Fermentation is a process of transforming one substance into another, carried out by microorganisms, such as bacteria and fungi, under certain circumstances, and which can occur under aerobic and/or anaerobic conditions.
- apple pomace
- cider
- vinegar
- probiotic beverage
- spirit
- alcoholic fermentation
- malolactic fermentation
1. Introduction
Apple species belong to the genus Malus of the Rosaceae family, and thousands of cultivars are grown all around the world. This is in fact one of the most important economic fruit species, according to the Food Agriculture Organization (FAO). The last available statistics from FAO are for the year 2018 and they report that at worldwide level the area for apple cultivation was 4904 thousand ha with a total production of 86,142 thousand tones, representing a trade value of around 8 billion US$ [1][2][3].
Apples are extensively consumed in all countries around the world, being very popular because of their appreciated taste, juiciness, color, texture and nutritional contribution. Additionally, they have a good preservation capacity, they are available year-round in markets, at relatively low prices and they are seen as a healthy food [4][5][6][7]. Besides being consumed fresh, apples can also be transformed into many different kinds of apple products, according to the processing technology used [1]. Some of these apple products include juices [8][9], dehydrated [10][11][12][13], canned [1][14] or purées [15][16]. Additionally, other apple products are obtained through fermentation processes, such as probiotic fermented apple juices [17][18][19] and cider [20][21][22], or fermented products obtained from apple pomace generated as industrial by-products [23][24][25][26].
Fermentation is a process of transforming one substance into another, carried out by microorganisms, such as bacteria and fungi, under certain circumstances, and which can occur under aerobic and/or anaerobic conditions. The specific product resulting from a certain fermentation process is determined by the type of microorganism, the processing conditions, and the substance in which the fermentation takes place [27][28]. A successful fermentation process depends on four basic points: the microorganism or microorganisms used, the culture medium, the way of conducting the process and the stages of product recovery [29][30][31]. Care must be taken to ensure good compatibility between the microorganism used and the culture medium, in order to promote the conditions for the microorganism to perform the necessary metabolic functions and thus obtain the desired result. If all these factors are adequately controlled, the growth of the microorganisms will be highly effective and in this way the product synthesized will be of good quality and the production yield will be satisfactory. Among the processing conditions that must be controlled for effective fermentation to take place are the pH, temperature, humidity, aeration (in the case of aerobic fermentation), medium bed thickness and agitation speed [32][33][34][35].
Apples are recognized as providing a high amount of bioactive compounds with health promoting benefits. They are major dietary sources of flavonoids, being particularly rich in the flavonol quercetin and its derivatives [36], which are bioactive compounds object of several studies that confirm their antioxidant [37], anti-inflammatory [38] and antimicrobial [39] properties, as well as antidepressive [40] and anticarcinogenic [41][42] effects. Additionally, these compounds also protect against arteriosclerosis [43], diabetes [44], and neurodegenerative [45], cardiovascular [46] and oral [47] diseases [48]. Other studies have demonstrated that apple compounds like phenolic acids [49], flavonols, flavones and anthocyanins [50], triterpenoids [51], pectin and pectic oligosaccharides [52] and apple polysaccharides [53] have a beneficial effect on colorectal cancer and intestinal inflammation [5]. However, the concentrations and type of bioactive molecules present in apples can vary noticeably according to species and cultivar, and depends on the climatic, agronomic, harvest and postharvest conditions as well as food processing operations and storage [36][54].
The operation of fermentation has a strong impact on the product’s properties, promoting a high degree of changes, some of them related with composition and nutritional value, others with organoleptic properties and others related with the effects on the human body, namely by impacting the bioactive compounds or by adding probiotic bacteria. A great deal of fermented food products acquire, through the action of the bacteria present, characteristics that improve their health effects. The study by Peng et al. [17] analyzed the properties of fermented cloudy juices obtained from different apple cultivars, and they observed important changes in the chemical composition, sensory profiles as well as bacterial counts, according to the cultivar. In the work by Roberts et al. [18] it was postulated that fermented apple juice could be successfully used as a functional food, which is particularly suitable for consumers who seek for non-dairy probiotic beverages.
2. Methodology
The methodology that was followed in the elaboration of this review included, in a first step, the selection of the topic to be addressed. For this, a previous search was conducted on the literature to evaluate whether such a review had already been undertaken, which demonstrated that this was a field in which it would be suitable to gather in a review paper the information available scattered around the scientific literature. After establishing the study subjects, a search was conducted on the following scientific databases: Web of Science, Science Direct, Scopus, DOAJ, Medline and Pubmed, by selecting appropriate keywords. Some criteria of inclusion were established for each of the read articles based on the relevance for the particular aspects focused on in this review, with the publication date being as recent as possible.
The bibliographic sources used for this review were analyzed using the software VOSviewer, resulting in the diagram presented in Figure 1, which resulted from the analysis of co-occurrence links between keywords, considering those keywords that appeared at least twice. In Figure 1, the size of the circles and the corresponding label represent the number of keyword occurrences, while the relations between the keywords are given by the proximity of circles/labels, and were established according to the number of sources in which those keywords occurred jointly [55]. The results in Figure 1 indicate that the most relevant keywords were cider (24 occurrences), fermentation (20), volatile compounds (14), polyphenols (13), quercetin (13), humans (13), apple pomace (11) and lactic acid bacteria (10).
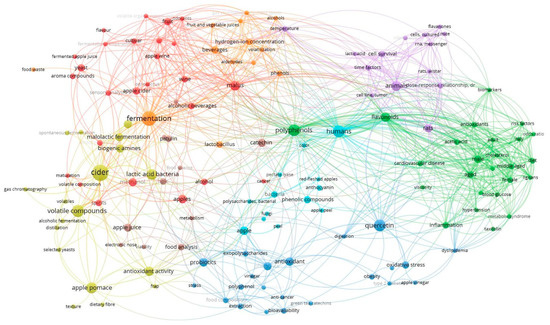
Figure 1. Analysis of co-occurrence links between keywords, selecting those that occurred at least twice.
3. Fermentation Technology Applied to Apple Products
Fermented apple beverages are very common in different countries around the world with some specificities. According to Figure 2, it is possible to produce many different products from apple using a fermentation process. Many of the products can be obtained directly from the fruit or the juice, while others are produced from apple pomace, which is the solid residue obtained after juice, cider, jam and vinegar production. This byproduct can be used as a raw material in many other food products, improving their commercial value and health benefits [56].
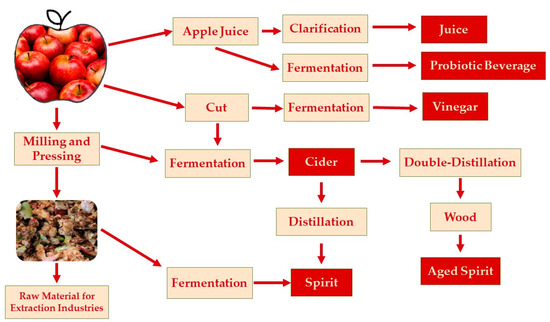
Figure 2. Fermented apple products.
3.1. Apple Pomace
Fruits that do not present good quality for consumption in natura generate large amounts of residues, composed of peel and pulp (95%), seeds (2% to 4%) and stems (1%) [57]. Apple pomace can be used as raw material in other food products, like for example apple pomace spirits [58], or used as a source from which to extract valuable components, such as pectin [59], aroma compounds [60], edible fibers [61][62], and antioxidant polyphenols [63], or to obtain protein-enriched feeds [64], to synthesize pectolytic enzymes [65], or to produce natural aroma compounds by fermentation [66].
The nutritional composition of apple pomace differs according to the fruit variety used in the industrial process and the juice extraction processes applied. Despite the above-mentioned differences, this residue presents high moisture content, high amount of carbohydrates such as cellulose, hemicellulose, lignin and simple sugars (glucose, fructose, and sucrose), small amounts of minerals, proteins, vitamins, besides being a natural source of pectic substances [67][68][69].
Given its nutritional value, apple pomace has been used for fermentation, mainly solid-state fermentation, because it contains all the nutrients necessary for the microorganisms to grow.
Apple pomace extract was used as a carbon source in an aerobic-fed batch process [70] for the production of baker’s yeast. The results of this work showed that the dough-raising capacity of the baker’s yeast grown on the apple pomace extract was kite the same as that of commercial yeast, so, apple pomace extract could be used as a substrate providing carbon for baker’s yeast production.
Wang et al. [71] studied the applicability of apple pomace as a natural stabilizer to increase the consistency and cohesiveness in yoghurt fermented with a mixture of Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus.
Given that apple pomace contains approximately 84.7% carbohydrate, 5.6% starch, and 54.2% total sugar [72], it could also be used in the production of alcoholic beverages or flavorings. Ricci et al. [73] in their studies concluded that the fermented apple pomace could be used as beer flavoring. In this case, the applicability of fermented apple pomace induced a more complex aroma profile, making it a viable option to aromatize alcoholic beverages, such as beer. Cider is mainly produced directly from the apple fermentation, as mentioned above; however, according to Li et al. [74], apple pomace can be added to this beverage as a fruity flavoring. Additionally, Madrera et al. [75] in their study concluded that fermented apple pomace can be used as a natural flavoring in beverages.
Apple pomace is also used to produce alcoholic beverages. The pomace spirits could be produced by fermentation of apple pomace with their indigenous microflora followed by a subsequent distillation [76], or by adding commercial yeasts. Madrera et al. [58] made apple spirits from dry apple pomace and the yeast strains used in this process were Saccharomyces cerevisiae, Hanseniaspora uvarum and wine dry yeast with ß-glucosidase enzyme [58]. The spirits were distilled twice, until an alcohol strength of 60% (v/v). In this case, it is very important to pay attention to the methanol content in the final beverages given the higher amount of pectins in the raw material [77].
3.2. Cider
The specific definition of cider is country dependent. In some countries where the cider production dates back centuries, cider definition and labelling is well defined, while in other countries, such as Eastern European countries, where the latest statistics place cider as the fastest growing market [78], the regulation is still adapting. According to European Cider and Fruit Wine Association, cider may be defined as an alcoholic beverage obtained exclusively by the complete or partial fermentation of fresh or concentrate apple juice. Apple cider alcohol content may vary within the range of 1.2–8.5% of alcohol by volume (ABV), and should maintain the character of the fermented apple juice. On the market there are also reduced-alcohol ciders, such as alcohol-free cider, containing less than 0.5% ABV, and low-alcohol cider, with alcohol content between 0.5 and 1.2% ABV. Among modern ciders, there are flavored ciders (that besides the apple base contain juices of other fruits, extracts and flavorings) and ice ciders (made without the addition of water, sugar or alcohol).
With the worldwide increase in the production of apples, processing the fruit into cider is becoming an important and a promising trend. The processing of apple cider consists of few steps: apple washing, apple sorting, crushing (small pieces of 4-5 mm thickness) and pressing in order to separate the apple juice, followed by clarification and depectinization, yeast inoculation, alcoholic fermentation, nutrient addition for the lactic acid bacteria (LAB), malolactic fermentation, stabilization and maturation (wood ageing, optional). The aroma composition of apple cider differs based on geographical provenance, apple cultivars, apple native bacterial diversity or types of microorganisms used for fermentation, as well as the processing methods applied.
Even though the consecrated cider assortments are the English and French ones, the cider market is continuously changing, and new variants are being developed. Recent studies have tested the use of unconventional apple cultivars such as dessert apples with good results in terms of quality and consumer preference [79][80]. Sparkling cider, a specialty product obtained by the secondary fermentation of ciders in bottles (“Champenoise method”) has been the subject of several studies [81][82][83].
Given the seasonality of apples, as a mainly cost-efficient alternative to fresh apple juice, the concentrated apple juice may be successfully used in cider making. When using concentrated apple juice, better results are obtained when the fermentation medium is supplemented with nutrients [84].
The pre-fermentation treatments made to apple juice prior to fermentation can significantly influence the quality of apple cider [85]. While centrifugation has only a minor effect on the phenolic content of apple juice, the oxygenation of juice strongly contributes to a decrease in all classes of native polyphenols [86], especially of catechins and procyanidins.
A key aspect during the alcoholic fermentation is the optimal consumption of nutrients by the yeast. By defining the optimal moment for biomass removal [87] or by applying the ultrasound-assisted fermentation [88], the degree of nutrient consumption for a high quality apple cider was determined.
Several fermentation yeasts have been tested to evaluate the cider volatile compounds: S. cerevisiae, Saccharomyces uvarum, Torulaspora delbrueckii, Hanseniaspora osmophila, H. uvarum, Starmerella bacillaris and Zygosaccharomyces bailii [89]. Ciders’ discrimination suggested possible strain-specific effects on the aroma fraction. Still, a recent study concluded that fermentation yeast might have little influence on the volatile profile of cider, but phenolic compositions of the juices and ciders might contribute to significant differences in astringency and bitterness sensory perceptions [90].
Apples and pears tend to have lower nutrient levels compared to grapes, which are also consumed by the S. cerevisiae yeasts during the alcoholic fermentation. Nutrient supplementation during malolactic fermentation is required to obtain a balanced apple cider, and consists of inactivating yeast mixtures rich in amino acids, mineral cofactors and vitamins. Those mainly responsible for malolactic fermentation in apple cider are the lactic acid bacteria (LAB)—Lactobacillus spp., Leuconostoc spp., Oenococcus spp. and Pediococcus spp. [91]. Many papers have studied the malolactic fermentation of wine [92][93][94][95][96], while few data are available regarding the LAB performances in cider processing [97][98][99]. Considering the performances of the dominant species involved in cider malolactic processes in the spontaneous cider production, the most adequate for use as a malolactic starter culture in cider production has been proved to be Oenococcus oeni, when compared to Lactobacillus collinoides and Pediococcus parvulus [98]. Good results were also obtained by using O. oeni for the deacidification of hard apple cider via the malolactic fermentation [99] or by the simultaneous malolactic fermentation using O. oeni with S. cerevisiae in the red-fleshed apple cider processing [100].
When aged in wood contact, alcoholic beverages get an improved quality and sensory profile. A study established an optimal dosage of medium toasted French and American oak chips of 4 g/L for 30 days of wood contact [101]. Still, even though it is a common practice, the wood aging of cider is not an intensively studied topic.
3.3. Vinegar
In ancient times, fermentation, namely acetic acid fermentation, was a practice used for food preservation. As apple harvesting regions are extremely large, apple being an easily adapted culture, apple production is overloaded in some parts of the globe. Apple-based processed products typically belong to the canning industry and the beverages industries, both alcoholic and non-alcoholic.
Considering the overproduction of apple cider, this product is often redirected to vinegar processing. Apple cider vinegar is consumed in Western European countries as a functional alcohol-free beverage. Apple vinegar, known as cider vinegar, is made with apple juice or concentrated apple juice via a double fermentation process—alcoholic fermentation followed by acetic fermentation.
Vinegar is highly consumed in the daily diet as a food flavoring agent, as a food preservative and with therapeutic objectives. Recent studies have found that acetic fermentation might improve the nutrients content and functionality. The alcoholic fermentation followed by the acetic acid fermentation change the nutritional profile of a beverage into a more complex one [102][103], even though the bio-accessibility of phenolic antioxidants from vinegar is a matter of great interest being subject of previous studies [104].
The composition is regulated by each state producers, considering mainly acidity, for which the minimum legal limit is 4% (w/v) acetic acid. Based on a traditional procedure, apple cider vinegar is made from fresh, crushed apples, then fermented, and matured in wooden barrels. Both fermentations—alcoholic and acetic—take place in the same barrel, with the spontaneous microflora (yeasts and acetic acid bacteria) contribution. The naturally occurring fermentation takes about 5–6 months to complete the entire fermentation process. The disadvantage in this case is the long process duration, incomplete or interrupted fermentation, and low acetic fermentation yield [105].
The chemical composition of apple cider, the raw material for apple vinegar production, may vary according to cultivar and harvesting areas. The European apple cider cultivars (at the optimal ripened stage) have total soluble solids ranging between 10 and 15 °Brix (average 9–11 °Brix), a titratable acidity of 0.12–0.31% malic acid, tannin content (responsible for the apples astringency) of 37 to 233 mg/100 mL, pectin content of 0.25–0.75% (the main responsible for the body or viscosity of apple juice), pH ranges 3.0–3.8. The optimal ripening stage is extremely important for the apple juice quality because unripe fruits will originate juices having lower total soluble solids and less aroma, a higher content of starch and acids and a bitter or astringent flavor, while overly mature apples will give lower yields due to the difficulty in extraction procedure, and exhibit a sweeter, but flatter flavor [105].
One solution for the increase of apple juice yield could be the use the pectolytic enzymes [106]. In traditional cider processing, a system with hydraulic, roller or pneumatic pressure is used to rupture and compress the cells until the recoverable juice is separated from cellular solids. This procedure presents some difficulties, such as increase of non-sugar solids that might cause haze and color formation. The prolonged process, compared to industrial one, along with the impossibility of total avoidance of air exposure, facilitates the microbial (yeasts, molds and acid tolerant bacteria) growth, causing the shortening of apple juice shelf-life. To avoid these disadvantages, the concentrated apple juice might be used instead [84]. When preparing fermentable juice, the apple concentrate is diluted with water and the mixture is supplemented with nutrients (ammonium phosphate and thiamine) prior to the alcoholic fermentation to support the vitality of yeast. Still, when using freshly pressed apples, different blending procedures can serve to define a specific flavor profile and chemical composition of the apple juice.
Alcoholic fermentation of apple juice is mainly carried out using S. cerevisiae yeast (pure culture inoculation or indigenous yeasts) and other indigenous yeast species such as H. uvarum (anamorph Kloeckera apiculata) that predominate at the beginning of fermentation process and are followed by S. cerevisiae at the end of fermentation, and Dekkera (anamorph Brettanomyces) species during the maturation phase. The final alcohol content varies depending on the initial apple sugar content, fermentation procedure or producer (5–10% ABV). The resulting apple cider is clarified and prepared for acetic fermentation. When cider vinegar is used to inoculate the new batch, one part of the ‘mother vinegar’ is added to five parts of apple cider. Increased acetic fermentation yields are recorded when pure culture of acetic acid bacteria is used. There are many processing methods for vinegar production, but only two are commercially used: the Orléans process—the traditional method for vinegar making, known as the “surface method”, and “submerged culture”, where oxygen is supplied in fermentation. The first method mentioned is recognized to make the best flavored apple cider vinegars, while the second is used to increase the acetic acid production and to decrease the duration of fermentation [107]. Cider vinegar might be stored and matured in wooden barrels in which case the product is impacted with respect to color and wood-derived flavor compounds [105]. Other treatments to assure the products’ stability are applied, such as ultrafiltration, pasteurization and use of chemical stabilization agents (sulphur dioxide, pectin, arabic gum, citric acid, potassium ferrocyanide).
3.4. Apple Spirit
According the European Regulation EC 110/2008 [108], fruit marc spirits is defined as a “drink with an alcoholic strength higher than 37.5% (v/v), and a quantity of volatile substances higher than 200 g per hectoliter of pure alcohol, that could be produced by the distillation of fermented fruit mash, juice or pomace”.
The final quality and aroma of the apple spirit, as well the methanol quantity, is related to the fruit used in the fermentation process, which could be influenced by the fruit variety, their geographical origin, ripening index and storage conditions until further processing [109][110][111].
To make apple spirit, firstly, it is necessary to extract the juice from the fruit, which is then fermented to produce cider. After that, it is necessary to produce a distillation (or double distillation) of the fermented juice. Although it is more usual on the market to find apple spirit without ageing in wood barrels, this process can be considered to improve the final quality of the beverage. Coldea et al. [112] in their study showed that apple spirit ageing for 60 days in wood impacted phenolic and volatile profiles regardless of the final beverage. The same authors concluded that the major volatile compounds were not affected by the ageing process except for 2-butanol, which increased over time. Wood ageing also accentuated some flavor compounds associated with the apple, such as isobutyl-acetate, hexyl-2-methylbutanoate and ethyl-nonaoate.
The most popular apple spirit is “Calvados”, which is produced by a combination of selected fruits with an appropriate sweet, tart and bitter flavor, that are fermented to produce cider. After that, the cider is double-distilled, followed by an ageing processes in wood [113].
The aroma profile of apple spirit is highly influenced by the cider [114]. When this process is longer, the apple distillate develops an aroma with more sweet and spicy characters, higher levels of ethyl acetate, ethyl succinate, ethyl lactate, and volatiles compounds derived from bacterial metabolism, such as 2-butanol, 4-ethylguaiacol, eugenol, and 2-propen-1-ol. Additionally, the yeast species influence the production and aroma of spirits namely in the aromatic composition [58].
To intensify the juice extraction before the fermentation process of apple spirit, some enzymes like pectinase can be added. However, this treatment could increase the methanol content in the beverage [115]. Additionally, if apple pomace was used in the spirit production, the methanol content could increase, given the higher quantities of pectin resulting from the apple seeds and peal. Concerning the legal limits of methanol and the problems with this compound for health, this must be very carefully monitored in the production process [77][116]. The selection of the yeast used in the fermentation processes could be also an important step. Some studies conclude that use of indigenous yeasts could be favorable for a low concentration in methanol in the final product [58]. Another process that could be used to reduce the methanol content of the apple spirit is to perform a pasteurization of fruit prior to alcoholic fermentation [110].
3.5. Probiotic Fermented Apples
Fermentation food technology is mainly used to change the flavor, odor and texture of the vegetables and fruits to increase their nutritional value, preservation capacity and decrease the needs of refrigeration and freezing [117]. Additionally, fermentation of foods could reduce some toxic compounds and produce antimicrobial substances, which increases the safety of the final product [118]. These fermented food products, with beneficial effects on human health, are usually called probiotics.
The aforementioned changes in aroma, flavor and texture are associated with the LAB that promote acidic taste, and are associated with proteolytic and lipolytic activities [119][120].
Ellendersen et al. [121] studied the best conditions in which to develop an apple juice (Gala variety) fermented with Lactobacillus casei. According the same authors, the developed beverage was characterized by a typical apple aroma from the raw material, a caramel color, and an acidic apple taste.
Dimitrovski et al. [122] developed a probiotic beverage made with apple juice and lactic acid bacterium Lactobacillus plantarum PCS 26 as fermentation agent. In this study, free and Ca-alginate-embedded bacteria were studied and authors concluded that apple juice is an appropriate raw material for the preparation of a functional drink with good sensory acceptance and appropriate shelf life.
Like for other food products, the final quality of probiotic fermented apples depends on fruits cultivars, which could influence mainly the taste and aroma of the final beverage [17]. This variation is explained by the fact that different varieties of apple have differing aroma and flavor characteristics due to their different compositions, namely total soluble sugars, organic acids and volatile compounds [123].
This entry is adapted from the peer-reviewed paper 10.3390/pr9020223
References
- Li, Y.; Zhang, X.; Nie, J.; Bacha, S.A.S.; Yan, Z.; Gao, G. Occurrence and co-occurrence of mycotoxins in apple and apple products from China. Food Control. 2020, 118, 107354.
- Zhang, L.; Hu, J.; Han, X.; Li, J.; Gao, Y.; Richards, C.; Zhang, C.; Tian, Y.; Liu, G.; Gul, H.; et al. A high-quality apple genome assembly reveals the association of a retrotransposon and red fruit colour. Nat. Commun. 2019, 10, 1–13.
- Food and Agriculture Organization. FAOSTAT. Available online: (accessed on 5 November 2020).
- Bars-Cortina, D.; Macià, A.; Iglesias, I.; Garanto, X.; Badiella, L.; Motilva, M.J. Seasonal Variability of the Phytochemical Composition of New Red-Fleshed Apple Varieties Compared with Traditional and New White-Fleshed Varieties. J. Agric. Food Chem. 2018, 66, 10011–10025.
- Bars-Cortina, D.; Martínez-Bardají, A.; Macià, A.; Motilva, M.J.; Piñol-Felis, C. Consumption evaluation of one apple flesh a day in the initial phases prior to adenoma/adenocarcinoma in an azoxymethane rat colon carcinogenesis model. J. Nutr. Biochem. 2020, 83, 108418.
- Mohebbi, S.; Babalar, M.; Zamani, Z.; Askari, M.A. Influence of early season boron spraying and postharvest calcium dip treatment on cell-wall degrading enzymes and fruit firmness in ‘Starking Delicious’ apple during storage. Sci. Hortic. 2020, 259, 108822.
- Bílková, A.; Baďurová, K.; Svobodová, P.; Vávra, R.; Jakubec, P.; Chocholouš, P.; Švec, F.; Sklenářová, H. Content of major phenolic compounds in apples: Benefits of ultra-low oxygen conditions in long-term storage. J. Food Compos. Anal. 2020, 92, 103587.
- Pruksasri, S.; Lanner, B.; Novalin, S. Nanofiltration as a potential process for the reduction of sugar in apple juices on an industrial scale. LWT 2020, 133, 110118.
- Sauceda-Gálvez, J.; Codina-Torrella, I.; Martinez-Garcia, M.; Hernández-Herrero, M.; Gervilla, R.; Roig-Sagués, A. Combined effects of ultra-high pressure homogenization and short-wave ultraviolet radiation on the properties of cloudy apple juice. LWT 2021, 136, 110286.
- Kidoń, M.; Grabowska, J. Bioactive compounds, antioxidant activity, and sensory qualities of red-fleshed apples dried by different methods. LWT 2021, 136, 110302.
- Feng, L.; Xu, Y.; Xiao, Y.; Song, J.; Li, D.; Zhang, Z.; Liu, C.; Liu, C.; Jiang, N.; Zhang, M.; et al. Effects of pre-drying treatments combined with explosion puffing drying on the physicochemical properties, antioxidant activities and flavor characteristics of apples. Food Chem. 2021, 338, 128015.
- Cruz, A.C.; Guiné, R.P.; Gonçalves, J.C. Drying Kinetics and Product Quality for Convective Drying of Apples (cvs. Golden Delicious and Granny Smith). Int. J. Fruit Sci. 2015, 15, 54–78.
- Guiné, R.P.; Cruz, A.C.; Mendes, M. Convective Drying of Apples: Kinetic Study, Evaluation of Mass Transfer Properties and Data Analysis using Artificial Neural Networks. Int. J. Food Eng. 2014, 10, 281–299.
- Dobiáš, J.; Voldřich, M.; Čurda, D. Heating of canned fruits and vegetables: Deaeration and texture changes. J. Food Eng. 2006, 77, 421–425.
- Lan, W.; Bureau, S.; Chen, S.; Leca, A.; Renard, C.M.; Jaillais, B. Visible, near- and mid-infrared spectroscopy coupled with an innovative chemometric strategy to control apple puree quality. Food Control. 2021, 120, 107546.
- Lan, W.; Jaillais, B.; Leca, A.; Renard, C.M.; Bureau, S. A new application of NIR spectroscopy to describe and predict purees quality from the non-destructive apple measurements. Food Chem. 2020, 310, 125944.
- Peng, W.; Meng, D.; Yue, T.; Wang, Z.; Gao, Z. Effect of the apple cultivar on cloudy apple juice fermented by a mixture of Lactobacillus acidophilus, Lactobacillus plantarum, and Lactobacillus fermentum. Food Chem. 2020, 340, 127922.
- Roberts, D.; Reyes, V.; Bonilla, F.; Dzandu, B.; Liu, C.; Chouljenko, A.; Sathivel, S. Viability of Lactobacillus plantarum NCIMB 8826 in fermented apple juice under simulated gastric and intestinal conditions. LWT 2018, 97, 144–150.
- Chen, C.; Lu, Y.; Yu, H.; Chen, Z.; Tian, H. Influence of 4 lactic acid bacteria on the flavor profile of fermented apple juice. Food Biosci. 2019, 27, 30–36.
- Sousa, A.; Vareda, J.; Pereira, R.; Silva, C.; Câmara, J.S.; Câmara, J.S.; Perestrelo, R. Geographical differentiation of apple ciders based on volatile fingerprint. Food Res. Int. 2020, 137, 109550.
- Lobo, A.P.; Bedriñana, R.P.; Madrera, R.R.; Valles, B.S. Aromatic, olfactometric and consumer description of sweet ciders obtained by cryo-extraction. Food Chem. 2021, 338, 127829.
- Cusano, E.; Cagliani, L.R.; Consonni, R.; Simonato, B.; Zapparoli, G. NMR-based metabolic profiling of different yeast fermented apple juices. LWT 2020, 118, 108771.
- Bortolini, D.G.; Benvenutti, L.; Demiate, I.M.; Nogueira, A.; Alberti, A.; Zielinski, A.A.F. A new approach to the use of apple pomace in cider making for the recovery of phenolic compounds. LWT 2020, 126, 109316.
- Madrera, R.R.; Bedriñana, R.P.; Valles, B.S. Enhancement of the nutritional properties of apple pomace by fermentation with autochthonous yeasts. LWT-Food Sci. Technol. 2017, 79, 27–33.
- Jin, Q.; Qureshi, N.; Wang, H.; Huang, H. Acetone-butanol-ethanol (ABE) fermentation of soluble and hydrolyzed sugars in apple pomace by Clostridium beijerinckii P. Fuel 2019, 244, 536–544.
- Zheng, Z.; Shetty, K. Enhancement of pea (Pisum sativum) seedling vigour and associated phenolic content by extracts of apple pomace fermented with Trichoderma spp. Process. Biochem. 2000, 36, 79–84.
- Katz, S.E.; Pollan, M. The Art of Fermentation: An In-Depth Exploration of Essential Concepts and Processes from around the World; Worth.it: White River Junction, VT, USA, 2012.
- Couto, S.R.; Sanromán, M. Ángeles Application of solid-state fermentation to food industry—A review. J. Food Eng. 2006, 76, 291–302.
- García, C.; Rendueles, M.; Díaz, L.A.G. Liquid-phase food fermentations with microbial consortia involving lactic acid bacteria: A review. Food Res. Int. 2019, 119, 207–220.
- Garro, M.S.; Rivas, F.P.; Garro, O.A. 3.10 - Solid State Fermentation in Food Processing: Advances in Reactor Design and Novel Applications. In Innovative Food Processing Technologies; Knoerzer, K., Muthukumarappan, K., Eds.; Elsevier: Oxford, UK, 2021; pp. 165–182.
- Hassan, A.; Nelson, B. Invited review: Anaerobic fermentation of dairy food wastewater. J. Dairy Sci. 2012, 95, 6188–6203.
- Reihani, S.F.S.; Khosravi-Darani, K. Influencing factors on single-cell protein production by submerged fermentation: A review. Electron. J. Biotechnol. 2019, 37, 34–40.
- Gavala, H.N.; Gavala, H.N.; Skiadas, I.V. Reactor systems for syngas fermentation processes: A review. Chem. Eng. J. 2018, 348, 732–744.
- Zhou, M.; Yan, B.; Wong, J.; Zhang, Y. Enhanced volatile fatty acids production from anaerobic fermentation of food waste: A mini-review focusing on acidogenic metabolic pathways. Bioresour. Technol. 2018, 248, 68–78.
- Peris, M.; Escuder-Gilabert, L. On-line monitoring of food fermentation processes using electronic noses and electronic tongues: A review. Anal. Chim. Acta 2013, 804, 29–36.
- Herranz, B.; Fernández-Jalao, I.; Álvarez, M.D.; Quiles, A.; Sánchez-Moreno, C.; Hernando, I.; De Ancos, B. Phenolic compounds, microstructure and viscosity of onion and apple products subjected to in vitro gastrointestinal digestion. Innov. Food Sci. Emerg. Technol. 2019, 51, 114–125.
- Wang, D.; Jiang, Y.; Sun-Waterhouse, D.-X.; Zhai, H.; Guan, H.; Rong, X.; Li, F.; Yu, J.-C.; Li, D. MicroRNA-based regulatory mechanisms underlying the synergistic antioxidant action of quercetin and catechin in H2O2-stimulated HepG2 cells: Roles of BACH1 in Nrf2-dependent pathways. Free. Radic. Biol. Med. 2020, 153, 122–131.
- Tian, C.; Liu, X.; Chang, Y.; Wang, R.; Lv, T.; Cui, C.; Liu, M. Investigation of the anti-inflammatory and antioxidant activities of luteolin, kaempferol, apigenin and quercetin. South Afr. J. Bot. 2021, 137, 257–264.
- Yong, H.; Bai, R.; Bi, F.; Liu, J.; Qin, Y.; Liu, J. Synthesis, characterization, antioxidant and antimicrobial activities of starch aldehyde-quercetin conjugate. Int. J. Biol. Macromol. 2020, 156, 462–470.
- Dimpfel, W. Rat electropharmacograms of the flavonoids rutin and quercetin in comparison to those of moclobemide and clinically used reference drugs suggest antidepressive and/or neuroprotective action. Phytomedicine 2009, 16, 287–294.
- Li, S.; Pei, Y.; Wang, W.; Liu, F.; Zheng, K.; Zhang, X. Quercetin suppresses the proliferation and metastasis of metastatic osteosarcoma cells by inhibiting parathyroid hormone receptor. Biomed. Pharmacother. 2019, 114, 108839.
- Imran, M.; Iqubal, M.K.; Imtiyaz, K.; Saleem, S.; Mittal, S.; Rizvi, M.M.A.; Ali, J.; Baboota, S. Topical nanostructured lipid carrier gel of quercetin and resveratrol: Formulation, optimization, in vitro and ex vivo study for the treatment of skin cancer. Int. J. Pharm. 2020, 587, 119705.
- Ishizawa, K.; Yoshizumi, M.; Kawai, Y.; Terao, J.; Kihira, Y.; Ikeda, Y.; Tomita, S.; Minakuchi, K.; Tsuchiya, K.; Tamaki, T. Pharmacology in Health Food: Metabolism of Quercetin In Vivo and Its Protective Effect Against Arteriosclerosis. J. Pharmacol. Sci. 2011, 115, 466–470.
- Ebrahimpour, S.; Zakeri, M.; Esmaeili, A. Crosstalk between obesity, diabetes, and alzheimer’s disease: Introducing quercetin as an effective triple herbal medicine. Ageing Res. Rev. 2020, 62, 101095.
- Sahoo, P.K.; Pradhan, L.K.; Aparna, S.; Agarwal, K.; Banerjee, A.; Das, S.K. Quercetin abrogates bisphenol A induced altered neurobehavioral response and oxidative stress in zebrafish by modulating brain antioxidant defence system. Environ. Toxicol. Pharmacol. 2020, 80, 103483.
- DiNicolantonio, J.J.; Mccarty, M.F. Targeting Casein kinase 2 with quercetin or enzymatically modified isoquercitrin as a strategy for boosting the type 1 interferon response to viruses and promoting cardiovascular health. Med. Hypotheses 2020, 142, 109800.
- Wang, Y.; Tao, B.; Wan, Y.; Sun, Y.; Wangab, L.; Sun, J.; Li, C. Drug delivery based pharmacological enhancement and current insights of quercetin with therapeutic potential against oral diseases. Biomed. Pharmacother. 2020, 128, 110372.
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337.
- Veeriah, S.; Miene, C.; Habermann, N.; Hofmann, T.; Klenow, S.; Sauer, J.; Böhmer, F.; Wölfl, S.; Pool-Zobel, B.L. Apple polyphenols modulate expression of selected genes related to toxicological defence and stress response in human colon adenoma cells. Int. J. Cancer 2008, 122, 2647–2655.
- Chang, H.; Lei, L.; Zhou, Y.; Ye, F.; Zhao, G. Dietary Flavonoids and the Risk of Colorectal Cancer: An Updated Meta-Analysis of Epidemiological Studies. Nutrients 2018, 10, 950.
- He, X.; Wang, Y.; Hu, H.; Zhang, Z. In Vitro and in Vivo Antimammary Tumor Activities and Mechanisms of the Apple Total Triterpenoids. J. Agric. Food Chem. 2012, 60, 9430–9436.
- Chung, W.S.F.; Meijerink, M.; Zeuner, B.; Holck, J.; Louis, P.; Meyer, A.S.; Wells, J.M.; Flint, H.J.; Duncan, S.H. Prebiotic potential of pectin and pectic oligosaccharides to promote anti-inflammatory commensal bacteria in the human colon. FEMS Microbiol. Ecol. 2017, 93.
- Li, Y.; Wang, S.; Sun, Y.; Xu, W.; Zheng, H.; Wang, Y.; Tang, Y.; Gao, X.; Song, C.; Long, Y.; et al. Apple polysaccharide protects ICR mice against colitis associated colorectal cancer through the regulation of microbial dysbiosis. Carbohydr. Polym. 2020, 230, 115726.
- Williams, D.J.; Edwards, D.; Hamernig, I.; Jian, L.; James, A.P.; Johnson, S.K.; Tapsell, L.C. Vegetables containing phytochemicals with potential anti-obesity properties: A review. Food Res. Int. 2013, 52, 323–333.
- Guiné, R.P.; Florença, S.G.; Barroca, M.J.; Anjos, O. The duality of innovation and food development versus purely traditional foods. Trends Food Sci. Technol. 2021, 109, 16–24.
- Lyu, F.; Luiz, S.F.; Azeredo, D.R.P.; Cruz, A.G.; Ajlouni, S.; Ranadheera, C.S. Apple Pomace as a Functional and Healthy Ingredient in Food Products: A Review. Processes 2020, 8, 319.
- Perussello, A.C.; Zhang, Z.; Marzocchella, A.; Tiwari, B.K. Valorization of Apple Pomace by Extraction of Valuable Compounds. Compr. Rev. Food Sci. Food Saf. 2017, 16, 776–796.
- Roberto, R.M.; Bedriñana, R.P.; Hevia, A.G.; Arce, M.B.; Valles, B.S. Production of spirits from dry apple pomace and selected yeasts. Food Bioprod. Process. 2013, 91, 623–631.
- May, C.D. Industrial pectins: Sources, production and applications. Carbohydr. Polym. 1990, 12, 79–99.
- Medeiros, A.B.; Pandey, A.; Freitas, R.J.; Christen, P.; Soccol, C.R. Optimization of the production of aroma compounds by Kluyveromyces marxianus in solid-state fermentation using factorial design and response surface methodology. Biochem. Eng. J. 2000, 6, 33–39.
- Grigelmo-Miguel, N.; Martín-Belloso, O. Comparison of Dietary Fibre from By-products of Processing Fruits and Greens and from Cereals. LWT Food Sci. Technol. 1999, 32, 503–508.
- Masoodi, F.; Sharma, B.; Chauhan, G. Use of apple pomace as a source of dietary fiber in cakes. Plant Foods Hum. Nutr. 2002, 57, 121–128.
- García, Y.D.; Valles, B.S.; Lobo, A.P. Phenolic and antioxidant composition of by-products from the cider industry: Apple pomace. Food Chem. 2009, 117, 731–738.
- Alibés, X.; Muñoz, F.; Rodriguez, J. Feeding value of apple pomace silage for sheep. Anim. Feed. Sci. Technol. 1984, 11, 189–197.
- Berovič, M.; Ostroveršnik, H. Production of Aspergillus niger pectolytic enzymes by solid state bioprocessing of apple pomace. J. Biotechnol. 1997, 53, 47–53.
- Daigle, P.; Gélinas, P.; Leblanc, D.; Morin, A. Production of aroma compounds by Geotrichum candidum on waste bread crumb. Food Microbiol. 1999, 16, 517–522.
- Villas-Boas, S.G.; Esposito, E.; De Mendonça, M.M. Novel lignocellulolytic ability of Candida utilis during solid-substrate cultivation on apple pomace. World J. Microbiol. Biotechnol. 2002, 18, 541–545.
- Jin, H.; Kim, H.-S.; Kim, S.-K.; Shin, M.-K.; Kim, J.-H.; Lee, J.-W. Production of heteropolysaccharide-7 by Beijerinckia indica from agro-industrial byproducts. Enzym. Microb. Technol. 2002, 30, 822–827.
- Canteri-Schemin, M.H.; Fertonani, H.C.R.; Waszczynskyj, N.; Wosiacki, G. Extraction of pectin from apple pomace. Braz. Arch. Biol. Technol. 2005, 48, 259–266.
- Bhushan, S.; Joshi, V. Baker’s Yeast Production under Fed Batch Culture from Apple Pomace. J. Sci. Ind. Res. 2006, 65, 72–76.
- Wang, X.; Kristo, E.; Lapointe, G. The effect of apple pomace on the texture, rheology and microstructure of set type yogurt. Food Hydrocoll. 2019, 91, 83–91.
- Dhillon, G.S.; Kaur, S.; Brar, S.K. Perspective of apple processing wastes as low-cost substrates for bioproduction of high value products: A review. Renew. Sustain. Energy Rev. 2013, 27, 789–805.
- Ricci, A.; Cirlini, M.; Guido, A.; Liberatore, C.M.; Ganino, T.; Lazzi, C.; Chiancone, B. From Byproduct to Resource: Fermented Apple Pomace as Beer Flavoring. Foods 2019, 8, 309.
- Li, S.; Nie, Y.; Ding, Y.; Zhao, J.; Tang, X. Effects of Pure and Mixed Koji Cultures with S accharomyces cerevisiae on Apple Homogenate Cider Fermentation. J. Food Process. Preserv. 2015, 39, 2421–2430.
- Madrera, R.R.; Bedriñana, R.P.; Valles, B.S. Production and characterization of aroma compounds from apple pomace by solid-state fermentation with selected yeasts. LWT 2015, 64, 1342–1353.
- Silva, M.; Macedo, A.; Malcata, F. Review: Steam distilled spirits from fermented grape pomace Revision: Bebidas destiladas obtenidas de la fermentación del orujo de uva. Food Sci. Technol. Int. 2000, 6, 285–300.
- Botelho, G.; Anjos, O.; Estevinho, L.M.; Caldeira, I. Methanol in Grape Derived, Fruit and Honey Spirits: A Critical Review on Source, Quality Control, and Legal Limits. Processes 2020, 8, 1609.
- AICV. European Cider Trends 2019; The European Cider & Fruit Wine Association: Brussels, Belgium, 2019.
- Nicolini, G.; Roman, T.; Carlin, S.; Malacarne, M.; Nardin, T.; Cossignani, L.; Larcher, R. Characterisation of single-variety still ciders produced with dessert apples in the Italian Alps. J. Inst. Brew. 2018, 124, 457–466.
- Ye, M.; Yue, T.; Yuan, Y. Changes in the profile of volatile compounds and amino acids during cider fermentation using dessert variety of apples. Eur. Food Res. Technol. 2014, 239, 67–77.
- Valles, B.S.; Bedriñana, R.P.; Queipo, A.L.; Alonso, J.J.M. Screening of cider yeasts for sparkling cider production (Champenoise method). Food Microbiol. 2008, 25, 690–697.
- Lobo, A.P.; Tascón, N.F.; Roberto, R.M.; Valles, B.S. Sensory and Foaming Properties of Sparkling Cider. J. Agric. Food Chem. 2005, 53, 10051–10056.
- Gomis, D.B.; Tamayo, D.M.; Valles, B.S.; Alonso, J.J.M. Detection of Apple Juice Concentrate in the Manufacture of Natural and Sparkling Cider by Means of HPLC Chemometric Sugar Analyses. J. Agric. Food Chem. 2004, 52, 201–203.
- Rosend, J.; Kaleda, A.; Kuldjärv, R.; Arju, G.; Nisamedtinov, I. The Effect of Apple Juice Concentration on Cider Fermentation and Properties of the Final Product. Foods 2020, 9, 1401.
- Tarko, T.; Duda-Chodak, A.; Sroka, P.; Januszek, M. Effect of Musts Oxygenation at Various Stages of Cider Production on Oenological Parameters, Antioxidant Activity, and Profile of Volatile Cider Compounds. Biomolecules 2020, 10, 890.
- Guyot, S.; Marnet, N.; Sanoner, P.; Drilleau, J.-F. Variability of the Polyphenolic Composition of Cider Apple (Malus domestica) Fruits and Juices. J. Agric. Food Chem. 2003, 51, 6240–6247.
- Nogueira, A.; Mongruel, C.; Simões, D.R.S.; Waszczynskyj, N.; Wosiacki, G. Effect of biomass reduction on the fermentation of cider. Braz. Arch. Biol. Technol. 2007, 50, 1083–1092.
- Al Daccache, M.; Koubaa, M.; Salameh, D.; Maroun, R.G.; Louka, N.; Vorobiev, E. Ultrasound-assisted fermentation for cider production from Lebanese apples. Ultrason. Sonochem. 2020, 63, 104952.
- Lorenzini, M.; Simonato, B.; Slaghenaufi, D.; Ugliano, M.; Zapparoli, G. Assessment of yeasts for apple juice fermentation and production of cider volatile compounds. LWT 2019, 99, 224–230.
- Laaksonen, O.; Kuldjärv, R.; Paalme, T.; Virkki, M.; Yang, B. Impact of apple cultivar, ripening stage, fermentation type and yeast strain on phenolic composition of apple ciders. Food Chem. 2017, 233, 29–37.
- Cousin, F.J.; Le Guellec, R.; Schlusselhuber, M.; Dalmasso, M.; LaPlace, J.M.; Cretenet, M. Microorganisms in Fermented Apple Beverages: Current Knowledge and Future Directions. Microorganisms 2017, 5, 39.
- Abrahamse, C.E.; Bartowsky, E. Timing of malolactic fermentation inoculation in Shiraz grape must and wine: Influence on chemical composition. World J. Microbiol. Biotechnol. 2011, 28, 255–265.
- Sumby, K.M.; Grbin, P.R.; Jiranek, V. Implications of new research and technologies for malolactic fermentation in wine. Appl. Microbiol. Biotechnol. 2014, 98, 8111–8132.
- Sumby, K.M.; Bartle, L.; Grbin, P.R.; Jiranek, V. Measures to improve wine malolactic fermentation. Appl. Microbiol. Biotechnol. 2019, 103, 2033–2051.
- Betteridge, A.; Grbin, P.; Jiranek, V. Improving Oenococcus oeni to overcome challenges of wine malolactic fermentation. Trends Biotechnol. 2015, 33, 547–553.
- Bartowsky, E.; Costello, P.; Chambers, P. Emerging trends in the application of malolactic fermentation. Aust. J. Grape Wine Res. 2015, 21, 663–669.
- Sánchez, A.; Coton, M.; Coton, E.; Herrero, M.; García, L.A.; Díaz, M. Prevalent lactic acid bacteria in cider cellars and efficiency of Oenococcus oeni strains. Food Microbiol. 2012, 32, 32–37.
- Sánchez, A.; Rodríguez, R.; Coton, M.; Coton, E.; Herrero, M.; García, L.A.; Díaz, M. Population dynamics of lactic acid bacteria during spontaneous malolactic fermentation in industrial cider. Food Res. Int. 2010, 43, 2101–2107.
- Reuss, R.; Stratton, J.; Smith, D.; Read, P.; Cuppett, S.; Parkhurst, A. Malolactic Fermentation as a Technique for the Deacidification of Hard Apple Cider. J. Food Sci. 2010, 75, C74–C78.
- Li, C.X.; Zhao, X.H.; Zuo, W.F.; Zhang, T.L.; Zhang, Z.Y.; Chen, X. The effects of simultaneous and sequential inoculation of yeast and autochthonous Oenococcus oeni on the chemical composition of red-fleshed apple cider. LWT 2020, 124, 109184.
- Fan, W.; Xu, Y.; Yu, A. Influence of Oak Chips Geographical Origin, Toast Level, Dosage and Aging Time on Volatile Compounds of Apple Cider. J. Inst. Brew. 2006, 112, 255–263.
- Budak, H.N.; Guzel-Seydim, Z.B. Antioxidant activity and phenolic content of wine vinegars produced by two different techniques. J. Sci. Food Agric. 2010, 90, 2021–2026.
- Mudura, E.; Coldea, T.; Socaciu, C.; Ranga, F.; Pop, C.; Rotar, A.; Pasqualone, A. Brown beer vinegar: A potentially functional product based on its phenolic profile and antioxidant activity. J. Serbian Chem. Soc. 2018, 83, 19–30.
- Bakir, S.; Toydemir, G.; Boyacioglu, D.; Beekwilder, J.; Capanoglu, E. Fruit Antioxidants during Vinegar Processing: Changes in Content and in Vitro Bio-Accessibility. Int. J. Mol. Sci. 2016, 17, 1658.
- Joshi, V.K.; Sharma, S. Cider Vinegar: Microbiology, Technology and QualityVinegars of the World. In Vinegars of the World; Springer: Milan, Italy, 2009; pp. 196–207. ISBN 978-88-470-0866-3.
- Alkorta, I.; Garbisu, C.; Llama, M.J.; Serra, J.L. Industrial applications of pectic enzymes: A review. Process. Biochem. 1998, 33, 21–28.
- Budak, N.H.; Aykin, E.; Seydim, A.C.; Greene, A.K.; Guzel-Seydim, Z.B. Functional Properties of Vinegar. J. Food Sci. 2014, 79, R757–R764.
- EC. Regulation (EC) No 110/2008 of the European Parliament and of the Council of 15 January 2008 on the Definition, Description, Presentation, Labelling and the Protection of Geographical Indications of Spirit Drinks and Repealing Council Regulation (EEC) No 1576/89; European Commission: Brussels, Belgium, 2008.
- Januszek, M.; Satora, P.; Tarko, T. Oenological Characteristics of Fermented Apple Musts and Volatile Profile of Brandies Obtained from Different Apple Cultivars. Biomolecules 2020, 10, 853.
- Hang, Y.D.; Woodams, E.E. Influence of apple cultivar and juice pasteurization on hard cider and eau-de-vie methanol content. Bioresour. Technol. 2010, 101, 1396–1398.
- Versini, G.; Franco, M.; Moser, S.; Barchetti, P.; Manca, G. Characterisation of apple distillates from native varieties of Sardinia island and comparison with other Italian products. Food Chem. 2009, 113, 1176–1183.
- Coldea, T.E.; Socaciu, C.; Mudura, E.; Socaci, S.A.; Ranga, F.; Pop, C.R.; Vriesekoop, F.; Pasqualone, A. Volatile and phenolic profiles of traditional Romanian apple brandy after rapid ageing with different wood chips. Food Chem. 2020, 320, 126643.
- Small, R.W.; Couturier, M.; Godfrey, M. Beverage Basics: Understanding and Appreciating Wine, Beer, and Spirits, 1st ed.; Wiley: Hoboken, NJ, USA, 2011.
- Roberto, R.M.; Lobo, A.P.; Alonso, J.J.M. Effect of cider maturation on the chemical and sensory characteristics of fresh cider spirits. Food Res. Int. 2010, 43, 70–78.
- Zhang, H.; Woodams, E.E.; Hang, Y.D. Influence of pectinase treatment on fruit spirits from apple mash, juice and pomace. Process. Biochem. 2011, 46, 1909–1913.
- Anjos, O.; Santos, A.J.; Estevinho, L.M.; Caldeira, I. FTIR–ATR spectroscopy applied to quality control of grape-derived spirits. Food Chem. 2016, 205, 28–35.
- Iyer, B.K.; Ananthanarayan, L. Effect of α-amylase addition on fermentation of idli—A popular south Indian cereal—Legume-based snack food. LWT 2008, 41, 1053–1059.
- Daeschel, M.A. Antimicrobial Substances from Lactic Acid Bacteria for Use as Food Preservatives. Food Technol. 1989, 43, 164–167.
- Leroy, F.; De Vuyst, L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci. Technol. 2004, 15, 67–78.
- Gomes, A.M.P.; Malcata, F.X. Bifidobacterium spp. and Lactobacillus acidophilus: Biological, biochemical, technological and therapeutical properties relevant for use as probiotics. Trends Food Sci. Technol. 1999, 10, 139–157.
- Ellendersen, L.D.S.N.; Granato, D.; Guergoletto, K.B.; Wosiacki, G. Development and sensory profile of a probiotic beverage from apple fermented with Lactobacillus casei. Eng. Life Sci. 2012, 12, 475–485.
- Dimitrovski, D.; Velickova, E.; Langerholc, T.; Winkelhausen, E. Apple juice as a medium for fermentation by the probiotic Lactobacillus plantarum PCS 26 strain. Ann. Microbiol. 2015, 65, 2161–2170.
- Braga, C.M.; Zielinski, A.A.F.; Da Silva, K.M.; De Souza, F.K.F.; Pietrowski, G.D.A.M.; Couto, M.; Granato, D.; Wosiacki, G.; Nogueira, A. Classification of juices and fermented beverages made from unripe, ripe and senescent apples based on the aromatic profile using chemometrics. Food Chem. 2013, 141, 967–974.
