1- and CDKA1;1-dominant negative forms, and CYCA higher-order mutants inhibit the division of GMCs [49,50,51,52]. MUTE upregulates cell cycle-related genets (CYCs and CDKs) by binding to their promoters to regulate the symmetric cell division of GMCs. FAMA binds to CDKB1;1 promoter regions, and FLP suppresses the expression of CDKB1;1 and CDKA1;1. As well, FAMA and FLP negatively regulate GMC symmetric division by repressing CDKs and CYCs [51,53,54]. Taken together, MUTE locks in the cells in the differentiation program, promotes regulators of the symmetric division of GMCs, and upregulates FLP and FAMA, which suppress regulators of the cell cycle, to control single symmetric cell division, as shown in Figure 3 [55].
This entry is adapted from the peer-reviewed paper 10.3390/plants10030432
