Non-alcoholic fatty liver disease (NAFLD) is the most frequent chronic liver disease in adults in developed countries, with a global prevalence as high as one billion. The pathogenesis of NAFLD is a multifactorial and multi-step process. Nowadays, a growing body of research suggests the considerable role of the endocannabinoid system (ECS) as a complex cell-signaling system in NAFLD development.
- endocannabinoid system
- endocannabinoid receptors
1. Introduction
The term “non-alcoholic fatty liver disease” (NAFLD) involves simple fat accumulation in the liver and may progress to steatohepatitis, fibrosis, cirrhosis, and, in some cases, hepatocellular carcinoma (HCC) [1]. Over the past decade, the prevalence of NAFLD (nearly 30% in the general adult population) is increasing worldwide with each passing year due to sedentary lifestyles and the unlimited availability of fat- and calorie-rich diets in modern western society [2]. The incidence of NAFLD increases with age, with a tendency to occur in men before 50 years of age and women after 50 years of age [3]. NAFLD is considered the most frequent liver disease in the world, the second most common cause of liver transplantation, and a primary cause of the development of hepatocellular carcinoma. Given these facts and the lack of effective treatment, NAFLD is a relevant problem for all health systems. Unsurprisingly, the pathogenesis of NAFLD is associated with fat deposition in the liver. Particularly, increased accumulation of triacylglycerols (TAG) is characteristic of NAFLD development. Steatosis occurs as a result of the imbalance between lipid storage (from accelerated free fatty acids (FFA) influx and de novo synthesis) and hepatic lipid clearance (decreased oxidation of FFA in the liver and decreased synthesis of low-density lipoproteins (VLDLs)). The complex and multifactorial process of NAFLD development was explained initially by the “two hits” model. The “first hit” included hepatic steatosis as a consequence of metabolic syndrome and excessive TAG deposition in hepatocytes. The “second hit” seemed to be necessary to develop non-alcoholic steatohepatitis (NASH) from NAFLD. However, this model was too simple to fully describe the complexity of NAFLD. In 2010, Tilg and Moschen proposed the “multiple hit” model, suggesting that different risk factors such as insulin resistance, adipocytes dysfunction, nutritional factors, gut microbiota, and genetic and epigenetic factors act simultaneously on both intrahepatic and extrahepatic pathways, which finally leads to steatosis or inflammation [4]. The previous model assumed that NAFLD always precedes inflammation. According to the “multiple hit” model, depending on which signaling pathways are activated by risk factors, hepatic lipid overload or NASH development may occur [5]. Currently, there are only a few specific pharmaceutical strategies available to treat NAFLD. However, none of them is ideal. Many of the promising results from rodent studies on phytocannabinoids and the endocannabinoid system (ECS) have fueled hopes of implementing novel therapeutic approaches and targets in humans. In our review, we aim to discuss the latest reports describing the changes in the ECS and its components on the development and progression of NAFLD. Furthermore, we will summarize the clinical studies analyzing the effects of natural cannabinoids in NAFLD treatment.
2. Phytocannabinoids
The Cannabis sativa plant is rich in a broad spectrum of phytochemicals including cannabinoids, terpenoids, sterols, and flavonoids. Phytocannabinoids (natural cannabinoids contained in the Cannabis plant) are considered to be the most active ingredients of marijuana that may be found in the human body of all the above mentioned. Most of the phytocannabinoids are found in female Cannabis inflorescence [6].
Among many phytocannabinoids found in Cannabis, the most studied agents are Δ9-tetrahydrocannabinol (THC), cannabidiol (CBD), and tetrahydrocannabivarin (THCV) [7].
There are plenty of Cannabis sativa varieties (cultivars) existing, and each one has an individual combination of bioactive compounds. In this case, especially important is the proportion of THC:CBD:THCV, which is the cause of the unique and different pharmacodynamic and medicinal properties of various Cannabis extracts [8]. Cannabis also contains a large number of acidic precursors of the aforementioned molecules, respectively: Δ9-tetrahydrocannabinolic acid (THCA), cannabidiolic acid (CBDA), and tetrahydrocannabivarinic acid (THCVA). These compounds may reveal interesting therapeutic properties, such as attenuation of body weight gain and amelioration of glucose-insulin homeostasis in a mouse model of HFD-induced obesity after administration of THCA [9]. However, the current knowledge in the field of phytocannabinoid acids is limited and requires further examination [10]. Therefore, the effect of medicinal Cannabis should be considered as the “entourage effect” of cannabis as a whole [6]. When analyzing the effects of the individual phytocannabinoids presented in our review, their complex pharmacology should be considered. The different effects on the response of several phytocannabinoids studied in vivo are possibly related to the competition and displacement of endogenous cannabinoids, with the different centers (orthosteric and allosteric) and with the biased signalling of cannabinoid target receptors [8]. Additionally, phytocannabinoids interact with each other. For instance, CBD has the ability to antagonize THC effects by CBR1 and non-CB1 receptor mechanisms of action. However, CBD may also potentiate some THC effects in an additive or synergistic fashion [11].
Promising research results regarding clinical application of cannabinoids in many morbidities and increasing acceptance of the clinical use of marijuana and its derivatives has led the pharmaceutical industry to research new compounds based on Cannabis [12]. This was accompanied by a growing awareness of the role of the endocannabinoid system in our body. Currently, THC and CBD are the two major active compounds of Cannabis. Table 1 provides a summary of the main opposing characteristics of these substances that account for the differential effects exerted on the human body.
| Feature | THC | CBD | Reference |
|---|---|---|---|
| Interaction with receptors: a) CB1R b) CB2R |
+ (partial agonist) - (weak antagonist) |
- (negative allosteric modulator) - (inverse agonist) |
[13][14][15] |
| Psychoactive effect | Yes | No | [16] |
| Appetite stimulation | Yes | No | [16] |
| Cardiovascular stimulation (inducing tachycardia and hypertension) | Yes | No | [16] |
| Anticonvulsant effect | Yes | No | [16] |
| Therapeutic indications approved by the FDA 1 | Anorexia associated with weight loss in AIDS 3 patients Nausea and vomiting associated with anticancer chemotherapy Multiple sclerosis spasticity |
Lennox-Gastaut syndrome Dravet syndrome |
[17][18][19][20] |
| Formulations available on US pharmaceutical market | Nabilone (trade name Cesamet) synthetic THC analog aviable as oral capsule Dronabinol (trade name Marinol)—synthetic formulation of the main THC constituent enantiomer found in Cannabis: [(−)-trans-Δ9-tetrahydrocannabinol] as an oily resin in capsules |
Epidiolex®—pharmaceutical formulation of CBD as an oral solution | [18][19][20] |
| Combination drugs available on US 2 pharmaceutical market | Nabiximol (trade name Sativex)—oral spray standardized in composition, formulation, and dose, delivering of 2.7 mg THC and 2.5 mg CBD per dose. | [21] | |
1 FDA, Food and Drug Administration; 2 US, United States; 3 AIDS, Acquired Immunodeficiency Syndrome.
3. Effects of Prolonged Cannabis Use in the Context of NAFLD and its Comorbidities
Progressive legalization of marijuana across the world allowed researchers to observe its interesting properties in encouraging or counteracting many metabolic and psychiatric diseases. Although Cannabis use is generally considered an unhealthy, addictive habit, there is growing strong evidence that it can be protective against the development of metabolic disturbances leading to hepatic steatosis and its progression.
This is a particularly interesting fact because dysregulation of the endocannabinoid system is undoubtedly one of the most important factors in the development of NAFLD. Moreover, Cannabis use is expected to increase over the coming years as a new therapeutic agent for many disorders.
On the other hand, it is well documented that chronic cannabis use (CCU) has been associated with metabolic disturbances ascertained as detrimental factors leading to NAFLD development. Firstly, CCU is undoubtedly linked with increased appetite and calorie overconsumption [22][23]. What is more, this appetite dysregulation is augmented by a higher intake of highly-palpable, unhealthy foods that are rich in refined sugar and fat [24][25][26]. On the other hand, a multitude of studies analyzing the metabolic effects of CCU observed plenty of beneficial effects that could counteract the development of NAFLD. It has been shown that prolonged Cannabis use is linked with decreased prevalence of insulin resistance [27][28] and hyperlipidemia and metabolic syndrome [29][30], as well as decreased frequency of diabetes mellitus occurrence [25]. In the aspect of CCU and obesity, an overwhelming majority of studies have shown a decreased prevalence of obesity among marijuana users. However, one study showed increased visceral adiposity in Cannabis smokers, but this study considered only 30 cannabis smokers [31][32][33]. Mounting evidence indicates that current or past marijuana use is associated with a lower risk of NAFLD development, regardless of the presence of metabolic risk factors [31][34][35][36]. At first glance, this association is contradictory to the role of ECS in NAFLD development. Although it is clearly proven that increased endocannabinoid tone in liver and brain relates to obesity and metabolic syndrome and contributes to the development of NAFLD, the complex effects and impact of phytocannabinoids in relation to NAFLD pathophysiology are not yet fully clear (Figure 1).
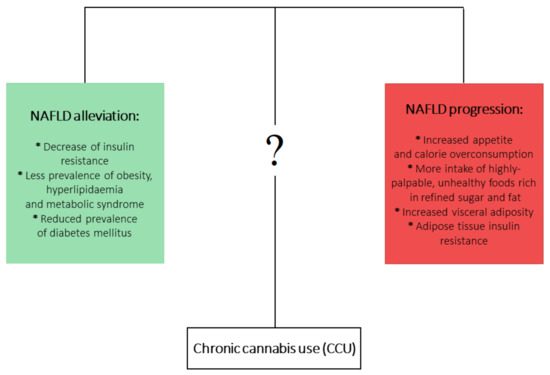
One of the possible mechanisms responsible for the positive influence of Cannabis use on the prevalence of NAFLD and other metabolic diseases may include the antagonistic action of CBD and THCV on CB1R [37][38]. Additionally, CBD has been described as acting as a negative allosteric modulator of CB1R in HEK 293A and in STHdhQ7/Q7 cells, two model systems that highly express CB1R [26]. Antagonism of CB1R improves the insulin sensitivity of hepatocytes [39], decreases intrahepatic triglyceride synthesis [40], and decreases secretion of very-low-density lipoprotein (VLDL) [41]. Diminished IR may contribute to improved hepatic steatosis, hepatomegaly, and metabolic syndrome by restoring the optimal hepatic glucose metabolism and decreasing liver fat accumulation [42].
Moreover, the anti-inflammatory effects of phytocannabinoids should be emphasized, as they can inhibit secretion of pro-inflammatory cytokines (TNF-a, IL-6) and adipokines (leptin) and lead to the upregulation of inflammatory mediators such as cell transcription factor (NF-kB) [43]. Excessive inflammatory response plays a robust role in NAFLD development and progression to NASH [44]. CBD has been shown to alleviate liver inflammation induced by a high fat-cholesterol (HFC) diet in mice by inhibition of NF-kB and can likewise restrain NLRP3 inflammasome activation, which both lead to a reduction in inflammatory response [45][46][47]. Another possible mechanism that is involved in the beneficial properties of Cannabis is development of tolerance and down-regulation of CB1R from repetitive THC use. THC should theoretically induce or worsen NAFLD by its agonistic role regarding CB1R. However, it has been proven that repetitive use of THC may result in decreased CB1R density, which may contribute to a dose-dependent inverse relationship between marijuana use and NAFLD occurrence [14][29][48]. Finally, the most important factor is that Cannabis is a source of not only THC, CBD, and THCV but also various other phytocannabinoids such as cannabidivarin (CBDV), cannabigerol (CBG), cannabigerovarian (CBGV), cannabigerolic acid (CBGA), and cannabinol (CBN). The therapeutic potential of these compounds remains largely unexplored. Thus, there is a need for further research directed at establishing whether phytocannabinoids are indeed ‘a neglected pharmacological treasure trove’ [14][49].
This entry is adapted from the peer-reviewed paper 10.3390/jcm10030393
References
- Kanwar, P.; Kowdley, K.V. The Metabolic Syndrome and Its Influence on Nonalcoholic Steatohepatitis. Clin. Liver Dis. 2016, 20, 225–243.
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012, 55, 2005–2023.
- Lonardo, A.; Bellentani, S.; Argo, C.K.; Ballestri, S.; Byrne, C.D.; Caldwell, S.H.; Cortez-Pinto, H.; Grieco, A.; Machado, M.V.; Miele, L.; et al. Epidemiological modifiers of non-alcoholic fatty liver disease: Focus on high-risk groups. Dig. Liver Dis. 2015, 47, 997–1006.
- Tilg, H.; Moschen, A.R. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology 2010, 52, 1836–1846.
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048.
- Jin, D.; Dai, K.; Xie, Z.; Chen, J. Secondary Metabolites Profiled in Cannabis Inflorescences, Leaves, Stem Barks, and Roots for Medicinal Purposes. Sci. Rep. 2020, 10, 1–14.
- Aizpurua-Olaizola, O.; Soydaner, U.; Öztürk, E.; Schibano, D.; Simsir, Y.; Navarro, P.; Etxebarria, N.; Usobiaga, A. Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis sativa Plants from Different Chemotypes. J. Nat. Prod. 2016, 79, 324–331.
- Wu, J. Cannabis, cannabinoid receptors, and endocannabinoid system: Yesterday, today, and tomorrow. Acta Pharmacol. Sin. 2019, 40, 297–299.
- Palomares, B.; Ruiz-Pino, F.; Navarrete, C.; Velasco, I.; Sánchez-Garrido, M.A.; Jimenez-Jimenez, C.; Pavicic, C.; Vazquez, M.J.; Appendino, G.; Bellido, M.L.; et al. Tetrahydrocannabinolic acid A (THCA-A) reduces adiposity and prevents metabolic disease caused by diet-induced obesity. Sci. Rep. 2019, 171, 113693.
- Franco, R.; Rivas-Santisteban, R.; Reyes-Resina, I.; Casanovas, M.; Pérez-Olives, C.; Ferreiro-Vera, C.; Navarro, G.; Sánchez de Medina, V.; Nadal, X. Pharmacological potential of varinic-, minor-, and acidic phytocannabinoids. Pharmacol. Res. 2020, 158, 104801.
- McPartland, J.M.; Duncan, M.; Di Marzo, V.; Pertwee, R.G. Are cannabidiol and Δ9-tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br. J. Pharmacol. 2015, 172, 737–753.
- Goyal, H.; Rahman, M.R.; Perisetti, A.; Shah, N.; Chhabra, R. Cannabis in liver disorders: A friend or a foe? Eur. J. Gastroenterol. Hepatol. 2018, 30, 1283–1290.
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.M.; Denovan-Wright, E.M. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br. J. Pharmacol. 2015, 172, 4790–4805.
- Pertwee, R.G. The diverse CB 1 and CB 2 receptor pharmacology of three plant cannabinoids: Δ 9-tetrahydrocannabinol, cannabidiol and Δ 9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215.
- Morales, P.; Hurst, D.P.; Reggio, P.H. Molecular Targets of the Phytocannabinoids: A Complex Picture. In Progress in the Chemistry of Organic Natural Products; Springer: Cham, Switzerland, 2017; Volume 103, pp. 103–131.
- Borgelt, L.M.; Franson, K.L.; Nussbaum, A.M.; Wang, G.S. The pharmacologic and clinical effects of medical cannabis. Pharmacotherapy 2013, 33, 195–209.
- Product Monograph. Eliquis®. 2010. ISBN 1866234234. Available online: (accessed on 20 November 2020).
- U.S. Food and Drug Administration. Cesamet (nabilone) Capsules. Nda 18-677/S-011; 2006. Available online: (accessed on 10 November 2020).
- U.S. Food and Drug Administration. Marinol (Dronabinol) Technical Sheet. Nda 18-651/s-021; 2004. Available online: (accessed on 10 November 2020).
- Greenwich Biosciences Inc. Full prescribing information of EPIDIOLEX. Prescr. Inf. Leafl.; 2018. Available online: (accessed on 10 November 2020).
- GW Pharma, L. Part III: Consumer Information Sativex®. Prod. Monogr.; 2015. Available online: (accessed on 10 November 2020).
- Kirkham, T.C. Cannabinoids and appetite: Food craving and food pleasure. Int. Rev. Psychiatry 2009, 21, 163–171.
- Foltin, R.W.; Fischman, M.W.; Byrne, M.F. Effects of smoked marijuana on food intake and body weight of humans living in a residential laboratory. Appetite 1988, 11, 1–14.
- Rodondi, N.; Pletcher, M.J.; Liu, K.; Hulley, S.B.; Sidney, S. Marijuana Use, Diet, Body Mass Index, and Cardiovascular Risk Factors (from the CARDIA Study). Am. J. Cardiol. 2006, 98, 478–484.
- Rajavashisth, T.B.; Shaheen, M.; Norris, K.C.; Pan, D.; Sinha, S.K.; Ortega, J.; Friedman, T.C. Decreased prevalence of diabetes in marijuana users: Cross-sectional data from the National Health and Nutrition Examination Survey (NHANES) III. BMJ Open 2012, 2.
- Smit, E.; Crespo, C.J. Dietary intake and nutritional status of US adult marijuana users: Results from the Third National Health and Nutrition Examination Survey. Public Health Nutr. 2001, 4, 781–786.
- Penner, E.A.; Buettner, H.; Mittleman, M.A. The impact of marijuana use on glucose, insulin, and insulin resistance among US adults. Am. J. Med. 2013, 126, 583–589.
- Carrieri, M.P. Cannabis use and reduced risk of insulin-resistance in HIV-HCV infected patients: A longitudinal analysis (ANRS HEPAVIH CO-13). Clin. Infect. Dis. 2015, 61, 40–48.
- Le Strat, Y.; Le Foll, B. Obesity and cannabis use: Results from 2 representative national surveys. Am. J. Epidemiol. 2011, 174, 929–933.
- Vidot, D.C.; Prado, G.; Hlaing, W.W.M.; Florez, H.J.; Arheart, K.L.; Messiah, S.E. Metabolic Syndrome Among Marijuana Users in the United States: An Analysis of National Health and Nutrition Examination Survey Data. Am. J. Med. 2016, 129, 173–179.
- Muniyappa, R.; Sable, S.; Ouwerkerk, R.; Mari, A.; Gharib, A.M.; Walter, M.; Courville, A.; Hall, G.; Chen, K.Y.; Volkow, N.D.; et al. Metabolic effects of chronic cannabis smoking. Diabetes Care 2013, 36, 2415–2422.
- Sansone, R.A.; Sansone, L.A. Marijuana and body weight. Innov. Clin. Neurosci. 2014, 11, 50–54.
- Hayatbakhsh, M.R.; O’Callaghan, M.J.; Mamun, A.A.; Williams, G.M.; Clavarino, A.; Najman, J.M. Cannabis use and obesity and young adults. Am. J. Drug Alcohol Abuse 2010, 36, 350–356.
- Kim, D.; Kim, W.; Kwak, M.S.; Chung, G.E.; Yim, J.Y.; Ahmed, A. Inverse association of marijuana use with nonalcoholic fatty liver disease among adults in the United States. PLoS ONE 2017, 12, e0186702.
- Vázquez-Bourgon, J.; Ortiz-García de la Foz, V.; Suarez-Pereira, I.; Iruzubieta, P.; Arias-Loste, M.T.; Setién-Suero, E.; Ayesa-Arriola, R.; Gómez-Revuelta, M.; Crespo, J.; Crespo Facorro, B. Cannabis consumption and non-alcoholic fatty liver disease. A three years longitudinal study in first episode non-affective psychosis patients. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 95, 109677.
- Adejumo, A.C.; Alliu, S.; Ajayi, T.O.; Adejumo, K.L.; Adegbala, O.M.; Onyeakusi, N.E.; Akinjero, A.M.; Durojaiye, M.; Bukong, T.N. Cannabis use is associated with reduced prevalence of non-alcoholic fatty liver disease: A cross-sectional study. PLoS ONE 2017, 12, e0176416.
- Thomas, A.; Baillie, G.L.; Phillips, A.M.; Razdan, R.K.; Ross, R.A.; Pertwee, R.G. Cannabidiol displays unexpectedly high potency as an antagonist of CB 1 and CB 2 receptor agonists in vitro. Br. J. Pharmacol. 2007, 150, 613–623.
- Thomas, A.; Stevenson, L.A.; Wease, K.N.; Price, M.R.; Baillie, G.; Ross, R.A.; Pertwee, R.G. Evidence that the plant cannabinoid Δ 9- tetrahydrocannabivarin is a cannabinoid CB 1 and CB 2 receptor antagonist. Br. J. Pharmacol. 2005, 146, 917–926.
- Osei-Hyiaman, D.; Liu, J.; Zhou, L.; Godlewski, G.; Harvey-White, J.; Jeong, W.I.; Bátkai, S.; Marsicano, G.; Lutz, B.; Buettner, C.; et al. Hepatic CB1 receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J. Clin. Investig. 2008, 118, 3160–3169.
- Osei-Hyiaman, D.; DePetrillo, M.; Pacher, P.; Liu, J.; Radaeva, S.; Bátkai, S.; Harvey-White, J.; Mackie, K.; Offertáler, L.; Wang, L.; et al. Endocannabinoid activation at hepatic CB 1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J. Clin. Investig. 2005, 115, 1298–1305.
- Tam, J.; Liu, J.; Mukhopadhyay, B.; Cinar, R.; Godlewski, G.; Kunos, G. Endocannabinoids in liver disease. Hepatology 2011, 53, 346–355.
- Mu, W.; Xuefang, C.; Liu, Y.; Qianzhou, L.; Gaolin, L.; Jigang, Z.; Xiaoyu, L. Potential nexus of non-alcoholic fatty liver disease and type 2 diabetes mellitus: Insulin resistance between hepatic and peripheral tissues. Front. Pharmacol. 2019, 9, 1566.
- Jorgačević, B.; Vučević, D.; Vesković, M.; Mladenović, D.; Vukićević, D.; Vukićević, R.J.; Todorović, V.; Radosavljević, T. The effect of cannabinoid receptor 1 blockade on adipokine and proinflammatory cytokine concentration in adipose and hepatic tissue in mice with nonalcoholic fatty liver disease. Can. J. Physiol. Pharmacol. 2019, 97, 120–129.
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11.
- Braunersreuther, V.; Viviani, G.L.; Mach, F.; Montecucco, F. Role of cytokines and chemokines in non-alcoholic fatty liver disease. World J. Gastroenterol. 2012, 18, 727–735.
- Huang, Y.; Wan, T.; Pang, N.; Zhou, Y.; Jiang, X.; Li, B.; Gu, Y.; Huang, Y.; Ye, X.; Lian, H.; et al. Cannabidiol protects livers against nonalcoholic steatohepatitis induced by high-fat high cholesterol diet via regulating NF-κB and NLRP3 inflammasome pathway. J. Cell. Physiol. 2019, 234, 21224–21234.
- Ribeiro, A.; Ferraz-De-Paula, V.; Pinheiro, M.L.; Vitoretti, L.B.; Mariano-Souza, D.P.; Quinteiro-Filho, W.M.; Akamine, A.T.; Almeida, V.I.; Quevedo, J.; Dal-Pizzol, F.; et al. Cannabidiol, a non-psychotropic plant-derived cannabinoid, decreases inflammation in a murine model of acute lung injury: Role for the adenosine A 2A receptor. Eur. J. Pharmacol. 2012, 678, 78–85.
- Hirvonen, J.; Goodwin, R.S.; Li, C.T.; Terry, G.E.; Zoghbi, S.S.; Morse, C.; Pike, V.W.; Volkow, N.D.; Huestis, M.A.; Innis, R.B. Reversible and regionally selective downregulation of brain cannabinoid CB 1 receptors in chronic daily cannabis smokers. Mol. Psychiatry 2012, 17, 642–649.
- Mechoulam, R. Plant cannabinoids: A neglected pharmacological treasure trove. Br. J. Pharmacol. 2005, 146, 913–915.
