Marine mammals are sentinels for the marine ecosystem and threatened by numerous factors including infectious diseases. One of the most frequently isolated bacteria are beta-hemolytic streptococci. However, knowledge on ecology and epidemiology of streptococcal species in marine mammals is very limited.
- streptococci
- infectious diseases
- marine mammals
1. Streptococcal Findings in Marine Mammals
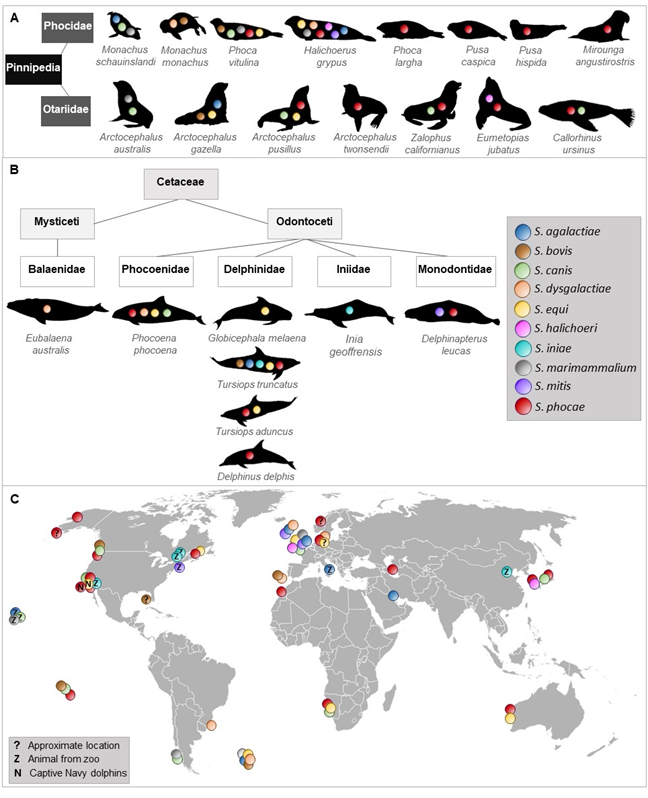
To the best of our knowledge, 10 streptococcal species were isolated and identified more than once from 23 species of Pinnipedia and Cetaceae worldwide (Figure 1).
Figure 1. Occurrence of streptococcal species described in different marine mammals. Streptococcal species that have been isolated and identified at least twice in pinnipeds (A) and cetaceans (B). (C) shows a world map indicating location of streptococcal species detected in marine mammals.
2. Streptococcus Agalactiae
S. agalactiae, also known as a bovine and human pathogen [1][2][3], was isolated from a wound and navel infection of grey seals (Halichoerus grypus) in North Rona in 1985 and 1986 [4] and from lesions of fight wounds, pneumonia, lymphadenitis as well as from lung and spleen samples of Antarctic fur seals (Arctocephalus gazella) from 1984–1987 on Bird Island, South Georgia [5]. Later, it was isolated from lesions and visceral organs (liver and lung) of a captive common bottlenose dolphin (Tursiops truncatus) that suffered from fatal necrotizing fasciitis and myositis [6]. One year later, the isolation of S. agalactiae from epaxial muscles of a wild stranded bottlenose dolphin was reported [7]. This strain caused 90% mortalities in tilapia in experimental infections and showed high similarity with strains associated with mullet kill in the concurrent Kuwait Bay. A mullet was found in the stomach of the dead dolphin, which might have served as a possible way of transmission. A study of human S. agalactiae strains from fish, seals, a dolphin, and a frog indicated zoonotic and anthroponotic hazard by causing severe disease in fish and compromising food security [8]. Between 2012 and 2014, S. agalactiae was isolated from a stranded grey seal on the British coast with ocular pathology[9]. In the Waikiki Aquarium, Honolulu, Haiwaii, S. agalactiae was isolated from two male healthy Hawaiian monk seals (M. schauinslandi) as part of their aerobic bacterial flora in the nasal cavity [10].
S. agalactiae is also known as serious fish pathogen [11][12][13]. In Brazil, high virulent strains were isolated from diseased Nile tilapia and transmission occurred by direct contact or through water [12]. Infection experiments confirmed the disease and revealed low LD50 for Nile tilapia. However, isolates from cattle did not cause any clinical signs in Nile tilapia and channel catfish indicating host specification and adaptation [14]. Human and bovine strains of S. agalactiae were able to cause disease in Nile tilapia, although there was no genetic relatedness of strains from fish, bovine, and human origin [15]. This suggests that the ability to cross host-specific barrier is not necessarily reflected by genetic linkage. Virulence gene profiling of S. agalctiae isolated from diseased tilapia in Thailand revealed a positive correlation of virulence genes content and pathogenicity [16]. Virulence genes for adhesion, invasion, and immune evasion were identified. Another study demonstrated that there were fish-specific genes or loci that were associated with disease in fish, while strains missing these regions were not able to cause morbidity in tilapia [17]. In addition, these fish-specific genes were mainly clustered in regions with signatures of mobile genetic elements and one fish-specific gene was found in the region, where the virulence genes rib or bca are in the human strain indicating genetic adaptation to the fish host.
3. Streptococcus Bovis
S. bovis has been isolated from the gastrointestinal tract and feces of cattle, sheep, goats [18], and dogs [19]. It has also been identified as human pathogen associated with endocarditis [20], meningitis [21], septic arthritis [22], bacteremia, and gastrointestinal disease [23]. Virulence factors associated with S. bovis infection were, for instance, extracellular proteins [24] and antigens [25]. S. bovis was detected in fur seals with pneumonia that was characterized by extensive polymorphonuclear infiltrations and necrosis or very widespread abscess formation and, frequently, by additionally fibrinous exudative pleurisy [5]. Together with S. phocae and S. canis it was also isolated from dead herpesvirus-positive harbor seal pups at the Smith Island, Washington [26]. A monk seal pup (Monachus monachus) found on the island Deserta Grande, Portugal died due to septicemia and S. bovis was isolated and considered as a potential causative agent [27]. In 2006, S. bovis isolation (together with S. equisimilis/mitis) from free-ranging bottlenose dolphins that were captured, sampled, and released in coastal Gulf of Mexico and Atlantic ocean waters was reported [28].
4. Streptococcus Canis
S. canis was first isolated from cows with mastitis and from dogs with different pathological findings [29], but can also cause infections in humans [30][31][32]. It has also been isolated from minks [33], feral cats [34] and cats [35]. Its virulence and pathogenicity were confirmed by the presence of virulence genes such as for fibronectin-binding protein, M proteins, protective antigen, and streptolysin [36][37][38][39]. The M protein of S. canis has also a high-affinity immunoglobulin G binding activity, which is not species-specific and facilitate S. canis to interact with different hosts [40].
A beta-hemolytic Streptococcus sp., biochemically similar to S. canis, was cultured from pyogranulomatus lesion of the laryngeal cartilages and epiglottis of an adult harbor seal (Phoca vitulina) [41]. S. canis was also isolated from peritoneal effusion of a captive California sea lion (Zalophus californianus) of the US Navy’s marine mammal program[42]and from a California sea lion with bilateral corneal ulceration of the London Zoo, UK [43]. During an increased mortality among South American fur seal (Arctocephalus australis) pups on Guafo Island, Chile, South America, S. canis together with S. marimammalium were isolated and associated with moderate to marked, multifocal, mucopurulent bronchopneumonia [44]. In August 1994, S. canis (and also S. phocae, S. equi subsp. zooepidemicus) was isolated from spleen, liver, and kidney of Cape fur seals (Arctocephalus pusillus pusillus) at Cape Cross, Namibia that suffered from respiratory infections and abortions associated with starvation [45]. Seven cases of stranded harbor porpoises (Phocoena phocoena) in England and Wales between 1990 and 1996 had a S. canis septicemia, which was isolated from lungs with pulmonary abscesses and enlarged pulmonary associated lymph nodes [46]. S. canis (together with S. phocae) was cultured from blood and lung samples of a dead, stranded Northern fur seal (Callorhinus ursinus) with necrotizing and fibrinous pneumonia infiltrated by band neutrophils with intraluminal abscess of bronchi at the coast of Niigata, Japan [47]. In 2005, the isolation of S. canis from two dead harbor seal pups on Smith Island, Washington was reported [26][48]. One died from omphalophlebitis and the other from omphalitis with subsequent peritonitis. S. canis was also isolated from the oral cavity of a male, healthy Hawaiian monk seal of the Waikiki Aquarium, Honolulu, Hawaii [10].
5. Streptococcus Dysgalactiae
S. dysgalactiae subsp. equisimilis, previously known as S. equisimilis [49] and found in humans [50]and different animals such as dogs, cows, pigs, and horses [51], was isolated from Antarctic fur seal pups with septicemia and rhinitis in South Georgia, UK between 1979–1982 [52] and 1986 from a grey seal cow on North Rona, Scotland [4]. In the years 1988 and 1989, an increased number of harbor porpoise carcasses was observed in North and Baltic Seas [53]. Thirty-five isolates of beta-hemolytic streptococci were classified in Lancefield group L and identified as S. dysgalactiae subsp. dysgalactiae. In 1997, S. dysgalactiae and S. dysgalactiae subsp. equisimilis isolates were found in a dead, wild monk seal pup in association with a septicemia on the island Deserta Grande, Portugal [27]. Three isolates identified as S. dysgalactiae subsp. dysgalactiae were obtained from phocid seals (harbor and grey seals) stranded in the North and Baltic Seas between 1995 and 2000 [54]. Between 2005–2011, pathologic and microbiological findings of a southern right whale (Eubalaena australis) calf from Brazil indicated that beta-hemolytic S. dysgalactiae septicemia was responsible for the death of the animal [55].
6. Streptococcus Equi
S. equi causes infections in horses [56] and was associated with canine infectious respiratory disease [57]. A systemic infection with S. equi in a horse handler has also been reported [58]. Further studies confirmed the zoonotic potential of S. equi [59][60]. In November 1978, a female North Atlantic pilot whale (Globicephala melaena) suffering from bronchopneumonia with lesions stranded on Metis Beach, Canada and S. equi (no further identification) was isolated from lung parenchyma, pharynx, and pericardial fluid [61]. A study from 1980 reported the isolation of S. equi subsp. zooepidemicus (previously S. zooepidemicus) from grey seals associated with mild, purulent pneumonia [62]. In 1994, it was isolated from the conjunctiva and trachea of two adult female Cape fur seals that had septicemic S. phocae in Namibia [45]. A total of 32 beta-hemolytic streptococcal isolates, collected during the phocine distemper outbreak in 2002 from 28 different harbor seals of the German North Sea, were identified as S. equi subsp. zooepidemicus [63]. Later, the same scientists isolated the same or at least very close related strains of S. equi subsp. zooepidemicus from grey seals and other harbor seals [64]. A retrospective study on 42 dead bottlenose dolphins from the US Navy Marine Mammal Program during 1980-2010 demonstrated an association of the isolation of S. equi subsp. zooepidemicus with pneumonia [65]. 16S rRNA sequences for S. equi (and S. phocae) were found in blow samples collected from four wild healthy Indo-Pacific bottlenose dolphins (T. aduncus) in Shark Bay (SB), Western Australia, in 2012 [66].
7. Streptococcus Halichoeri
S. halichoeri, characterized as non-hemolytic and classified in Lancefield group B, was first isolated from dead grey seals in Iverness and Cornwall, UK [67] and few years later, in 2012, also from the kidney of a stranded, female Stellar sea lion (Eumetopias jubatus) in South Korea [68]. Also, in 2012, a severe case of a human infection with S. halichoeri was reported [69]. The patient had no contact to seals, but to fish, which could have been a possible transmission route. However, this was not tested. Another human infection was reported in 2018, where a man suffered from skin cellulitis due to S. halichoeri [70]. Shewmaker et al. [71]compared human and seal strains and concluded two subspecies S. halichoeri subsp. halichoeri for the seal isolates and S. halichoeri subsp. hominis for strains associated with human infections. The core genome of 20 S. halichoeri isolates from different hosts including dogs and minks contained 19 different streptococcal virulence factors, of which most were associated with adherence followed by proteases and toxins emphasizing its pathogenic potential [72][73].
8. Streptococcus Iniae
S. niae was described as new species in 1976, when it was first isolated from a captive Amazon freshwater dolphin (Inia geoffrensis) suffering from a dermatologic syndrome called “golf ball disease” in the Steinhart Aquarium, San Francisco, USA [74]. Further isolates were obtained from captive freshwater dolphins housed at the Niagara Falls Aquarium in New York, USA two years later [[75], and in 1983 from a captive Amazon River dolphin at the Pittsburgh Zoo, USA that also developed the “golf ball disease”[76]. In 2015, a common dolphin died due to bacterial septicemia at Beijing aquarium, China, where S. iniae was isolated from hilar lymph nodes and pancreas of the dolphin [77].
S. iniae is also a serious fish pathogen [78][79]. Virulence mechanisms include a capsule with antiphagocytic function [80], the cytotoxin ß-hemolysin streptolysin S [81], an extracellular nuclease and s secreted nucleotidase that play an important role in immune evasion [82], a polysaccharide deacetylase involved in adherence, invasion, lysozyme resistance and survival in fish blood [83], and M-like protein [84]. Comparative genomics revealed genetic differences between strains from different hosts including I. geoffrensis and identified two plasticity zones that reflect adaptation to specific host environments [85]. Furthermore, the dolphin isolates differed from the fish and human isolates in lacking a capsule, forming denser and thicker biofilms, increased ability to withstand oxidative stress and were genetically highly divergent to the other isolates [86]. In addition, there were conserved mutation rates and mismatch/oxidized-guanine repair systems within phylogenetic clades, but significant differences between major phylogenetic lineages. Mutators might facilitate adaptation to novel hosts including immune escape. This indicates that S. iniae has the genetic repertoire to adapt very well to many different hosts.
9. Streptococcus Marimammalium
S. marimammalium was first isolated from the lung of a dead harbor seal and a dead grey seal in Iverness, Scotland [87]. In 2007/2008, it was also isolated (together with S. agalactiae and S. canis) from nasal and oral swabs of two healthy Hawaiian monk seals from the Waikiki Aquarium, Honolulu, Hawaii [10]. In 2016, it was also isolated from South American Fur Seal Pups with moderate to marked, multifocal, mucopurulent bronchopneumonia on Guafo Island, Chile, South America [44]. To our knowledge, nothing is known about virulence factors and pathogenicity of S. marimammalium.
10. Streptococcus Mitis
S. mitis is mainly known as member of the human oral cavity [88][89] and as opportunistic pathogen causing endocarditis and bloodstream infections in neutropenic and immunocompromised patients [90][91][92]. It is closely related to the human pathogen S. pneumoniae and its genome contain virulence genes involved in colonization and adherence, which might also be important for commensals to interact with host cells [93]. However, genes for hyaluronidase and capsular genes were absent.
S. mitis was isolated in 1985 from a blowhole swab of a captive, healthy white whale (Delphinapterus leucas) 139 days after captivity at the Mystic Marinelife Aquarium Connecticut, USA [94]. In 1985, it was isolated from lesions of a grey seal with peritonitis in North Rona, Scotland [4] and between 2012–2014 from clinically normal eyes of two grey seals stranded on the British coast [9]. These findings suggest that S. mitis might also be a commensal in some marine mammals. The commensalism and pathogenesis of S. mitis is reviewed by Mitchel, 2011 [95].
11. Streptococcus Phocae
S. phocae was first isolated and identified from lung, liver, spleen, and kidney samples of harbor seals suffering from pneumonia with areas of consolidation, purulent exudate in the bronchi, interlobular edema, and emphysema during a phocine distemper virus outbreak in northwestern Europe [34]. Later, S. phocae was also isolated from diseased Atlantic salmon[96][97], stranded southern sea otters [98], and as gut commensal of Indian white shrimp [99]. Two subspecies are described, S. phocae subsp. salmonis for isolates from Atlantic salmon and S. phocae subsp. phocae for isolates from seals [100].
In August 1994, beta-hemolytic streptococci with high similarity to S. phocae were isolated from spleen, liver, and kidney of Cape fur seals at Cape Cross, Namibia that suffered from respiratory infections and abortions [45]. A total of 69 S. phocae isolates were obtained from harbor and grey seals from the North and the Baltic Sea investigated between 1995 and 2000 [54]. A study on phocid seals (harbor and grey seals) that were older than 19 months from the North Sea of Schleswig-Holstein, Germany reported two S. phocae isolates from intestines of phocid seals with intestinal displacements [101]. During diagnostic evaluation by the Animal Health Center, Abbotsford, British Columbia, Canada S. phocae was isolated from harbor seals with an increase of prevalence since 2000, ringed seal (P. hispida) pups from arctic Canada and two stranded harbor porpoises from Washington State [102]. In spring and summer 2000, more than 10,000 Caspian seals (Pusa caspica) were found dead with canine distemper virus infection as primary diagnosis [103]. The investigated animals suffered from broncho-interstitial pneumonia, lymphocytic necrosis and depletion in lymphoid organs, and the presence of typical intracytoplasmic inclusion bodies in multiple epithelia. S. phocae was isolated from three of eight animals. Between 2001 and 2003, vaginal and preputial swabs of California Sea Lions were collected for investigations of genital bacterial infections and urogenital carcinoma [104]. S. phocae was isolated from three specimen of cancer and three specimens of non-cancer animals stranded along the central and northern California coast. In November 2007, a short-beaked common dolphin (Delphinus delphis) stranded at La Graciosa, Canary Islands [105]. Diagnostic evaluation revealed bacterial septicemia, fibrino-necrotizing to pyogranulomatous dermatitis and panniculitis, embolic pneumonia, neutrophilic and lymphoplasmacytic meningo-choroiditis, random neutrophilic hepatitis, lymphoplasmacytic myocarditis and epicarditis, necrotizing adrenalitis, suppurative endometritis, and multicentric reactive lymphadenopathy cutaneous purulent nodules in the tail fluke, vegetative mitral valve endocarditis, and presumed postpartum pyometra. S. phocae could be cultured from lung, brain, and adrenal gland tissue. Morbillivirus was detected in the epithelium of the choroid plexus of the fourth ventricle. In November 2009, a female spotted seal (Phoca largha) stranded at Kotzebue Sound, Alaska and was diagnosed with pyometra [106]. S. phocae was isolated from the purulent discharge in uterine contents. Three Navy bottlenose dolphins (T. truncatus) developed in the time between 2009 and 2010 a strangles-like syndrome associated with S. phocae, which was isolated after the animals showed clinical signs such as inflammatory hemogram, neutrophilic leukocytosis, and unilateral cervical lymphadenopathy [107]. Between 2004 to 2010 S. phocae could be isolated from five harbor seal pups of the Smith Island in Washington, USA that were also tested positive for phocine herpes virus [26]. S. phocae was also isolated from five cases of bacterial septicemia of white whales stranded in St. Lawrence Estuary between 1983 to 2012 [108]. Necropsy of a total of 241 harbor porpoises stranded at the eastern Pacific and western Atlantic coasts of Canada between 1988 to 2011 revealed bacterial septicemia with S. phocae isolation [109]. In winter 2012, an adult female Stellar sea lion stranded in South Korea and S. phocae was cultured from the liver [68]. The cause of death was unknown. During 85 postmortem investigations of marine mammals of the northeastern Pacific and arctic Canada stranded between 2007–2012 resulted in S. phocae isolates from harbor seals (n = 61), ringed seals (n = 5), harbor porpoises (n = 5), California sea lion (n = 7), Stellar sea lion (n = 3), Guadalupe fur seal (Arctocephalus twonsendii, n = 1) and elephant seal (Mirounga angustirostris, n = 1) [110]. Sequencing of 16S rRNA V4 hyper variable regions of blow samples collected from four wild healthy Indo-Pacific bottlenose dolphins (T. aduncus) in Shark Bay (SB), Western Australia, in 2012 identified S. phocae (and S. equi) [66]. In February 2014, S. phocae was isolated from a carcass of a subadult male northern seal at the coast of Niigata, Japan that suffered from necrotizing and fibrinous pneumonia with diffuse abscesses of all lung lobes and massive necrosis of kidney and liver [47]. Between 2010 to 2017 the health of captive and stranded Alaskan ice seals were investigated and S. phocae isolates were obtained from blood, abscess, and lymph node samples from ringed seals [111]. Harbor seals stranded at the coast of San Juan County, Washington, USA between 2002 to 2018 were examined and from one adult female animal a fatal septicemia caused by S. phocae was reported [112].
While the presence of an antiphagocytic capsule and virulence of S. phocae subsp. salmonis to Atlantic salmon has been demonstrated in infectivity experiments [96][113][114], whole genome analyses of S. phocae subsp. phocae identified typical streptococcal virulence factors such as fibronectin-binding proteins, the toxin streptolysin S and genes encoding for a capsule [115]. Invasion of fish and mammalian cell lines by S. phocae subsp. phocae has also been shown and confirmed its pathogenic potential [113].
However, S. phocae subsp. phocae also seems to be a commensal of the oral cavity of grey seals as revealed by microbiome analyses and 16S rRNA sequencing. A transmission of S. phocae to harbor porpoises via bites is also indicated [116] and S. phocae might be an opportunistic pathogen, at least for seals.
12. Streptococcus Viridans Group
In very few studies, streptococci isolated from marine mammals were identified as members of the S. viridans group (viridans streptococci), which includes streptococci that are usually alpha-hemolytic and inhabit the oral cavity, intestinal, and vaginal tract [117][118][119]. This group is very heterogeneous and includes species such as S. anginosus, S. mitis, S. sanguinis, S. mutans, and S. salivarius, which can also cause endocarditis [120], bacteremia [121], and respiratory infections [122].
Viridans streptococci were isolated from superficial abscesses, wounds, ocular and urethral discharges, and umbilici of live and from lung and liver samples of dead elephant seals, California sea lions and harbor seals stranded between January 1994 and December 1995 along the California Coast [123]. Viridans streptococci were isolated in mixed cultures with Arcanobacterium phocae from California sea lions, harbor seals, Northern elephant seals, sea otter and common dolphin stranded along the central California coast between 1994 and 2000 [124]. In Beluga whales that stranded at Cook Inlet (Alaska, USA) between 1998 and 2013 an isolate was identified as member of the S. viridans group [125]. Also, viridans streptococci were isolated from gastric fluid samples of free-ranging bottlenose dolphins from the southeastern United States during a catch and release health assessment between 2003 to 2005 [126].
13. One-Time only Detections of Streptococcal Species from Marine Mammals
In studies described above, streptococcal species have been isolated and identified at least twice or more. In the following, reports on one-time only descriptions of streptococcal species are summarized.
S. uberis was found in dead free-ranging male Antarctic fur seals with pneumonia and extensive polymorphonuclear infiltrations and necrosis or very widespread abscess formation and frequently there was an associated fibrinous exudative pleurisy [5]. S. oralis was isolated and identified by API strips from three swabs taken from healthy eyes of free-ranging grey seals stranded on the British coast between November 2012 and February 2014 [9]. In a metagenome dataset of blood, muscle, and fecal samples of a living stranded sperm whale (Physeter catodon) S. anginosus, S. pneumoniae, and S. suis were detected in blood and fecal samples, but not in the muscles [127]. The animal died 79 h after rescue. S. intermedius was detected in blow samples of free-ranging and presumably healthy grey whales from Magdalena Bay and the Gulf of California by polymerase chain reaction [128].
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms9020350
References
- Anthony, B.F.; Okada, D.M. The emergence of group B streptococci in infections of the newborn infant. Annu. Rev. Med. 1977, 28, 355–369.
- Musser, J.M.; Mattingly, S.J.; Quentin, R.; Goudeau, A.; Selander, R.K. Identification of a high-virulence clone of type III Streptococcus agalactiae (group B Streptococcus) causing invasive neonatal disease. Proc. Natl. Acad. Sci. USA 1989, 86, 4731–4735, doi:10.1073/pnas.86.12.4731.
- Keefe, G.P. Streptococcus agalactiae mastitis: A review. Can. Vet. J. 1997, 38, 429–437.
- Baker, J.R. Further studies on grey seal (Halichoerus grypus) pup mortality on North Rona. Br. Vet. J. 1988, 144, 497–506, doi:10.1016/0007-1935(88)90090-5.
- Baker, J.R.; McCann, T.S. Pathology and bacteriology of adult male antarctic fur seals, Arctocephalus gazella, dying at Bird Island, South Georgia. Br. Vet. J. 1989, 145, 263–275, doi:10.1016/0007-1935(89)90079-1.
- Zappulli, V.; Mazzariol, S.; Cavicchioli, L.; Petterino, C.; Bargelloni, L.; Castagnaro, M. Fatal necrotizing fasciitis and myositis in a captive common bottlenose dolphin (Tursiops truncatus) associated with Streptococcus agalactiae. J. Vet. Diagn. Investig. 2005, 17, 617–622, doi:10.1177/104063870501700620.
- Evans, J.J.; Pasnik, D.J.; Klesius, P.H.; Al-Ablani, S. First report of Streptococcus agalactiae and Lactococcus garvieae from a wild bottlenose dolphin (Tursiops truncatus). J. Wildl. Dis. 2006, 42, 561–569, doi:10.7589/0090-3558-42.3.561.
- Delannoy, C.M.; Crumlish, M.; Fontaine, M.C.; Pollock, J.; Foster, G.; Dagleish, M.P.; Turnbull, J.F.; Zadoks, R.N. Human Streptococcus agalactiae strains in aquatic mammals and fish. BMC Microbiol. 2013, 13, 41, doi:10.1186/1471-2180-13-41.
- Fleming, M.; Bexton, S. Conjunctival flora of healthy and diseased eyes of grey seals (Halichoerus grypus): Implications for treatment. Vet. Rec. 2016, 179, 99.
- Kissel, L.N.; Bankowski, M.J.; Koyamatsu, T.L.; Nagai, R.Y.; Seifried, S.E.; Crow, G.L. Aerobic microorganisms identified over a fourteen-month period from the upper respiratory tract of captive Hawaiian monk seals (Monachus schauinslandi). Aquat. Mamm. 2011, 37, 377.
- Evans, J.J.; Klesius, P.H.; Gilbert, P.M.; Shoemaker, C.A.; Sarawi, M.A.A.; Landsberg, J.; Duremdez, R.; Marzouk, A.A.; Zen-ki, S.A. Characterization of β-haemolytic Group B Streptococcus agalactiae in cultured seabream, Sparus auratus L., and wild mullet, Liza klunzingeri (Day), in Kuwait. J. Fish Dis. 2002, 25, 505–513, doi:10.1046/j.1365-2761.2002.00392.x.
- Mian, G.F.; Godoy, D.T.; Leal, C.A.G.; Yuhara, T.Y.; Costa, G.M.; Figueiredo, H.C.P. Aspects of the natural history and viru-lence of S. agalactiae infection in Nile tilapia. Vet. Microbiol. 2009, 136, 180–183, doi:10.1016/j.vetmic.2008.10.016.
- Duremdez, R.; Al-Marzouk, A.; Qasem, J.A.; Al-Harbi, A.; Gharabally, H. Isolation of Streptococcus agalactiae from cultured silver pomfret, Pampus argenteus (Euphrasen), in Kuwait. J. Fish Dis. 2004, 27, 307–310.
- Garcia, J.C.; Klesius, P.H.; Evans, J.J.; Shoemaker, C.A. Non-infectivity of cattle Streptococcus agalactiae in Nile tilapia, Oreo-chromis niloticus and channel catfish, Ictalurus punctatus. Aquaculture 2008, 281, 151–154, doi:10.1016/j.aquaculture.2008.05.028.
- Pereira, U.P.; Mian, G.F.; Oliveira, I.C.M.; Benchetrit, L.C.; Costa, G.M.; Figueiredo, H.C.P. Genotyping of Streptococcus aga-lactiae strains isolated from fish, human and cattle and their virulence potential in Nile tilapia. Vet. Microbiol. 2010, 140, 186–192, doi:10.1016/j.vetmic.2009.07.025.
- Kannika, K.; Pisuttharachai, D.; Srisapoome, P.; Wongtavatchai, J.; Kondo, H.; Hirono, I.; Unajak, S.; Areechon, N. Molecu-lar serotyping, virulence gene profiling and pathogenicity of Streptococcus agalactiae isolated from tilapia farms in Thailand by multiplex PCR. J. Appl. Microbiol. 2017, 122, 1497–1507, doi:10.1111/jam.13447.
- Delannoy, C.M.J.; Zadoks, R.N.; Crumlish, M.; Rodgers, D.; Lainson, F.A.; Ferguson, H.W.; Turnbull, J.; Fontaine, M.C. Ge-nomic comparison of virulent and non-virulent Streptococcus agalactiae in fish. J. Fish Dis. 2016, 39, 13–29, doi:10.1111/jfd.12319.
- Higginbottom, C.; Wheater, D.W.F. The incidence of Streptococcus bovis in cattle. J. Agric. Sci. 1954, 44, 434–442, doi:10.1017/S0021859600045299.
- Greetham, H.L.; Giffard, C.; Hutson, R.A.; Collins, M.D.; Gibson, G.R. Bacteriology of the Labrador dog gut: A cultural and genotypic approach. J. Appl. Microbiol. 2002, 93, 640–646, doi:10.1046/j.1365-2672.2002.01724.x.
- Moellering, R.C.; Watson, B.K.; Kunz, L.J. Endocarditis due to group D streptococci: Comparison of disease caused by Strep-tococcus bovis with that produced by the enterococci. Am. J. Med. 1974, 57, 239–250, doi:10.1016/0002-9343(74)90448-3.
- Fikar, C.R.; Levy, J. Streptococcus bovis meningitis in a neonate. Am. J. Dis. Child. 1979, 133, 1149–1150, doi:10.1001/archpedi.1979.02130110057009.
- García‐Porrúa, C.; González‐Gay, M.A.; Monterroso, J.R.; Sánchez‐Andrade, A.; González‐Ramirez, A. Septic arthritis due to Streptococcus bovis as presenting sign of ‘silent’ colon carcinoma. Rheumatology 2000, 39, 338–339, doi:10.1093/rheumatology/39.3.338.
- Murray, H.W.; Roberts, R.B. Streptococcus bovis bacteremia and underlying gastrointestinal disease. Arch. Intern Med. 1978, 138, 1097–1099, doi:10.1001/archinte.1978.03630320037013.
- Vanrobaeys, M.; De Herdt, P.; Ducatelle, R.; Creten, W.; Haesebrouck, F. Extracellular proteins and virulence in Streptococcus bovis isolates from pigeons. Vet. Microbiol. 1997, 59, 59–66, doi:10.1016/S0378-1135(97)00174-0.
- Vanrobaeys, M.; De Herdt, P.; Haesebrouck, F.; Ducatelle, R.; Devriese, L.A. Secreted antigens as virulence associated mark-ers in Streptococcus bovis strains from pigeons. Vet. Microbiol. 1996, 53, 339–348, doi:10.1016/S0378-1135(96)01254-0.
- Huggins, J.L.; Leahy, C.L.; Calambokidis, J. Causes and patterns of harbor seal (Phoca vitulina) pup mortality at Smith Island, Washington, 2004–2010. Northwest Nat. 2013, 94, 198–208, doi:10.1898/12-14.1.
- Neves, H.C.; Pires, R. Recuperation of a Mediterranean monk seal pup, Monachus monachus, in Desertas Islands, Madeira archipelago: Conditions for its success. In Arquipelago.Life and Marine Sciences; Ponta Delgada, Portugal, 2001; Supplement 2 Part B, pp. 111–116, ISSN 0873-4704.
- Buck, J.D.; Wells, R.S.; Rhinehart, H.L.; Hansen, L.J. Aerobic microorganisms associated with free-ranging bottlenose dol-phins in coastal Gulf of Mexico and Atlantic Ocean waters. J. Wildl. Dis. 2006, 42, 536–544, doi:10.7589/0090-3558-42.3.536.
- Devriese, L.A.; Hommez, J.; Kilpper-Bälz, R.; Schleifer, K.-H. Streptococcus canis sp. nov.: A species of Group G streptococci from animals. Int. J. Syst. Evol. Microbiol. 1986, 36, 422–425, doi:10.1099/00207713-36-3-422.
- Galpérine, T.; Cazorla, C.; Blanchard, E.; Boineau, F.; Ragnaud, J.-M.; Neau, D. Streptococcus canis infections in humans: Ret-rospective study of 54 patients. J. Infect. 2007, 55, 23–26, doi:10.1016/j.jinf.2006.12.013.
- Bert, F.; Lambert-Zechovsky, N. Septicemia caused by streptococcus canis in a human. J. Clin. Microbiol. 1997, 35, 777–779.
- Lam, M.M.; Clarridge, J.E.; Young, E.J.; Mizuki, S. The other Group G Streptococcus: Increased detection of Streptococcus canis ulcer infections in dog owners. J. Clin. Microbiol. 2007, 45, 2327–2329, doi:10.1128/JCM.01765-06.
- Chalmers, G.; McLean, J.; Hunter, D.B.; Brash, M.; Slavic, D.; Pearl, D.L.; Boerlin, P. Staphylococcus spp., Streptococcus canis, and Arcanobacterium phocae of healthy Canadian farmed mink and mink with pododermatitis. Can. J. Vet. Res. 2015, 79, 129–135.
- Hariharan, H.; Matthew, V.; Fountain, J.; Snell, A.; Doherty, D.; King, B.; Shemer, E.; Oliveira, S.; Sharma, R.N. Aerobic bacteria from mucous membranes, ear canals, and skin wounds of feral cats in Grenada, and the antimicrobial drug suscepti-bility of major isolates. Comp. Immunol. Microbiol. Infect. Dis. 2011, 34, 129–134, doi:10.1016/j.cimid.2010.05.001.
- Timoney, J.F.; Velineni, S.; Ulrich, B.; Blanchard, P. Biotypes and ScM types of isolates of Streptococcus canis from diseased and healthy cats. Vet. Rec. 2017, 180, 358.
- DeWinter, L.M.; Low, D.E.; Prescott, J.F. Virulence of Streptococcus canis from canine streptococcal toxic shock syndrome and necrotizing fasciitis. Vet. Microbiol. 1999, 70, 95–110, doi:10.1016/S0378-1135(99)00128-5.
- Fulde, M.; Rohde, M.; Polok, A.; Preissner, K.T.; Chhatwal, G.S.; Bergmann, S. Cooperative plasminogen recruitment to the surface of Streptococcus canis via M protein and enolase enhances bacterial survival. mBio 2013, 4, doi:10.1128/mBio.00629-12.
- Eichhorn, I.; Linden, M.; van der Jarek, M.; Fulde, M. Draft genome sequence of zoonotic Streptococcus canis isolate G361. Genome Announc. 2017, 5, doi:10.1128/genomeA.00967-17.
- Yang, J.; Liu, Y.; Xu, J.; Li, B. Characterization of a new protective antigen of Streptococcus canis. Vet. Res. Commun. 2010, 34, 413–421.
- Bergmann, S.; Eichhorn, I.; Kohler, T.P.; Hammerschmidt, S.; Goldmann, O.; Rohde, M.; Fulde, M. SCM, the M protein of Streptococcus canis binds immunoglobulin G. Front. Cell Infect. Microbiol. 2017, 7, doi:10.3389/fcimb.2017.00080.
- Stroud, R.K.; Roffe, T.J. Causes of death in marine mammals stranded along the oregon coast. J. Wildl. Dis. 1979, 15, 91–97, doi:10.7589/0090-3558-15.1.91.
- Van Bonn, W.G.; Ridgway, S.H.; Williams, B.H. Chronic refractory emesis associated with a colonic lesion in a california sea lion (Zalophus californianus). J. Zoo Wildl. Med. 1995, 26, 286–292.
- Williams, D.L.; MacGregor, S.; Sainsbury, A.W. Evaluation of bacteria isolated from infected eyes of captive, non-domestic animals. Vet. Rec. 2000, 146, 515–518, doi:10.1136/vr.146.18.515.
- Seguel, M.; Gutiérrez, J.; Hernández, C.; Montalva, F.; Verdugo, C. Respiratory mites (Orthohalarachne diminuata) and β-hemolytic streptococci-associated bronchopneumonia outbreak in South American fur seal pups (Arctocephalus australis). J. Wildl. Dis. 2018, 54, 380–385, doi:10.7589/2017-09-214.
- Henton, M.M.; Zapke, O.; Basson, P.A. Streptococcus phocae infections associated with starvation in Cape fur seals: Case re-port. J. S. Afr. Vet. Assoc. 1999, 70, 98–99.
- Jepson, P.D.; Baker, J.R.; Kuiken, T.; Simpson, V.R.; Kennedy, S.; Bennett, P.M. Pulmonary pathology of harbour porpoises (Phocoena phocoena) stranded in England. Vet. Rec. 2000, 146, 721–728.
- Iwao, H.; Yanagisawa, M.; Kino, S.; Takamori, J.; Okamoto, M. Two beta-hemolytic streptococci Streptococcus canis and S. phocae isolated from a northern fur seal with septicemia from Niigata, Japan. In Proceedings of the 46th Annual Conference of the International Association for Aquatic Animal Medicine, Chicago, IL, USA, April 6-10, 2015. Available online: https://www.vin.com/doc/?id=7009572 (accessed on July 6. 2020).
- Leahy, C.L. Causes and Patterns of Harbor Seal (Phoca vitulina) Pup Mortality at Smith Island, Washington, 2004–2009. Ph.D. Thesis, Evergreen State College, Olympia, WA, USA, 2010.
- Vandamme, P.; Pot, B.; Falsen, E.; Kersters, K.; Devriese, L.A. Taxonomic study of Lancefield streptococcal Groups C, G, and L (Streptococcus dysgalactiae) and proposal of S. dysgalactiae subsp. equisimilis subsp. nov. Int. J. Syst. Evol. Microbiol. 1996, 46, 774–781, doi:10.1099/00207713-46-3-774.
- Hughes, J.M.; Wilson, M.E.; Brandt, C.M.; Spellerberg, B. Human infections due to Streptococcus dysgalactiae subspecies equi-similis. Clin. Infect. Dis. 2009, 49, 766–772, doi:10.1086/605085.
- Jensen, A.; Kilian, M. Delineation of Streptococcus dysgalactiae, its subspecies, and its clinical and phylogenetic relationship to Streptococcus pyogenes. J. Clin. Microbiol. 2012, 50, 113–126, doi:10.1128/JCM.05900-11.
- Baker, J.R.; Doidge, D.W. Pathology of the antarctic fur seal (Arctocephalus gazella) in South Georgia. Br. Vet. J. 1984, 140, 210–219, doi:10.1016/0007-1935(84)90084-8.
- Swenshon, M.; Lämmler, C.; Siebert, U. Identification and molecular characterization of beta-hemolytic streptococci isolated from harbor porpoises (Phocoena phocoena) of the North and Baltic Seas. J. Clin. Microbiol. 1998, 36, 1902–1906, doi:10.1128/JCM.36.7.1902-1906.1998.
- Vossen, A.; Abdulmawjood, A.; Lämmler, C.; Weiß, R.; Siebert, U. Identification and molecular characterization of beta-hemolytic streptococci isolated from harbor seals (Phoca vitulina) and grey seals (Halichoerus grypus) of the German North and Baltic Seas. J. Clin. Microbiol. 2004, 42, 469–473, doi:10.1128/JCM.42.1.469-473.2004.
- Bianchi, M.V.; Ehlers, L.P.; Vargas, T.P.; Lopes, B.C.; Taunde, P.A.; de Cecco, B.S.; Henker, L.C.; Vielmo, A.; Lorenzett, M.P.; Riboldi, C.I.; et al. Omphalitis, urachocystitis and septicemia by Streptococcus dysgalactiae in a southern right whale calf Eu-balaena australis, Brazil. Dis. Aquat. Organ. 2018, 131, 227–232, doi:10.3354/dao03293.
- Boyle, A.G.; Timoney, J.F.; Newton, J.R.; Hines, M.T.; Waller, A.S.; Buchanan, B.R. Streptococcus equi infections in horses: Guidelines for treatment, control, and prevention of strangles—Revised consensus statement. J. Vet. Intern Med. 2018, 32, 633–647, doi:10.1111/jvim.15043.
- Chalker, V.J.; Brooks, H.W.; Brownlie, J. The association of Streptococcus equi subsp. zooepidemicus with canine infectious res-piratory disease. Vet. Microbiol. 2003, 95, 149–156, doi:10.1016/S0378-1135(03)00155-X.
- Breiman, R.F.; Silverblatt, F.J. Systemic Streptococcus equi infection in a horse handler—A case of human strangles. West. J. Med. 1986, 145, 385–386.
- Minces, L.R.; Brown, P.J.; Veldkamp, P.J. Human meningitis from Streptococcus equi subsp. zooepidemicus acquired as zoono-ses. Epidemiol. Infect. 2011, 139, 406–410, doi:10.1017/S0950268810001184.
- Eyre, D.W.; S. Kenkre, J.; Bowler, I.C.J.W.; McBride, S.J. Streptococcus equi subspecies zooepidemicus meningitis—A case report and review of the literature. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 1459–1463, doi:10.1007/s10096-010-1037-5.
- Higgins, R.; Claveau, R.; Roy. R. Bronchopneumonia caused by Streptococcus equi in a North Atlantic pilot whale (Globicepha-la melaena). J. Wildl. Dis. 1980, 16, 319–321.
- Baker, J.R. The pathology of the grey seal (Haliochoerus grypus). II. Juveniles and adults. Br. Vet. J. 1980, 136, 443–447, doi:10.1016/S0007-1935(17)32185-1.
- Akineden, Ö.; Hassan, A.A.; Alber, J.; El-Sayed, A.; Estoepangestie, A.T.S.; Lämmler, C.; Weiss, R.; Siebert, U. Phenotypic and genotypic properties of Streptococcus equi subsp. zooepidemicus isolated from harbor seals (Phoca vitulina) from the German North Sea during the phocine distemper outbreak in 2002. Vet. Microbiol. 2005, 110, 147–152, doi:10.1016/j.vetmic.2005.06.010.
- Akineden, Ö.; Alber, J.; Lämmler, C.; Weiss, R.; Siebert, U.; Foster, G.; Tougaard, S.; Brasseur, S.M.J.M.; Reijnders, P.J.H. Re-latedness of Streptococcus equi subsp. zooepidemicus strains isolated from harbour seals (Phoca vitulina) and grey seals (Halicho-erus grypus) of various origins of the North Sea during 1988–2005. Vet. Microbiol. 2007, 121, 158–162, doi:10.1016/j.vetmic.2006.11.015.
- Venn-Watson, S.; Daniels, R.; Smith, C. Thirty year retrospective evaluation of pneumonia in a bottlenose dolphin Tursiops truncatus population. Dis. Aquat. Organ. 2012, 99, 237–242, doi:10.3354/dao02471.
- Nelson, T.M.; Wallen, M.M.; Bunce, M.; Oskam, C.L.; Lima, N.; Clayton, L.; Mann, J. Detecting respiratory bacterial commu-nities of wild dolphins: Implications for animal health. Mar. Ecol. Prog. Ser. 2019, 622, 203–217, doi:10.3354/meps13055.
- Lawson, P.A.; Foster, G.; Falsen, E.; Davison, N.; Collins, M.D. Streptococcus halichoeri sp. nov., isolated from grey seals (Hali-choerus grypus). Int. J. Syst. Evol. Microbiol. 2004, 54, 1753–1756, doi:10.1099/ijs.0.63082-0.
- Lee, K.; Kim, J.-Y.; Jung, S.C.; Lee, H.-S.; Her, M.; Chae, C. First isolation of Streptococcus halichoeri and Streptococcus phocae from a steller sea lion (Eumetopias jubatus) in South Korea. J. Wildl. Dis. 2015, 52, 183–185, doi:10.7589/2015-05-112.
- Foo, R.M.; Chan, D. A Fishy Tale: A man with empyema caused by Streptococcus halichoeri. J. Clin. Microbiol. 2014, 52, 681–682, doi:10.1128/JCM.03055-13.
- Giudice, P.D.; Plainvert, C.; Hubiche, T.; Tazi, A.; Fribourg, A.; Poyart, C. Infectious cellulitis caused by Streptococcus hali-choeri. Acta Derm. Venereol. 2018, 98, 378–379, doi:10.2340/00015555-2837.
- Shewmaker, P.L.; Whitney, A.M.; Humrighouse, B.W. Phenotypic, genotypic, and antimicrobial characteristics of Streptococ-cus halichoeri isolates from humans, proposal to rename Streptococcus halichoeri as Streptococcus halichoeri subsp. halichoeri, and description of Streptococcus halichoeri subsp. hominis subsp. nov., a bacterium associated with human clinical infections. J. Clin. Microbiol. 2016, 54, 739–744, doi:10.1128/JCM.03214-15.
- Aaltonen, K.; Kant, R.; Eklund, M.; Raunio-Saarnisto, M.; Paulin, L.; Vapalahti, O.; Grönthal, T.; Rantala, M.; Sironen, T. Streptococcus halichoeri: Comparative genomics of an emerging pathogen. Int. J. Genomics 2020, doi:10.1155/2020/8708305.
- Eklund, M.; Aaltonen, K.; Sironen, T.; Raunio-Saarnisto, M.; Grönthal, T.; Nordgren, H.; Pitkälä, A.; Vapalahti, O.; Rantala, M. Comparison of Streptococcus halichoeri isolates from canine and fur animal infections: Biochemical patterns, molecular characteristics and genetic relatedness. Acta Vet. Scand. 2020, 62, 26, doi:10.1186/s13028-020-00525-3.
- Pier, G.B.; Madin, S.H. Streptococcus iniae sp. nov., a beta-hemolytic Streptococcus isolated from an Amazon freshwater dol-phin, Inia geoffrensis. Int. J. Syst. Evol. Microbiol. 1976, 26, 545–553, doi:10.1099/00207713-26-4-545.
- Pier, G.B.; Madin, S.H.; Al-Nakeeb, S. Isolation and characterization of a second isolate of Streptococcus iniae. Int. J. Syst. Evol. Microbiol. 1978, 28, 311–314, doi:10.1099/00207713-28-2-311.
- Bonar, C.J.; Wagner, R.A. A Third report of “golf ball disease” in an amazon river dolphin (Inia geoffrensis) associated with Streptococcus iniae. J. Zoo Wildl. Med. 2003, 34, 296–301, doi:10.1638/1042-7260(2003)034[0296:ATROGB]2.0.CO;2.
- Song, Z.; Yue, R.; Sun, Y.; Liu, C.; Khan, S.H.; Li, C.; Zhao, Y.; Zhou, X.; Yang, L.; Zhao, D. Fatal bacterial septicemia in a bottlenose dolphin Tursiops truncatus caused by Streptococcus iniae. Dis. Aquat. Organ. 2017, 122, 195–203, doi:10.3354/dao03069.
- Weinstein, M.R.; Litt, M.; Kertesz, D.A.; Wyper, P.; Rose, D.; Coulter, M.; McGeer, A.; Facklam, R.; Ostach, C.; Willey, B.M.; et al. Invasive infections due to a fish pathogen, Streptococcus iniae. N. Engl. J. Med. 1997, 337, 589–594, doi:10.1056/NEJM199708283370902.
- Rahmatullah, M.; Ariff, M.; Kahieshesfandiari, M.; Daud, H.M.; Zamri-Saad, M.; Sabri, M.Y.; Amal, M.N.A.; Ina-Salwany, M.Y. Isolation and pathogenicity of Streptococcus iniae in cultured red hybrid tilapia in Malaysia. J. Aquat. Anim. Health 2017, 29, 208–213, doi:10.1080/08997659.2017.1360411.
- Locke, J.B.; Colvin, K.M.; Datta, A.K.; Patel, S.K.; Naidu, N.N.; Neely, M.N.; Nizet, V.; Buchanan, J.T. Streptococcus iniae capsule impairs phagocytic clearance and contributes to virulence in fish. J. Bacteriol. 2007, 189, 1279–1287, doi:10.1128/JB.01175-06.
- Locke, J.B.; Colvin, K.M.; Varki, N.; Vicknair, M.R.; Nizet, V.; Buchanan, J.T. Streptococcus iniae β-hemolysin streptolysin S is a virulence factor in fish infection. Dis. Aquat. Organ. 2007, 76, 17–26, doi:10.3354/dao076017.
- Soh, K.Y.; Loh, J.M.S.; Hall, C.; Proft, T. Functional analysis of two novel Streptococcus iniae virulence factors using a zebrafish infection model. Microorganisms 2020, 8, 1361, doi:10.3390/microorganisms8091361.
- Milani, C.J.E.; Aziz, R.K.; Locke, J.B.; Dahesh, S.; Nizet, V.; Buchanan, J.T. The novel polysaccharide deacetylase homologue Pdi contributes to virulence of the aquatic pathogen Streptococcus iniae. Microbiology 2010, 156, 543–554, doi:10.1099/mic.0.028365-0.
- Locke, J.B.; Aziz, R.K.; Vicknair, M.R.; Nizet, V.; Buchanan, J.T. Streptococcus iniae M-like protein contributes to virulence in fish and is a target for live attenuated vaccine development. PLoS ONE 2008, 3, e2824, doi:10.1371/journal.pone.0002824.
- Liu, T.; Wang, Y.; Wang, K. Comparative genome analysis of Streptococcus iniae DX09 reveals new insights into niche adapta-tion and competitive host colonisation ability. Oncotarget 2017, 5, doi:10.18632/oncotarget.23198.
- Silayeva, O.; Engelstädter, J.; Barnes, A.C. Evolutionary epidemiology of Streptococcus iniae: Linking mutation rate dynamics with adaptation to novel immunological landscapes. bioRxiv 2020, 355412, doi:10.1101/355412.
- Lawson, P.A.; Foster, G.; Falsen, E.; Collins, M.D. Streptococcus marimammalium sp. nov., isolated from seals. Int. J. Syst. Evol. Microbiol. 2005, 55, 271–274, doi:10.1099/ijs.0.63342-0.
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the normal bacterial flora of the oral cavity. J. Clin. Mi-crobiol. 2005, 43, 5721–5732, doi:10.1128/JCM.43.11.5721-5732.2005.
- Smith, D.J.; Anderson, J.M.; King, W.F.; Houte, J. van, Taubman, M.A. Oral streptococcal colonization of infants. Oral Micro-biol. Immunol. 1993, 8, 1–4, doi:10.1111/j.1399-302X.1993.tb00535.x.
- Matsui, N.; Ito, M.; Kuramae, H.; Inukai, T.; Sakai, A.; Okugawa, M. Infective endocarditis caused by multidrug-resistant Streptococcus mitis in a combined immunocompromised patient: An autopsy case report. J. Infect. Chemother. 2013, 19, 321–325.
- Husain, E.; Whitehead, S.; Castell, A.; Thomas, E.E.; Speert, D.P. Viridans streptococci bacteremia in children with malignan-cy: Relevance of species identification and penicillin susceptibility. Pediatr. Infect. Dis. J. 2005, 24, 563–566, doi:10.1097/01.inf.0000164708.21464.03.
- Marron, A.; Carratalà, J.; González-Barca, E.; Fernández-Sevilla, A.; Alcaide, F.; Gudiol, F. Serious complications of bactere-mia caused by viridans streptococci in neutropenic patients with cancer. Clin. Infect. Dis. 2000, 31, 1126–1130, doi:10.1086/317460.
- Denapaite, D.; Brückner, R.; Nuhn, M.; Reichmann, P.; Henrich, B.; Maurer, P.; Schähle, Y.; Selbmann, P.; Zimmermann, W.; Wambutt, R.; et al. The genome of Streptococcus mitis B6—What is a commensal? PLoS ONE 2010, 5, e9426, doi:10.1371/journal.pone.0009426.
- Buck, J.D.; Shepard, L.L.; Bubucis, P.M.; Spotte, S.; McClave, K.; Cook, R.A. Microbiological characteristics of white whale (Delphinapterus leucas) from capture through extended captivity. Can. J. Fish Aquat. Sci. 1989, 46, 1914–1921, doi:10.1139/f89-241.
- Mitchell, J. Streptococcus mitis: Walking the line between commensalism and pathogenesis. Mol. Oral Microbiol. 2011, 26, 89–98, doi:10.1111/j.2041-1014.2010.00601.x.
- Romalde, J.L.; Ravelo, C.; Valdés, I.; Magariños, B.; de la Fuente, E.; Martín, C.S.; Avendaño-Herrera, R.; Toranzo, A.E. Strep-tococcus phocae, an emerging pathogen for salmonid culture. Vet. Microbiol. 2008, 130, 198–207, doi:10.1016/j.vetmic.2007.12.021.
- Gibello, A.; Mata, A.I.; Blanco, M.M.; Casamayor, A.; Domínguez, L.; Fernández-Garayzabal, J.F. First identification of Strep-tococcus phocae isolated from Atlantic salmon (Salmo salar). J. Clin. Microbiol. 2005, 43, 526–527, doi:10.1128/JCM.43.1.526-527.2005.
- Imai, D.; Jang, S.; Miller, M.; Conrad, P.A. Characterization of beta-hemolytic streptococci isolated from southern sea otters (Enhydra lutris nereis) stranded along the California coast. Vet. Microbiol. 2009, 136, 378–381, doi:10.1016/j.vetmic.2008.11.009.
- Satish, R.K.; Arul, V. Purification and characterization of phocaecin PI80: An anti-listerial bacteriocin produced by Strepto-coccus phocae PI80 Isolated from the gut of Peneaus indicus (Indian white shrimp). J. Microbiol. Biotechnol. 2009, 19, 1393–1400.
- Avendaño-Herrera, R.; Balboa, S.; Castro, N.; González-Contreras, A.; Magariños, B.; Fernández, J.; Toranzo, A.E.; Romalde, J.L. Comparative polyphasic characterization of Streptococcus phocae strains with different host origin and description of the subspecies Streptococcus phocae subsp. salmonis subsp. nov. Int. J. Syst. Evol. Microbiol. 2014, 64, 1775–1781, doi:10.1099/ijs.0.056978-0.
- Ludes-Wehrmeister, E.; Wohlsein, P.; Prenger-Berninghoff, E.; Ewers, C.; Woelfing, B.; Lehnert, K.; Siebert, U. Intestinal dis-placements in older harbour and grey seals. Dis. Aquat. Organ. 2020, 138, 215–225, doi:10.3354/dao03455.
- Raverty, S.A.; Gaydos, J.K.; Nielsen, O.; Ross, P.S. Pathologic and clinical implications of Streptococcus phocae isolated from pinnipeds along coastal Washington State, British Columbia and Arctic Canada. In Proceedings of the 35th Annual Confer-ence of the International Association of Aquatic Animal Medicine, Galveston, TX, USA, April 4-9, 2004. Available online: https://www.vin.com/doc/?id=6696240 (accessed on July 6, 2020).
- Kuiken, T.; Kennedy, S.; Barrett, T.; Van de Bildt, M.W.G.; Borgsteede, F.H.; Brew, S.D.; Codd, G.A.; Duck, C.; Deaville, R.; Eybatov, T.; et al. The 2000 canine distemper epidemic in caspian seals (Phoca caspica): Pathology and analysis of contributory factors. Vet. Pathol. 2006, 43, 321–338, doi:10.1354/vp.43-3-321.
- Johnson, S.; Lowenstine, L.; Gulland, F.; Jang, S.; Imai, D.; Almy, F.; DeLong, R.; Gardner, I. Aerobic bacterial flora of the vagina and prepuce of California sea lions (Zalophus californianus) and investigation of associations with urogenital carcino-ma. Vet. Microbiol. 2006, 114, 94–103, doi:10.1016/j.vetmic.2005.11.045.
- Díaz-Delgado, J.; Sierra, E.; Vela, A.I.; Arbelo, M.; Zucca, D.; Groch, K.R.; Fernández, A. Coinfection by Streptococcus phocae and Cetacean morbillivirus in a short-beaked common dolphin Delphinus delphis. Dis. Aquat. Organ. 2017, 124, 247–252, doi:10.3354/dao03124.
- Hueffer, K.; Lieske, C.L.; McGilvary, L.M.; Hare, R.F.; Miller, D.L.; O’Hara, T.M. Streptococcus phocae isolated from a spotted seal (Phoca largha) with pyometra in Alaska. J. Zoo Wildl. Med. 2011, 42, 108–112, doi:10.1638/2010-0064.1.
- Ruiz, C.L.; Jensen, E.D.; Johnson, S.P.; Lutmerding, B.A.; Smith, C.R.; Meegan, J.M.; Emory-Gomez, F.M.; Venn-Watson, S. Strangles-like clinical presentation of Streptococcus phocae in three bottlenose dolphins (Tursiops truncatus). In Proceedings of the 42nd Annual Conference of the International Association for Aquatic Animal Medicine, Las Vegas, NV, USA: May 7-11, 2011. Available online: https://www.vin.com/doc/?id=6698522 (accessed on January 11, 2021).
- Lair, S.; Martineau, D.; Measures, L.N. Causes of Mortality in St. Lawrence Estuary Beluga (Delphinapterus leucas), from 1983 to 2012; Canadian Science Advisory Secretariat: Ottawa, ON, Canada, 2014.
- Fenton, H.; Daoust, P.-Y.; Forzán, M.J.; Vanderstichel, R.V.; Ford, J.K.B.; Spaven, L.; Lair, S.; Raverty, S. Causes of mortality of harbor porpoises Phocoena phocoena along the Atlantic and Pacific coasts of Canada. Dis. Aquat. Organ. 2017, 122, 171–183, doi:10.3354/dao03080.
- Taurisano, N.D.; Butler, B.P.; Stone, D.; Hariharan, H.; Fields, P.J.; Ferguson, H.W.; Haulena, M.; Cotrell, P.; Nielsen, O.; Raverty, S. Streptococcus phocae in marine mammals of Northeastern Pacific and Arctic Canada: A retrospective analysis of 85 postmortem investigations. J. Wildl. Dis. 2017, 54, 101–111, doi:10.7589/2016-09-208.
- Goertz, C.E.C.; Reichmuth, C.; Thometz, N.M.; Ziel, H.; Boveng, P. Comparative health assessments of Alaskan ice seals. Front. Vet. Sci. 2019, 6, doi:10.3389/fvets.2019.00004.
- Ashley, E.A.; Olson, J.K.; Adler, T.E.; Raverty, S.; Anderson, E.M.; Jeffries, S.; Gaydos, J.K. Causes of mortality in a harbor seal (Phoca vitulina) population at equilibrium. Front. Mar. Sci. 2020, 7, doi:10.3389/fmars.2020.00319.
- Martin, M.C.-S.; González‐Contreras, A.; Avendaño‐Herrera, R. Infectivity study of Streptococcus phocae to seven fish and mammalian cell lines by confocal microscopy. J. Fish Dis. 2018, 35, 431–436, doi:10.1111/j.1365-2761.2012.01361.x.
- Salazar, S.; Oliver, C.; Yáñez, A.J.; Avendaño-Herrera, R. Comparative analysis of innate immune responses to Streptococcus phocae strains in Atlantic salmon (Salmo salar) and rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2016, 51, 97–103, doi:10.1016/j.fsi.2016.02.005.
- Avendaño-Herrera, R.; Poblete-Morales, M. Genome sequence of Streptococcus phocae subsp. phocae Strain ATCC 51973T isolated from a harbor seal (Phoca vitulina). Genome Announc. 2015, 3, doi:10.1128/genomeA.01307-15.
- Gilbert, M.J.; IJsseldijk, L.L.; Rubio-García, A.; Gröne, A.; Duim, B.; Rossen, J.; Zomer, A.L.; Wagenaar, J.A. After the bite: Bacterial transmission from grey seals (Halichoerus grypus) to harbour porpoises (Phocoena phocoena). R. Soc. Open Sci. 2020, doi:10.1098/rsos.192079.
- Coykendall, A.L. Classification and identification of the viridans streptococci. Clin. Microbiol. Rev. 1989, 2, 315–328, doi:10.1128/CMR.2.3.315.
- Facklam, R.R. Physiological differentiation of viridans streptococci. J. Clin. Microbiol. 1977, 5, 184–201.
- Beighton, D.; Hardie, J.M.; Whiley, R.A. A scheme for the identification of viridans streptococci. J. Med. Microbiol. 1991, 35, 367–372, doi:10.1099/00222615-35-6-367.
- Douglas, C.W.I.; Heath, J.; Hampton, K.K.; Preston, F.E. Identity of viridans streptococci isolated from cases of infective en-docarditis. J. Med. Microbiol. 1993, 39, 179–182, doi:10.1099/00222615-39-3-179.
- Salavert, M.; Gomez, L.; Rodriguez-Carballeira, M.; Xercavins, M.; Freixas, N.; Garau, J. Seven-year review of bacteremia caused by Streptococcus milleri and other viridans streptococci. Eur. J. Clin. Microbiol. Infect. Dis. 1996, 15, 365–371, doi:10.1007/BF01690091.
- Noguchi, S.; Yatera, K.; Kawanami, T.; Yamasaki, K.; Naito, K.; Akata, K.; Shimabukuro, I.; Ishimoto, H.; Yoshii, C.; Mukae, H. The clinical features of respiratory infections caused by the Streptococcus anginosus group. BMC Pulm. Med. 2015, 15, 133, doi:10.1186/s12890-015-0128-6.
- Thornton, S.M.; Nolan, S.; Gulland, F.M.D. Bacterial isolates from california sea lions (Zalophus californianus), harbor seals (Phoca vitulina), and northern elephant seals (Mirounga angustirostris) admitted to a rehabilitation center along the Central California Coast, 1994–1995. J. Zoo Wildl. Med. 1998, 29, 171–176.
- Johnson, S.P.; Jang, S.; Gulland, F.M.D.; Miller, M.A.; Casper, D.R.; Lawrence, J.; Herrera, J. Characterization and clinical manifestations of Arcanobacterium phocae infections in marine mammals stranded along the Central California Coast. J. Wildl. Dis. 2003, 39, 136–144, doi:10.7589/0090-3558-39.1.136.
- Burek-Huntington, K.A.; Dushane, J.L.; Goertz, C.E.C.; Measures, L.N.; Romero, C.H.; Raverty, S.A. Morbidity and mortality in stranded Cook Inlet beluga whales Delphinapterus leucas. Dis. Aquat. Organ. 2015, 114, 45–60, doi:10.3354/dao02839.
- Morris, P.J.; Johnson, W.R.; Pisani, J.; Bossart, G.D.; Adams, J.; Reif, J.S.; Fair, P.A. Isolation of culturable microorganisms from free-ranging bottlenose dolphins (Tursiops truncatus) from the southeastern United States. Vet. Microbiol. 2011, 148, 440–447, doi:10.1016/j.vetmic.2010.08.025.
- Li, C.; Tan, X.; Bai, J.; Xu, Q.; Liu, S.; Guo, W.; Yu, C.; Fan, G.; Lu, Y.; Zhang, H.; et al. A survey of the sperm whale (Physeter catodon) commensal microbiome. PeerJ 2019, 7, e7257, doi:10.7717/peerj.7257.
- Rocha Goselin, A. Caracterización de la Carga Microbiana y Parasitaria de tres Especies de Misticetos en las ostas de la Península de Baja California, México. 2009. Available online: http://www.repositoriodigital.ipn.mx//handle/123456789/13932 (accessed on 9 July 2020).