Honey is a natural by-product from the flower nectar and aerodigestive tract of honey bees, which contains various complex biochemical components. Fructose (36%) and glucose (31%) are the main carbohydrate constituents of honey.
- metabolic syndrome
- honey
- obesity
- hyperglycaemia
1. The Mechanisms of Honey in Reversing Metabolic Changes
Given the role of inflammation and oxidative stress in the development of metabolic syndrome, honey, with the capacity to quench these processes, could prevent metabolic syndrome. The therapeutic effects of honey large depend on the antioxidant and anti-inflammatory properties of its polyphenol and flavonoid content. The phenolic acid and flavonoid profiles of honey vary based on factors, such as climate, geographic factors, and floral abundance [1]. A study on the total phenolic contents and colour intensity of different types of Malaysian honey reported a positive correlation between total phenolic content (TPC) and colour intensity of honey, whereby Kelulut honey had the highest value of TPC of 784.3 mg GAE/kg and other honey, such as Tualang, Pineapple and Borneo, had TPC values of 589.2, 602.4, and 510.4 mg GAE/kg, respectively [2]. The polyphenols and flavonoids are potent antioxidants because they can donate hydrogen and hydrogen groups to scavenge free radicals in oxidative stress. For example, quercetin, caffeic acid (CA), and chlorogenic acid are polyphenols that possess iron-chelating and iron-stabilizing properties, which prevents free radical formation, making them great antioxidants [3][4][5]. Furthermore, polyphenols, such as apigenin, quercetin, and kaempferol, may also exert their anti-inflammatory properties by modulation of enzymes involved in proinflammatory activities, such as nuclear factor-kappa B (NF-κB), activator protein-1 (AP-1), or nuclear factor erythroid 2-related factor 2 (Nrf2) [6][7][8]. These bioactive compounds may contribute synergistically to the antimetabolic effects of honey.
2. Anti-Inflammatory and Antioxidant Properties of Honey
As illustrated in the previous section, metabolic syndrome is closely linked with oxidative stress and inflammation. Studies have shown that honey can protect against the activation of NF-κB, the key transcription factor of inflammation. An in vitro study reported that 5–20% manuka honey inhibited the activation of NF-κB and AP-1 in H. pylori-induced NF-κB and AP-1 DNA-binding activity in gastric epithelial cells and downregulated the expression of cyclooxygenase-2 (COX-2) [9]. Hussein and colleagues also reported the suppression of NF-κB (p65 and p50 gene expression) by Gelam honey at 1.0 and 2.0 g/kg body weight for 7 days in a Carrageenan-induced paw oedema model in rats. Gelam honey at 1 or 2 g/kg also inhibited the nuclear transcription of NF-κB, followed by a subsequent reduction in COX-2 and TNFα [10]. Another animal study by Aziz and colleagues also observed a significant decrease in NF-κB, IL-1β, and TNFα expression, as well as a significant increase in the antioxidant CAT after 28 days of treatment with 1 and 2 g/kg body weight SBH in diabetic rats [11].
On the other hand, honey could activate the nuclear localization of Nrf2, which is the key regulator of the cellular antioxidant defence. Activation of Nrf2, in turn, facilitates the transcription of several Nrf2 target genes that control antioxidant defence and autophagy. Honey activates AMPK and endogenous enzymatic antioxidants, such as SOD, CAT, and GPX [12]. Manuka honey was reported to prevent oxidative damage and preserve mitochondrial functionality via activation of the AMPK/Nrf2 signalling pathway, with a subsequent increase in the expression of antioxidant enzymes, such as SOD and CAT [13]. Another study by Ranneh and colleagues reported that SBH suppressed lipopolysaccharide (LPS)-induced chronic subclinical systemic inflammation (CSSI) and oxidative stress in rats. SBH also reduced NF-κB, p65, and p38 MAPK and upregulated Nrf2 expression in the liver, kidney, heart, and lungs [14]. A study by Sabitha and colleagues reported that p-CA suppressed ethanol-induced oxidative stress and apoptosis by suppressing CYP2E1 and stimulating Nrf2 and its target protein expression in rat liver tissue. Therefore, p-CA is an effective antioxidant by enhancing Nrf2 signalling [15]. Furthermore, honey is also known to reduce malondialdehyde (MDA) levels, which is a product of lipid peroxidation with high reactivity and toxicity, making it one of the most reliable biomarkers of oxidative stress [16]. A study found that supplementation of Tualang honey 1 g/kg for 12 weeks in spontaneously hypertensive rats reduced malondialdehyde (MDA) levels and downregulated the activity of glutathione-S-transferase (GST) and CAT, while it moderately upregulated Nrf2 mRNA expression [17].
3. Antiobesity Properties of Honey
An in vitro study on Pineapple honey showed a significantly reduced lipid droplet size by 33.78% to 70.36% and reduced lipid accumulation in treated 3T3-L1 adipocytes, suggesting honey might limit the storage of lipids in adipocytes [18]. Malaysian Gelam and Acacia honey were reported to reduce weight gain and BMI in rats with obesity induced by a high-fat diet (HFD). Rats that were fed Gelam honey ad libitum for 4 weeks also showed a reduction in the adiposity index compared to the HFD group, showing Gelam honey can prevent excessive adipose tissue formation [19]. Similar results were demonstrated by Rafie and colleagues using stingless bee honey (SBH) from Heterotrigona itama. The rats showed reduced BMI, percentage body weight gain, adiposity index, and relative liver weight after a 6-week honey supplementation (500–1000 mg/kg) [20]. Ramli and colleagues also observed a reduction in body fat and omental fat mass in rats supplemented with 1 g/kg/day of Kelulut honey for 8 weeks [21]. Romero-Silva and colleagues observed a significantly lower weight gain as well as smaller fat cells in rats supplemented with a hypercaloric diet containing 20% honey (unknown source) compared with the sucrose-fed rats for 8 weeks [22].
In addition, human interventional studies also found a similar finding marked by a reduction in body weight, body fat, and lipid profile after consumption of 70 g of natural unprocessed honey collected from Iran for 30 days [23]. Furthermore, a randomised open-labelled controlled clinical study by Pai and colleagues reported a significant reduction in body weight, BMI, WC, hip circumference, and lipid profile in obese patients treated with unprocessed and processed honey (collected from India). The honey was supplemented at 48 g for 48 days in these patients [24].
Honey is rich in polyphenols known to reduce body weight and fat mass, thus explaining its antiobesity properties. A study by Liao and colleague reported that a reduction in body weight as well as a decrease in the ratio of various adipose tissue mass (epididymal, retroperitoneal, and mesenteric fat) and body weight in mice given a high-fat diet with 0.02% and 0.08% w/w CA for 6 weeks [25]. A similar study with the supplementation of 50 µg/day of quercetin for 8 weeks in mice fed a high-fat diet also reported a reduction in fat mass and body weight [26]. Another animal study reported CA and chlorogenic acid supplementation (0.02% w/w) for 8 weeks in mice fed a high-fat diet significantly reduced BW by 8% and 16%, respectively. Furthermore, the weight of the epididymal white adipose tissue of mice supplemented with CA and chlorogenic acid was lower than the control group by 22% and 46%, respectively. In this experiment, supplementation with CA and chlorogenic acid also reduced plasma leptin, indicating the alleviation of leptin resistance [27]. Meanwhile, an in vitro study concerning the effects of GA on lipolysis found that GA (250 µM 48 and 72 h) inhibits proliferation but induces apoptosis in 3T3-L1 preadipocytes. As evidence, cells treated with 50 µM GA for 12 h showed an increase in Fas (CD95)/Fas Ligand (FasL; CD95L) and p53 expression. These proteins are involved in the extracellular pathway in apoptotic signalling [28]. However, the actions of these polyphenols might be different compared to honey because the content and their interactions with other compounds need to be considered.
Besides, fructo-oligosaccharides (FOSs) are also known to alter lipid metabolism. FOSs in honey are resistant to human digestive enzymes and could act as probiotics. Various studies reported that the administration of these fructans promotes a reduction in weight gain and energy intake in rats [29]. A study from Kaume et al. observed that dietary supplementation of 5% FOSs (w/w) could significantly lower total lipids by 12% with a subsequent reduction in liver weight in obese Zucker rats [30]. Daubioul and colleagues also observed that the body weight of FOS-fed Zucker rats was significantly lower than control rats after 4 weeks of treatment. A histological examination of the liver revealed a reduction in fat cells of the rats fed with 10% FOS (w/w). Besides, FOS also decreases malic enzyme (ME) activity, a lipogenic key enzyme in providing NADPH for fatty acid elongation by FAS. Therefore, FOS can inhibit the synthesis of long-chain fatty acid [31]. Similarly, Agheli and colleagues reported a reduction of FAS activity after FOS supplementation in insulin-resistant Sprague-Dawley rats [32]. Delzane and Kok showed a reduction in the activity of lipogenic enzymes, such as acetyl-CoA carboxylase (ACC), ME, and FAS after FOS supplementation by modifying the gene expression of these enzymes. Of note, FAS mRNA was reduced by 40% in FOS-fed rats compared to the control group [33].
A summary of the antiobesity properties of honey is presented in Figure 1.
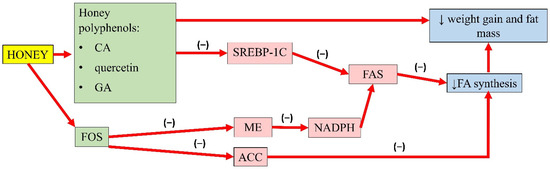
Figure 1. Summary of the antiobesity properties of honey. CA, caffeic acid; GA, gallic acid; FOS, fructo-oligosaccharides; SREBP-1C, sterol regulatory element-binding transcription factor 1C; ME, malic enzyme; ACC, acetyl-CoA carboxylase; FAS, fatty acid synthase; NADPH, nicotinamide adenine dinucleotide phosphate; FA, fatty acid; (−), inhibit; ↓, reduce.
4. Antihyperglycaemic Properties of Honey
Honey is a sweet substance with a relatively low glycaemic index, making it a suitable sugar substitute. The fructose present in honey contributes to its sweet taste. The major source of fructose used by the food industry as a sweetener is derived from cane sugar or high-fructose corn syrup. Fructose is a potent and acute regulator of liver glucose uptake and glycogen synthesis. It forms TG more effectively and is more lipogenic than glucose despite having a similar chemical structure [34]. In the liver, fructose bypasses the regular steps of glycolysis, catalyzed by glucokinase or hexokinase and phosphofructokinase. Instead, fructose is transported by insulin-independent glucose transporter (GLUT-5) and is metabolized to fructose-1-phosphate by the enzyme fructokinase or ketohexokinase [35]. High fructose intake results in postprandial hypertriglyceridaemia and an increase in visceral adipose deposition. This event exacerbates hepatic triglyceride accumulation, protein kinase C activation, and hepatic insulin resistance due to the continuous portal delivery of fatty acid to the liver [36]. On the other hand, honey can normalise circulating glucose levels because its fructose content can prolong gastric emptying and lowers food intake [12]. The slow absorption of fructose within the intestinal tracks might prolong interaction between fructose and the intestinal receptor, which might result in satiety [37].
The hypoglycaemic properties of honey have been illustrated in rodent models of diabetes, healthy subjects, and diabetic patients. These effects might be contributed by the components of honey, such as fructose, and phenolic acids. A study reported that administration of 1.0 to 2.0 g/kg of Nigerian honey for 3 weeks significantly reduced hyperglycaemia in alloxan-induced diabetic rats [38]. Another animal study also reported similar findings with 1.0 and 2.0 g/kg body weight of SBH from Geniotrigona thoracica in diabetic rat models by suppressing FBG levels after 28 days of treatment. Additionally, histopathological changes, expression of oxidative stress, inflammation, and apoptosis markers within the pancreatic islet were improved in conjunction with the increase in the expression of insulin in the islet [11].
In a human intervention study, 30 days of 70 g of honey (collected from Iran) was reported to reduce FBG compared to overweight individuals fed with sucrose [23]. In obese girls, supplementation of 15 g honey for 6 months caused a reduction in BMI and the area under the concentration–time curve (AUC) in an oral glucose tolerance test and insulin [39]. Agrawal and colleagues observed a higher degree of tolerance to honey with a significantly lower glucose level in patients with diabetes or impaired glucose tolerance after consumption of 90 g of unprocessed natural honey from India in 300 mL of water, at a 30-min interval up to 2 h [40]. A case-control study in Egypt involving children and adolescents with type 1 diabetes mellitus also recorded a lower glycaemic index and incremental index in both the diabetic and control group after honey (unspecified source) consumption at a calculated dose (dose in g = subjects’ body weight in kg × 1.75 with a maximum of 75 g) diluted in 200 mL of water, every 30 min postprandial for 2 h [41].
Honey could modulate the key components of the insulin signalling pathway, P13k/Akt [12]. The development of insulin resistance is characterised by an increase in NF-κB, MAPK, and IRS-1 serine phosphorylation. However, pretreatment of oxidative stress-induced HIT-T15 cells with Gelam honey extract (20, 40, 60, and 80 µg/mL) and quercetin (20, 40, 60, and 80 µM) for 24 h, prior to stimulation with 20 and 50 mM glucose, reported an increase in the expression of Akt and decreased expression of IRS-1 serine phosphorylation, NF-κB, and MAPK [42]. In addition, a study by Tapia and colleagues also observed lower serum glucose in rats fed with 10% honey ad libitum for 4 months. Interestingly, long-term honey consumption in the presence of a high-fat diet did not significantly increase the insulin concentration. Honey was reported as the sweetener that increased phosphorylation of IRS-tyrosine and Akt and lowered the protein abundance of NF-κB, which indicates better insulin signalling. It was also observed that honey significantly increased white adipose tissue GLUT-4 expression in rats, an insulin-sensitive glucose transporter that moves glucose into the adipose tissue, indicating better insulin sensitivity [43]. A summary of the antihyperglycaemic properties of honey is presented in Figure 2.
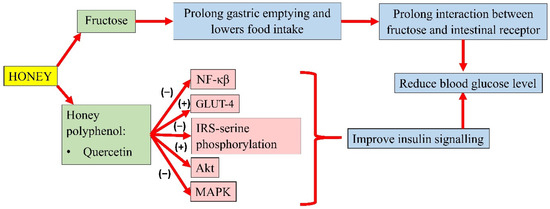
Figure 2. Summary of the antihyperglycemic properties of honey. NF-κB, nuclear factor kappa B; GLUT-4, insulin-independent glucose transporter 4; IRS-serine phosphorylation, inhibiting insulin receptor substrate-serine phosphorylation; Akt, protein kinase B; MAPK, mitogen-activated protein kinase; (−), inhibit; (+), increase.
5. Antihyperlipidaemic Properties of Honey
Many studies on the hypolipidaemic effects of honey have been conducted. A study by Samat et al. reported that the consumption of Gelam and Acacia honey (dose unspecified) for four weeks reduced TG and cholesterol in rats fed a high-fat diet (HFD) [19]. In another report, supplementation of SBH from Geniotrigona thoracica honey 1.0 and 2.0 g/kg body weight for 28 days could increase the HDL-C level but reduce TG, TC, and LDL-C levels in streptozotocin-nicotinamide-induced diabetic male rats [11]. This beneficial effect was replicated in human studies, whereby the consumption of 70 g of natural unprocessed honey dissolved in 250 mL of tap water for 30 days in overweight and obese individuals caused a 3.3%, 4.3%, and 19% reduction in TC, LDL-C, and TG [23].
The natural compounds in honey contribute to its lipid-lowering effects. Polyphenols, such as CA and p-coumaric acid (P-CA), commonly seen in all honey possess numerous bioactive properties including antioxidant, anti-inflammatory, and lipid-lowering actions [37]. Studies on CA and p-coumaric acid have shown that both compounds can reduce the mRNA expression of SREBP-1c and FAS and inhibit their activity [25][44].
FFA is the main player in the synthesis of TG in hepatocytes. In metabolic syndrome, lipolysis in adipose tissue increases, resulting in enhanced FFA delivery to the liver [45]. Both SREBP-1c and FAS are key regulators to FFA synthesis, and their dysregulation is the primary source of hypertriglyceridaemia [46]. In recent studies, phenolic compounds can activate 5′adenosine monophosphate-activated protein kinase (AMPK), which mediates the reduction in SREBP-1c protein expression by preventing SREBP-1c nuclear translocation, subsequently suppressing FAS synthase expression [44][47].
According to Liao and colleagues, CA reduced the TG and cholesterol content in oleic acid-induced hepatic lipogenesis in HepG2. Furthermore, CA also enhanced the phosphorylation of AMPK and ACC, which are lipid oxidation-related proteins. CA also downregulated the lipogenesis gene expression of SREBP-1 and its target gene FAS in the presence of oleic acid [25].
Gallic acid (GA) and catechin can also be found in honey. Chang and colleagues’ study showed that oleic acid significantly increased FAS, SREBP-1, and phosphorylated AMPK expression in high-fat diet-treated mice. An extract containing GA and catechin reduced FAS expression by 6% and SREBP-1 by 23%. This observation indicated that GA and catechin might attenuate hepatic lipid accumulation by regulating FA and TG synthesis [48]. Another study by Kim and colleagues demonstrated that p-CA (up to 40 µg) increased the phosphorylation of AMPK and ACC, and the expression of carnitine palmitoyltransferase-1a in HepG2 cells, suggesting enhanced fatty acid β-oxidation. Furthermore, p-CA also reduced lipid accumulation in HepG2 cells, implying its ability to attenuate FA synthesis. The authors also implied that p-CA might inhibit the lipid uptake of HepG2 cells [44]. The effects of these individual compounds might not fully recapitulate the antihyperlipidaemic potential of honey, as they might act in synergy with other components to achieve the overall effects. A summary of the antihyperlipidaemic properties of honey is presented in Figure 3.
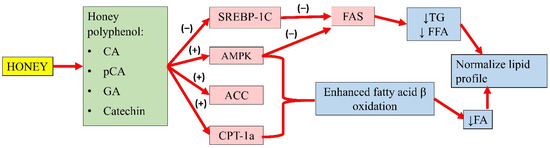
Figure 3. Summary of the antihyperlipidaemic properties of honey. CA, caffeic acid; p-CA, p-coumaric acid; GA, gallic acid; SREBP-1c, sterol regulatory element-binding transcription factor 1c; AMPK, 5′adenosine monophosphate-activated protein kinase; ACC, acetyl-CoA carboxylase; CPT-1a, carnitine palmitoyltransferase-1a; FAS, fatty acid synthase; TG, serum triglyceride; FFA, free fatty acid; FA, fatty acid; (−), inhibit; (+), increase; ↓, reduce.
6. Antihypertensive Properties of Honey
Flavonoids present in honey, such as quercetin and kaempferol, show promising results in the treatment of cardiovascular diseases [49]. A study by Sanchez and colleagues reported that treatment with 10 mg/kg of quercetin for 13 weeks lowered blood pressure and heart rate in spontaneously hypertensive rats. This was achieved by upregulating eNOS and p47 protein expression and reducing NADPH-oxidase-mediated superoxide anion generation, which attenuated endothelial dysfunction [50]. Another in vitro study showed that quercetin (>0.1 µM) increased the conductance of calcium-activated potassium channel (BKca) currents in rat coronary artery rings. Given that BKca regulates coronary artery tone in vivo, quercetin could induce vasodilatation [51]. Kuhlmann and colleagues reported quercetin improves endothelial dysfunction by inducing BKca-dependant endothelial hyperpolarisation in human endothelial cells derived from the umbilical cord vein (HUVEC) (with maximum effect achieved at 50 µM/L). This event led to an influx of extracellular calcium ions, resulting in increased NO production [52]. Similarly, an in vitro study demonstrated that quercetin exerted vasodilatory effects on the human umbilical artery. In a double-blind randomised placebo-controlled study, healthy volunteers were given capsules containing placebo, 200, or 400 mg quercetin randomly in 3 consecutive weeks. The result showed that quercetin increased the brachial diameter [53].
In addition, quercetin also reduced endothelial proliferation by 56% and increased cyclic guanosine monophosphate (cGMP) level by 5 fold due to NO formation [52]. An in vitro study by Shen and colleagues indicated that quercetin improved endothelial dysfunction in the mouse abdominal aorta and aortic ring. At a dose of 5 and 10 µM, quercetin significantly increased acetylcholine-mediated endothelial-dependant relaxation in the presence of hypochlorous acid (HOCl) by 34% and 78%, respectively. Incubation with 10 µM quercetin 2 h prior to treatment with HOCl also restored eNOS activity in aortic tissue. Quercetin activated eNOS activity, subsequently increasing NO production [54].
Meanwhile, pre-treatment of kaempferol in HUVEC was reported to suppress the expression of NF-κB by LPS significantly. In addition, TNFα was increased in LPS-stimulated endothelial cells, which was significantly decreased with kaempferol. Altogether, the results suggest that kaempferol improves barrier integrity, and inhibits the activity of cell adhesion and migration to endothelial cells by inhibiting NF-κB expression and TNFα production, thereby promoting its benefits in the treatment of vascular inflammatory disease [55]. The individual effects of these bioactive compounds in honey and their interactions contribute to its overall antihypertensive effects. A summary of the antihypertensive properties of honey is presented in Figure 4.
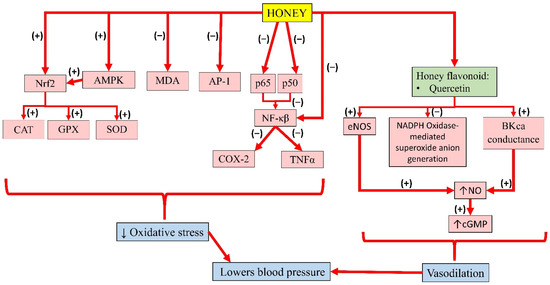
Figure 4. Summary of the antihypertensive properties of honey. Nrf2, nuclear factor erythroid 2-related factor 2; AMPK, 5′adenosine monophosphate-activated protein kinase; MDA, malondialdehyde; AP-1, activator protein-1; p65, subunit 65 protein of NF-κB; p50, subunit 50 protein of NF-κB; NF-κB, nuclear factor kappa B; CAT, catalase; GPX, glutathione peroxidase; SOD, superoxide dismutase; COX-2, cyclooxygenase-2; TNFα, tumour necrosis factor-alpha; eNOS, endothelial nitric oxide synthase; NADPH, nicotinamide adenine dinucleotide phosphate; BKca, large-conductance calcium-activated potassium channel; NO, nitric oxide; cGMP, cyclic guanosine monophosphate; (−), inhibit; (+), increase; ↓, reduce; ↑, increase.
This entry is adapted from the peer-reviewed paper 10.3390/molecules26040808
References
- Cheung, Y.; Meenu, M.; Yu, X.; Xu, B. Phenolic acids and flavonoids profiles of commercial honey from different floral sources and geographic sources. Int. J. Food Prop. 2019, 22, 290–308.
- Kek, S.P.; Chin, N.L.; Yusof, Y.A.; Tan, S.W.; Chua, L.S. Total Phenolic Contents and Colour Intensity of Malaysian Honeys from the Apis spp. and Trigona spp. Bees. Agric. Agric. Sci. Procedia 2014, 2, 150–155.
- Andjelković, M.; Van Camp, J.; De Meulenaer, B.; Depaemelaere, G.; Socaciu, C.; Verloo, M.; Verhe, R. Iron-chelation properties of phenolic acids bearing catechol and galloyl groups. Food Chem. 2006, 98, 23–31.
- Hussein, S.Z.; Yusoff, K.M.; Makpol, S.; Yusof, Y.A.M. Antioxidant capacities and total phenolic contents increase with gamma irradiation in two types of Malaysian honey. Molecules 2011, 16, 6378.
- Leopoldini, M.; Russo, N.; Chiodo, S.; Toscano, M. Iron chelation by the powerful antioxidant flavonoid quercetin. J. Agric. Food Chem. 2006, 54, 6343–6351.
- Qin, Y.; Zhao, D.; Zhou, H.G.; Wang, X.H.; Zhong, W.L.; Chen, S.; Gu, W.G.; Wang, W.; Zhang, C.H.; Liu, Y.R.; et al. Apigenin inhibits NF-κB and snail signaling, EMT and metastasis in human hepatocellular carcinoma. Oncotarget 2016, 7, 41421–41431.
- Patil, R.H.; Babu, R.L.; Naveen Kumar, M.; Kiran Kumar, K.M.; Hegde, S.M.; Nagesh, R.; Ramesh, G.T.; Sharma, S.C. Anti-Inflammatory Effect of Apigenin on LPS-Induced Pro-Inflammatory Mediators and AP-1 Factors in Human Lung Epithelial Cells. Inflammation 2016, 39, 138–147.
- Sharma, A.; Parikh, M.; Shah, H.; Gandhi, T. Modulation of Nrf2 by quercetin in doxorubicin-treated rats. Heliyon 2020, 6, e03803.
- Abdel-Latif, M.M.M.; Abouzied, M.M. Molecular Mechanisms of Natural Honey Against H. pylori Infection Via Suppression of NF-κB and AP-1 Activation in Gastric Epithelial Cells. Arch. Med. Res. 2016, 47, 340–348.
- Hussein, S.Z.; Yusoff, K.M.; Makpol, S.; Anum, Y.; Yusof, M. Gelam Honey Attenuates Carrageenan-Induced Rat Paw Inflammation via NF- k B Pathway. PLoS ONE 2013, 8.
- Aziz, M.S.A.; Giribabu, N.; Rao, P.V.; Salleh, N. Pancreatoprotective effects of Geniotrigona thoracica stingless bee honey in streptozotocin-nicotinamide-induced male diabetic rats. Biomed. Pharmacother. 2017, 89, 135–145.
- Ahmed, S.; Sulaiman, S.A.; Baig, A.A.; Ibrahim, M.; Liaqat, S.; Fatima, S.; Jabeen, S.; Shamim, N.; Othman, N.H. Honey as a Potential Natural Antioxidant Medicine: An Insight into Its Molecular Mechanisms of Action. Oxid. Med. Cell. Longev. 2018, 2018.
- Alvarez-Suarez, J.M.; Giampieri, F.; Cordero, M.; Gasparrini, M.; Forbes-Hernández, T.Y.; Mazzoni, L.; Afrin, S.; Beltrán-Ayala, P.; González-Paramás, A.M.; Santos-Buelga, C.; et al. Activation of AMPK/Nrf2 signalling by Manuka honey protects human dermal fibroblasts against oxidative damage by improving antioxidant response and mitochondrial function promoting wound healing. J. Funct. Foods 2016, 25, 38–49.
- Ranneh, Y.; Akim, A.M.; Hamid, H.A.; Khazaai, H.; Fadel, A.; Mahmoud, A.M. Stingless bee honey protects against lipopolysaccharide induced-chronic subclinical systemic inflammation and oxidative stress by modulating Nrf2, NF-κB and p38 MAPK. Nutr. Metab. 2019, 16, 15.
- Sabitha, R.; Nishi, K.; Gunasekaran, V.; Annamalai, G.; Agilan, B.; Ganeshan, M. P-Coumaric acid ameliorates ethanol-induced kidney injury by inhibiting inflammatory cytokine production and NF- and kappa;B signaling in rats. Asian Pac. J. Trop. Biomed. 2019.
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438.
- Erejuwa, O.O.; Sulaiman, S.A.; Ab Wahab, M.S.; Sirajudeen, K.N.S.; Salleh, S.; Gurtu, S. Honey supplementation in spontaneously hypertensive rats elicits anti-hypertensive effect via amelioration of renal oxidative stress. Oxid. Med. Cell. Longev. 2012, 2012, 374037.
- Samat, S.; Salleh, M.A.M.; Adam, Z.; Ismail, W.I.W. Pineapple honey inhibits adipocytes proliferation and reduces lipid droplet accumulation in 3T3-L1 adipocytes. Malaysian Appl. Biol. 2019, 48, 21–26.
- Samat, S.; Kanyan Enchang, F.; Nor Hussein, F.; Wan Ismail, W.I. Four-Week Consumption of Malaysian Honey Reduces Excess Weight Gain and Improves Obesity-Related Parameters in High Fat Diet Induced Obese Rats. Evid. Based Complement. Altern. Med. 2017, 2017, 9.
- Rafie, A.Z.M.; Syahir, A.; Ahmad, W.A.N.W.; Mustafa, M.Z.; Mariatulqabtiah, A.R. Supplementation of Stingless Bee Honey from Heterotrigona itama Improves Antiobesity Parameters in High-Fat Diet Induced Obese Rat Model. Evidence-based Complement. Altern. Med. 2018, 2018, 1–10.
- Ramli, N.Z.; Chin, K.-Y.; Zarkasi, K.A.; Ahmad, F. The Beneficial Effects of Stingless Bee Honey from Heterotrigona itama against Metabolic Changes in Rats Fed with High-Carbohydrate and High-Fat Diet. Int. J. Environ. Res. Public Health 2019, 16, 4987.
- Romero-Silva, S.; Angel Martinez, R.M.P.; Romero-Romero, L.; Rodriguez, O.; Gerardo Salas, G.C.; Morel, N.; Javier Lopez-Munoz, F.; Angel Lima-Mendoza, L.; Bravo, G. Effects of Honey Against the Accumulation of Adipose Tissue and the Increased Blood Pressure on Carbohydrate-Induced Obesity in Rat. Lett. Drug Des. Discov. 2011, 8, 69–75.
- Yaghoobi, N.; Al-Waili, N.; Ghayour-Mobarhan, M.; Parizadeh, S.M.R.; Abasalti, Z.; Yaghoobi, Z.; Yaghoobi, F.; Esmaeili, H.; Kazemi-Bajestani, S.M.R.; Aghasizadeh, R.; et al. Natural honey and cardiovascular risk factors; effects on blood glucose, cholesterol, triacylglycerole, CRP, and body weight compared with sucrose. Sci. World J. 2008, 8, 463–469.
- Pai, S.; Shivappa, C.B.; Surendra, A. Original Article Anti-obesity and Anti-hyperlipidemic activity of Processed Honey A Randomised, Open labeled, Controlled Clinical Study. J. Res. Tradit. Med. 2018, 4, 40–48.
- Liao, C.-C.; Ou, T.-T.; Wu, C.-H.; Wang, C.-J. Prevention of Diet-Induced Hyperlipidemia and Obesity by Caffeic Acid in C57BL/6 Mice through Regulation of Hepatic Lipogenesis Gene Expression. J. Agric. Food Chem. 2013, 61, 11082–11088.
- Henagan, T.M.; Lenard, N.R.; Gettys, T.W.; Stewart, L.K. Dietary quercetin supplementation in mice increases skeletal muscle PGC1α expression, improves mitochondrial function and attenuates insulin resistance in a time-specific manner. PLoS ONE 2014, 9, 1–11.
- Cho, A.S.; Jeon, S.M.; Kim, M.J.; Yeo, J.; Seo, K., II; Choi, M.S.; Lee, M.K. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem. Toxicol. 2010, 48, 937–943.
- Hsu, C.L.; Lo, W.H.; Yen, G.C. Gallic acid induces apoptosis in 3T3-L1 pre-adipocytes via a fas- and mitochondrial-mediated pathway. J. Agric. Food Chem. 2007, 55, 7359–7365.
- Erejuwa, O.O.; Sulaiman, S.A.; Ab Wahab, M.S. Oligosaccharides might contribute to the antidiabetic effect of honey: A review of the literature. Molecules 2011, 17, 248–266.
- Kaume, L.; Gilbert, W.; Gadang, V.; Devareddy, L. Dietary Supplementation of Fructooligosaccharides Reduces Hepatic Steatosis Associated with Insulin Resistance in Obese Zucker Rats. Funct. Foods Health Dis. 2011, 1, 199–213.
- Daubioul, C.A.; Taper, H.S.; De Wispelaere, L.D.; Delzenne, N.M. Dietary oligofructose lessens hepatic steatosis, but does not prevent hypertriglyceridemia in obese Zucker rats. J. Nutr. 2000, 130, 1314–1319.
- Agheli, N.; Kabir, M.; Berni-canani, S.; Petitjean, E.; Boussairi, A.; Luo, J.; Bornet, F.; Slama, G.; Rizkalla, S.W. Biochemical and Molecular Roles of Nutrients Plasma Lipids and Fatty Acid Synthase Activity Are Regulated by Short-. J. Nutr. 1998, 128, 1283–1288.
- Delzenne, N.M.; Kok, N.N. Nutritional and Health Benefits of Inulin and Oligofructose. J. Nutr. 1999, 129, 1402S–1406S.
- Khitan, Z.; Kim, D.H. Fructose: A key factor in the development of metabolic syndrome and hypertension. J. Nutr. Metab. 2013, 2013, 682673.
- Ferder, L.; Ferder, M.D.; Inserra, F. The role of high-fructose corn syrup in metabolic syndrome and hypertension. Curr. Hypertens. Rep. 2010, 12, 105–112.
- Basciano, H.; Federico, L.; Adeli, K. Fructose, insulin resistance, and metabolic dyslipidemia. Nutr. Metab. 2005, 2, 5.
- Afroz, R.; Tanvir, E.M.; Zheng, W.H.; Little, P.J. Molecular Pharmacology of Honey Journal of Clinical & Experimental Molecular Pharmacology of Honey. J. Clin. Exp. Pharmacol. 2016, 6, 1–13.
- Erejuwa, O.O.; Nwobodo, N.N.; Akpan, J.L.; Okorie, U.A.; Ezeonu, C.T.; Ezeokpo, B.C.; Nwadike, K.I.; Erhiano, E.; Abdul Wahab, M.S.; Sulaiman, S.A. Nigerian Honey Ameliorates Hyperglycemia and Dyslipidemia in Alloxan-Induced Diabetic Rats. Nutrients 2016, 8, 95.
- Farakla, I.; Koui, E.; Arditi, J.; Papageorgiou, I.; Bartzeliotou, A.; Papadopoulos, G.E.; Mantzou, A.; Papathanasiou, C.; Dracopoulou, M.; Papastamataki, M.; et al. Effect of honey on glucose and insulin concentrations in obese girls. Eur. J. Clin. Invest. 2019, 49, 1–8.
- Agrawal, O.P.; Pachauri, A.; Yadav, H.; Urmila, J.; Goswamy, H.M.; Chapperwal, A.; Bisen, P.S.; Prasad, G.B.K.S. Subjects with impaired glucose tolerance exhibit a high degree of tolerance to honey. J. Med. Food 2007, 10, 473–478.
- Abdulrhman, M.; El-Hefnawy, M.; Hussein, R.; El-Goud, A.A. The glycemic and peak incremental indices of honey, sucrose and glucose in patients with type 1 diabetes mellitus: Effects on C-peptide level-a pilot study. Acta Diabetol. 2011, 48, 89–94.
- Batumalaie, K.; Zaman Safi, S.; Mohd Yusof, K.; Shah Ismail, I.; Devi Sekaran, S.; Qvist, R. Effect of gelam honey on the oxidative stress-induced signaling pathways in pancreatic hamster cells. Int. J. Endocrinol. 2013, 2013, 367312.
- Sánchez-Tapia, M.; Martínez-Medina, J.; Tovar, A.R.; Torres, N. Natural and Artificial Sweeteners and High Fat Diet Modify Differential Taste Receptors, Insulin, and TLR4-Mediated Inflammatory Pathways in Adipose Tissues of Rats. Nutrients 2019, 11, 880.
- Kim, J.-H.; Kang, S.-I.; Shin, H.-S.; Yoon, S.-A.; Kang, S.-W.; Ko, H.-C.; Kim, S.-J. Sasa quelpaertensis and p-coumaric acid attenuate oleic acid-induced lipid accumulation in HepG2 cells. Biosci. Biotechnol. Biochem. 2013, 77, 1595–1598.
- Choi, S.S.; Diehl, A.M. Hepatic triglyceride synthesis and nonalcoholic fatty liver disease. Curr. Opin. Lipidol. 2008, 19, 295–300.
- Shimano, H.; Sato, R. SREBP-regulated lipid metabolism: Convergent physiology-divergent pathophysiology. Nat. Rev. Endocrinol. 2017, 13, 710–730.
- Xie, Q.; Quan, Y.; Liao, D.; Zhang, B.; Pan, X. Reactive oxygen species induce cell death via Akt signaling in rat osteoblast-like cell line ROS 17/2.8. Toxicol. Ind. Health 2013, 31, 1236–1242.
- Chang, J.-J.; Chung, D.-J.; Lee, Y.-J.; Wen, B.-H.; Jao, H.-Y.; Wang, C.-J. Solanum nigrum Polyphenol Extracts Inhibit Hepatic Inflammation, Oxidative Stress, and Lipogenesis in High-Fat-Diet-Treated Mice. J. Agric. Food Chem. 2017, 65, 9255–9265.
- Terzo, S.; Mulè, F.; Amato, A. Honey and obesity-related dysfunctions: A summary on health benefits. J. Nutr. Biochem. 2020, 82, 1–8.
- Sánchez, M.; Galisteo, M.; Vera, R.; Villar, I.C.; Zarzuelo, A.; Tamargo, J.; Pérez-Vizcaíno, F.; Duarte, J. Quercetin downregulates NADPH oxidase, increases eNOS activity and prevents endothelial dysfunction in spontaneously hypertensive rats. J. Hypertens. 2006, 24, 75–84.
- Cogolludo, A.; Frazziano, G.; Briones, A.M.; Cobeño, L.; Moreno, L.; Lodi, F.; Salaices, M.; Tamargo, J.; Perez-Vizcaino, F. The dietary flavonoid quercetin activates BKCa currents in coronary arteries via production of H2O2. Role in vasodilatation. Cardiovasc. Res. 2007, 73, 424–431.
- Kuhlmann, C.R.W.; Schaefer, C.A.; Kosok, C.; Abdallah, Y.; Walther, S.; Lüdders, D.W.; Neumann, T.; Tillmanns, H.; Schäfer, C.; Piper, H.M.; et al. Quercetin-induced induction of the NO/cGMP pathway depends on Ca 2+-activated K+ channel-induced hyperpolarization-mediated Ca2+-entry into cultured human endothelial cells. Planta Med. 2005, 71, 520–524.
- Perez, A.; Gonzalez-Manzano, S.; Jimenez, R.; Perez-Abud, R.; Haro, J.M.; Osuna, A.; Santos-Buelga, C.; Duarte, J.; Perez-Vizcaino, F. The flavonoid quercetin induces acute vasodilator effects in healthy volunteers: Correlation with beta-glucuronidase activity. Pharmacol. Res. 2014, 89, 11–18.
- Shen, Y.; Croft, K.D.; Hodgson, J.M.; Kyle, R.; Lee, I.L.E.; Wang, Y.; Stocker, R.; Ward, N.C. Quercetin and its metabolites improve vessel function by inducing eNOS activity via phosphorylation of AMPK. Biochem. Pharmacol. 2012, 84, 1036–1044.
- Kim, T.H.; Ku, S.K.; Lee, I.C.; Bae, J.S. Anti-inflammatory effects of kaempferol-3-O-sophoroside in human endothelial cells. Inflamm. Res. 2012, 61, 217–224.
