The insular cortex is an important information integration center. Numerous imaging studies have documented increased activity of the insular cortex in the presence of neuropathic pain; however, the specific role of this region remains controversial.
- neuropathic pain
- insular cortex
- analgesic response
1. Introduction
Neuropathic pain occurs when the somatosensory nervous system is damaged due to disease or trauma. Its main symptoms include hyperalgesia, allodynia, and spontaneous pain. Hyperalgesia and allodynia refer to increased pain perception in response to stimuli that usually do and do not cause pain, respectively [1]. Neuropathic pain is difficult to cure, and the long-term suffering that it causes can affect an individual’s quality of life and psychological state. For this reason, it is often comorbid with mood disorders, such as depression and anxiety [2]. Nonsteroidal anti-inflammatory drugs (NSAIDs) are effective for nociceptive pain, but have little effect on neuropathic pain [3].
Mu-opioid receptor (MOR) agonists, such as morphine, are the gold standard of analgesia for various types of pain, such as cancer and severe acute pain [4]. However, for neuropathic pain, the effect is not as obvious [5]. Some researchers believe that the delta-opioid receptor (DOR) may be an important target for the treatment of neuropathic pain [6][7]. Repeated administrations of SCN80, a DOR agonist, could improve some symptoms of neuropathic pain in rats with chronic constriction injury (CCI) [8]. The exploration of analgesic drugs indicates that there are many difficulties in the treatment of neuropathic pain. In many cases, antidepressants and anticonvulsants are used to relieve the symptoms of neuropathic pain [9].
Neurosurgical inventions are also options for the treatment of neuropathic pain [10], such as lesion of the dorsal root entry zone (DREZ) [11] and motor cortex neuromodulation [12]. Responses to neuropathic pain treatment differ among individuals, and sex-based differences in analgesia responses have been reported [13]. Exploration of the mechanisms underlying neuropathic pain from a broad range of perspectives may aid in the identification of better ways to relieve this condition.
As the insular cortex is relatively hidden, the embedding of a cannula or an electrode in the insular lobe for laboratory animal research is difficult. Thus, the insula is more mysterious to neuroscientists than other cortical regions. The rapid development of imaging technology has enabled researchers to explore the functions of the insula. Anatomic studies have confirmed that the insula is connected with many brain structures, such as the somatosensory cortex, limbic system, and frontal lobe [14]. Imaging studies suggest that the insular functions are diverse and include those related to interoception and emotion, reward and motivation, cognition, and decision-making [15].
Some researchers have suggested that the insula is an important information integration center [16] or an area of cross-modal integration [17]. The insular cortex has also been found to participate in the processing of empathy and awareness, and it may tell us “how we feel now” and even “who we are” [18]. In addition, the insula participates in the processing of pain, a complex multidimensional experience [19]. Studies have found that the insula is involved in the processing of autonomic responses to noxious stimuli [20] and in the affective–motivational component of pain [21]; however, increasing evidence indicates that the involvement of the insula in pain processing is more complex [22][23][24].
2. Role of the Insula in Neuropathic Pain
The role of the insula in neuropathic pain has been of interest for decades. The historical progress is shown in Figure 1. In 1956, a study reported a patient with neuropathic pain who had some small soft fused lesions in the insular cortex and parietal operculum [25][26]. Although this result was from an autopsy, it still suggests the relationship between the insular lobe and neuropathic pain. The development of imaging technology in the 1990s promoted progress in the research of the insular cortex. In 1995, Hsieh et al. found increased regional cerebral blood flow (rCBF) in the bilateral anterior insula and other brain regions in patients with painful mononeuropathy using positron emission tomography (PET) [27].
In 1999, Treede et al. proposed that the insular cortex may be involved in the affective–motivational dimensions of pain [19]. However, this may not be enough to explain the special role of the insula in neuropathic pain. The processing of synesthesia is an important feature of the insula. A 2006 study reported the synesthesia of neuropathic pain and odor and the associated insular activation using functional magnetic resonance imaging (fMRI) [28]. Since 2007, people have noticed the relationship between analgesia and the insula [29], as well as the molecules related to the insula and neuropathic pain [30]. In the past decade, neural network research related to the insular lobe has also provided a great deal of evidence revealing the pathogenesis of neuropathic pain [31]. In recent years, researchers have found that empathy may affect neuropathic pain and that the insular lobe is also involved [32].
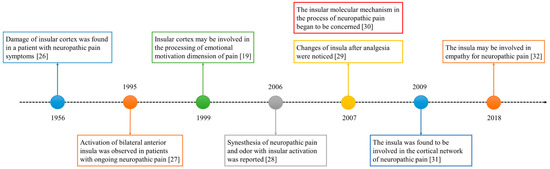
Figure 1. The development timeline of the insular lobe and neuropathic pain.
Although many studies have found that the insular cortex is essential for pain processing, it is still difficult to explain how it participates in pain. In this review, we introduce the structural and functional changes of the insular lobe in the neuropathic pain state and the related molecular mechanisms. There are other brain regions involved in pain, such as the thalamus, primary somatosensory cortex (SI), secondary somatosensory cortex (SII), anterior cingulate cortex (ACC), and frontal cortex [20][33]. Each brain region plays an important and unique role in the occurrence and development of neuropathic pain. However, to clearly describe the insular cortex, we ignore the changes in other brain areas during neuropathic pain.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22052648
References
- Jensen, T.S.; Finnerup, N.B. Allodynia and hyperalgesia in neuropathic pain: Clinical manifestations and mechanisms. Lancet Neurol. 2014, 13, 924–935.
- Wright, M.E.; Rizzolo, D. An update on the pharmacologic management and treatment of neuropathic pain. JAAPA 2017, 30, 13–17.
- Cohen, S.P.; Mao, J.R. Neuropathic pain: Mechanisms and their clinical implications. Bmj-Brit. Med. J. 2014, 348.
- Drdla, R.; Gassner, M.; Gingl, E.; Sandkuhler, J. Induction of synaptic long-term potentiation after opioid withdrawal. Science 2009, 325, 207–210.
- Martinez-Navarro, M.; Maldonado, R.; Banos, J.E. Why mu-opioid agonists have less analgesic efficacy in neuropathic pain? Eur. J. Pain 2019, 23, 435–454.
- Nadal, X.; Banos, J.E.; Kieffer, B.L.; Maldonado, R. Neuropathic pain is enhanced in delta-opioid receptor knockout mice. Eur. J. Neurosci. 2006, 23, 830–834.
- Castany, S.; Carcole, M.; Leanez, S.; Pol, O. The antinociceptive effects of a delta-opioid receptor agonist in mice with painful diabetic neuropathy: Involvement of heme oxygenase 1. Neurosci. Lett. 2016, 614, 49–54.
- Vicario, N.; Parenti, R.; Arico, G.; Turnaturi, R.; Scoto, G.M.; Chiechio, S.; Parenti, C. Repeated activation of delta opiod receptors counteracts nerve injury-induced TNF-alpha up-regulation in the sciatic nerve of rats with neuropathic pain: A possible correlation with delta opiod receptors-mediated antiallodinic effect. Mol. Pain 2016, 12.
- Gilron, I.; Baron, R.; Jensen, T. Neuropathic pain: Principles of diagnosis and treatment. Mayo Clin. Proc. 2015, 90, 532–545.
- Pereira, E.A.; Aziz, T.Z. Neuropathic pain and deep brain stimulation. Neurotherapeutics 2014, 11, 496–507.
- Haninec, P.; Kaiser, R.; Mencl, L.; Waldauf, P. Usefulness of screening tools in the evaluation of long-term effectiveness of DREZ lesioning in the treatment of neuropathic pain after brachial plexus injury. BMC Neurol. 2014, 14, 225.
- Meeker, T.J.; Keaser, M.L.; Khan, S.A.; Gullapalli, R.P.; Seminowicz, D.A.; Greenspan, J.D. Non-invasive Motor Cortex Neuromodulation Reduces Secondary Hyperalgesia and Enhances Activation of the Descending Pain Modulatory Network. Front. Neurosci. 2019, 13, 467.
- Gensel, J.C.; Donahue, R.R.; Bailey, W.M.; Taylor, B.K. Sexual Dimorphism of Pain Control: Analgesic Effects of Pioglitazone and Azithromycin in Chronic Spinal Cord Injury. J. Neurotrauma 2019, 36, 2372–2376.
- Mufson, E.J.; Mesulam, M.M. Insula of the old world monkey. II: Afferent cortical input and comments on the claustrum. J. Comp. Neurol. 1982, 212, 23–37.
- Uddin, L.Q.; Nomi, J.S.; Hebert-Seropian, B.; Ghaziri, J.; Boucher, O. Structure and Function of the Human Insula. J. Clin. Neurophysiol. 2017, 34, 300–306.
- Kaplan, C.M.; Schrepf, A.; Vatansever, D.; Larkin, T.E.; Mawla, I.; Ichesco, E.; Kochlefl, L.; Harte, S.E.; Clauw, D.J.; Mashour, G.A.; et al. Functional and neurochemical disruptions of brain hub topology in chronic pain. Pain 2019, 160, 973–983.
- Gogolla, N. The insular cortex. Curr. Biol. 2017, 27, R580–R586.
- Craig, A.D. How do you feel—Now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009, 10, 59–70.
- Treede, R.D.; Kenshalo, D.R.; Gracely, R.H.; Jones, A.K. The cortical representation of pain. Pain 1999, 79, 105–111.
- Schnitzler, A.; Ploner, M. Neurophysiology and functional neuroanatomy of pain perception. J. Clin. Neurophysiol. 2000, 17, 592–603.
- Schreckenberger, M.; Siessmeier, T.; Viertmann, A.; Landvogt, C.; Buchholz, H.G.; Rolke, R.; Treede, R.D.; Bartenstein, P.; Birklein, F. The unpleasantness of tonic pain is encoded by the insular cortex. Neurology 2005, 64, 1175–1183.
- Singer, T.; Seymour, B.; O’Doherty, J.; Kaube, H.; Dolan, R.J.; Frith, C.D. Empathy for pain involves the affective but not sensory components of pain. Science 2004, 303, 1157–1162.
- Kong, J.; White, N.S.; Kwong, K.K.; Vangel, M.G.; Rosman, I.S.; Gracely, R.H.; Gollub, R.L. Using fMRI to dissociate sensory encoding from cognitive evaluation of heat pain intensity. Hum. Brain Mapp. 2006, 27, 715–721.
- Henderson, L.A.; Gandevia, S.C.; Macefield, V.G. Somatotopic organization of the processing of muscle and cutaneous pain in the left and right insula cortex: A single-trial fMRI study. Pain 2007, 128, 20–30.
- Garcia-Larrea, L. Insights gained into pain processing from patients with focal brain lesions. Neurosci. Lett. 2012, 520, 188–191.
- Biemond, A. The conduction of pain above the level of the thalamus opticus. AMA Arch. Neurol. Psychiatry 1956, 75, 231–244.
- Hsieh, J.C.; Belfrage, M.; Stone-Elander, S.; Hansson, P.; Ingvar, M. Central representation of chronic ongoing neuropathic pain studied by positron emission tomography. Pain 1995, 63, 225–236.
- Villemure, C.; Wassimi, S.; Bennett, G.J.; Shir, Y.; Bushnell, M.C. Unpleasant odors increase pain processing in a patient with neuropathic pain: Psychophysical and fMRI investigation. Pain 2006, 120, 213–220.
- Maihöfner, C.; Ringler, R.; Herrndobler, F.; Koppert, W. Brain imaging of analgesic and antihyperalgesic effects of cyclooxygenase inhibition in an experimental human pain model: A functional MRI study. Eur. J. Neurosci. 2007, 26, 1344–1356.
- Maarrawi, J.; Peyron, R.; Mertens, P.; Costes, N.; Magnin, M.; Sindou, M.; Laurent, B.; Garcia-Larrea, L. Differential brain opioid receptor availability in central and peripheral neuropathic pain. Pain 2007, 127, 183–194.
- Cauda, F.; Sacco, K.; Duca, S.; Cocito, D.; D’Agata, F.; Geminiani, G.C.; Canavero, S. Altered resting state in diabetic neuropathic pain. PLoS ONE 2009, 4, e4542.
- Zaniboni, C.R.; Pelarin, V.; Baptista-de-Souza, D.; Canto-de-Souza, A. Empathy for Pain: Insula Inactivation and Systemic Treatment With Midazolam Reverses the Hyperalgesia Induced by Cohabitation With a Pair in Chronic Pain Condition. Front. Behav. Neurosci. 2018, 12, 278.
- Seifert, F.; Schuberth, N.; De Col, R.; Peltz, E.; Nickel, F.T.; Maihöfner, C. Brain activity during sympathetic response in anticipation and experience of pain. Hum. Brain. Mapp. 2013, 34, 1768–1782.
