A biosensor is an integrated receptor-transducer device, which can convert a biological response into an electrical signal.
- biosensors
- nanomaterials
- nanobiosensing
- gold nanoparticles
1. Biosensor
1.1. Design and Principle
A biosensor is a device or probe that integrates a biological element, such as an enzyme or antibody, with an electronic component to generate a measurable signal. The electronic component detects, records, and transmits information regarding a physiological change or the presence of various chemical or biological materials in the environment. Biosensors come in different sizes and shapes and can detect and measure even low concentrations of specific pathogens, or toxic chemicals, and pH levels. A typical biosensor comprises (a) an analyte, (b) bioreceptor, (c) transducer, (d) electronics, and (e) display (Figure 2) [1].
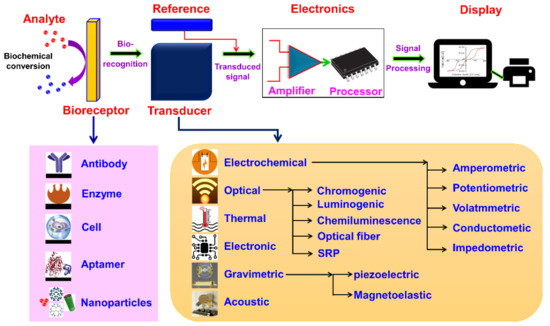
Figure 2. Schematic diagram of typical biosensor consisting of bioreceptor, transducer, electronic system (amplifier and processor), and display (PC or printer) and various types of bioreceptors and transducers used in the biosensors are also shown.
-
(a) Analyte: A substance of interest whose constituents are being identified or detected (e.g., glucose, ammonia, alcohol, and lactose).
-
(b) Bioreceptor: A biomolecule (molecule) or a biological element that can recognize the target substrate (i.e., an analyte) is known as bioreceptor (e.g., enzymes, cells, aptamers, deoxyribonucleic acid (DNA or RNA), and antibodies). The process of signal production (in the form of light, heat, pH, charge or mass change, plant or animal tissue, and microbial products) during the interaction between bioreceptor and analyte is called biorecognition.
-
(c) Transducer: A device that transforms energy from one form to another. The transducer is a key element in a biosensor. It converts the biorecognition event into a measurable signal (electrical) that connects with the quantity or in the presence of a chemical or biological target. This process of energy conversion is known as signalization. Transducers generate either optical or electrical signals proportional to the number of analyte–bioreceptor interactions. According to the operating principle, transducers are broadly categorized as electrochemical, optical, thermal, electronic, and gravimetric transducers.
-
(d) Electronics: The transduced signal is processed and prepared for the display. The electrical signals obtained from the transducer are amplified and converted into digital form. The processed signals are quantified by the display unit.
-
(e) Display: The display unit is composed of a user interpretation system, such as a computer or a printer that generates the output so that the corresponding response can be readable and understandable by the user. Depending on the end-user prerequisite, the output can be in the form of a numerical, graphical, or tabular value, or a figure.
1.2. Evolution of Biosensors
The evolution of biosensors has been classified into three generations based on the attachment of the components, that is, according to the method of integration of the bio-recognition element (bioreceptor) to the transducer. In the first generation (Ist gen), the biosensors measure the content of the analytes and products of the bioreceptor reactions, which diffuse to the surface of the transducer and produce an electric response. This type of sensor is also called mediator-less amperometric biosensors. Leland Charles Clark Jr., the father of biosensors, described components of a biosensor in his first report. This report, published in 1956, was about an electrode that can measure the oxygen concentration in blood [2]. In 1962, Clark experimentally described the employment of an amperometric enzyme electrode for glucose detection [3]. In 1967, Clark’s work was modified by Updike and Hicks, who realized the first functional enzyme electrode-based on glucose oxidase immobilized on an oxygen sensor [4].
In 1969, Guilbault and Montalvo demonstrated and reported the first potentiometric enzyme electrode-based sensor for detecting urea [5]. In 1973, Guilbault and Lubrano described glucose and a lactate enzyme sensor based on hydrogen peroxide detection at a platinum electrode [6]. A heat-sensitive enzyme sensor known as ”thermistor” was developed by the Klaus Mosbach group in 1974 [7]. In 1975, Lubbers and Opitz extended the concept to make an optical biosensor for alcohol [8]. In the second generation (IInd gen), individual components such as auxiliary enzymes and co-reactants (artificial or partially toxic mediators or nanomaterials), are integrated into the biological component layer of the biosensor with the view of enhancing analytical efficiency. These types of sensors are called mediator amperometric biosensors. In 1976, Clemens et al. incorporated an electrochemical glucose biosensor in a “bedside artificial pancreas” [9][10]. VIA Medical introduced a novel semi-continuous catheter-based blood glucose analyzer and, later in 1976, La Roche presented the lactate analyzer LA 640, which was used for electron transport from lactate dehydrogenase to an electrode [11]. In the third generation (IIIrd gen), the bioreceptor molecule becomes an integral part of the base sensing element, that is, biosensors progressed toward employing enzymes and mediators on the same electrode rather than freely diffusing mediators in the electrolyte. A direct interaction was established between the enzymes and electrode through the transfer of electrons, without any requirement of intermediate stages like in nanomaterials. Besides the interaction, low design cost and feasibility of having repeated measurements are the advantages of this generation of biosensor [12]. In 1983, Liedberg identified dependency reactions in real-time using the surface plasmon resonance (SPR) technique [13]. The blood glucose level was measured in 1987 with a pen-sized detector by Cambridge, USA. Figure 3 shows the three generations of the biosensors and Table 1 presents the timeline for the historical development of biosensors.

Figure 3. Three generations of the biosensor construction (MOX: Oxidized mediator; MRed: Reduced mediator).
Table 1. Development of biosensors in different timelines.
| Year | Development of Biosensor |
|---|---|
| 1906 | M. Cramer observed electric potential arising between parts of the fluid [14] |
| 1909 | Soren Sorensen developed the concept of pH and pH scale [15] |
| 1909–1922 | Griffin and Nelson were the first to demonstrate the immobilization of the enzyme invertase on aluminum hydroxide and charcoal [16][17] |
| 1922 | W.S. Hughes discovered a pH measurement electrode [18] |
| 1956 | Leland C. Clark, Jr invented the first oxygen electrode [2] |
| 1962 | Leland C. Clark, Jr et al. experimentally demonstrated an amperometric enzyme electrode for detecting glucose [3] |
| 1967 | Updike and Hicks and realized the first functional enzyme electrode based on glucose oxidase immobilized onto an oxygen sensor [4] |
| 1969 | Guilbault and Montalvo demonstrated and reported the first potentiometric enzyme electrode-based sensor for the detecting urea [5] |
| 1970 | Discovery of ion-sensitive field-effect transistor (ISFET) by Bergveld [19] |
| 1973 | Guilbault and Lubrano defined glucose and a lactate enzyme sensor based on hydrogen peroxide detection at a platinum electrode [6] |
| 1974 | K. Mosbach and B. Danielsson developed enzyme thermistor [7] |
| 1975 | D.W. Lubbers and N. Opitz demonstrated fiber-optic biosensor for carbon dioxide and oxygen detection [8] |
| 1975 | First commercial biosensor for glucose detection by YSI [20][21] |
| 1975 | Suzuki et al. First demonstrated microbe-based immunosensor [22] |
| 1976 | Clemens et al. demonstrated first bedside artificial pancreas [9] |
| 1980 | Peterson demonstrated the first fiber-optic pH sensor for in vivo blood gases [23] |
| 1982 | Fiber-optic biosensor for glucose detection by Schultz [24] |
| 1983 | Liedberg et al. observed surface plasmon resonance (SPR) immunosensor [13] |
| 1983 | Roederer and Bastiaans developed the first immunosensor based on piezoelectric detection [25] |
| 1984 | First mediated amperometric biosensor: ferrocene used with a glucose oxidase for glucose detection [12] |
| 1990 | SPR-based biosensor by Pharmacia Biacore [26] |
| 1992 | Handheld blood biosensor by i-STAT [26] |
| 1999 | Poncharal et al. demonstrated the first nanobiosensor [27] |
| 2018 | S. Girbi et al. demonstrated nerve-on-chip type biosensor for assessment of nerve impulse conduction [28] |
1.3. Characteristics of Biosensors
To develop a highly effective and capable biosensor system, certain static and dynamic requirements are necessary. Based on these specifications, the performance of the biosensors can be optimized for commercial uses [29][30][31].
-
(a) Selectivity: Selectivity is a crucial feature to consider when selecting a bioreceptor for a biosensor. A bioreceptor can detect a particular target analyte molecule in a sample comprised of admixture spices and unwanted contaminants.
(b) Sensitivity: The minimum amount of analyte that can be correctly detected/identified in a minimum number of steps and in low concentrations (ng/mL or fg/mL) to verify the existence of analyte traces in the sample.
(c) Linearity: Linearity contributes to the accuracy of the measured results. The higher the linearity (straight line), the higher the substrate concentration detection.
(d) Response time: The time is taken for obtaining 95% of the results.
(e) Reproducibility: Reproducibility is characterized by precision (similar output when the sample is measured more than once) and accuracy (capability of a sensor to generate a mean value closer to the actual value when the sample is measured every time). It is the ability of the biosensor to produce identical results whenever the same sample is measured more than once.
(f) Stability: Stability is one of the key characteristics in biosensor applications where continuous monitoring is required. Stability is the extent of vulnerability to environmental disturbances inside and outside the biosensing device. The factors that affect stability are the affinity of the bioreceptor (the extent of binding of the analyte to the bioreceptor) and the degradation of the bioreceptor over time.
1.4. Classification of Biosensors
Classification of biosensors is a diverse and multidisciplinary field. Various criteria are involved in the classification of biosensors and the outline classification scheme is shown in Figure 4.

Figure 4. Classification of biosensors based on various bioreceptors and transducers used.
As discussed earlier, bioreceptors are considered as the primary component in biosensor construction. Based on the bioreceptor, biosensors are classified as enzymatic biosensors (most common biosensor class), immunosensors (possess high specificity and sensitivity and are specifically useful in diagnosis), aptamer or nucleic acid-based biosensors (possess high specificity for microbial strains and nucleic acid-containing analyte), and microbial or whole-cell biosensors. The second classification is made on the basis of the transducer and sensors are categorized as electrochemical (which is further grouped as potentiometric, amperometric, impedance and conductometric), electronic biosensor, thermal biosensor, optical, and mass-based or gravimetric. Another classification includes bioreceptor-analyte combinations, which are limited. Some classifications are made depending on the detection system (optical, electrical, electronic, thermal, mechanical, and magnetic) and the technology (nano, surface plasmon resonance (SPR), biosensors-on-chip (lab-on-chip), electrometers, and deployable).
2. Nanomaterial-Based Biosensors (Nanobiosensors)
With advances in nanotechnology, research and development in the field of biosensors have become open and multidisciplinary. Exploring NMs, such as NPs (metal- and oxide-based), NWs, NRs, CNTs, QDs, and nanocomposites (dendrimers), for different characteristics provides the possibility of improving the performance of biosensors and increase the power of detection through size and morphology control. Different types of NMs-based biosensors (nanobiosensors) are shown in Figure 11.
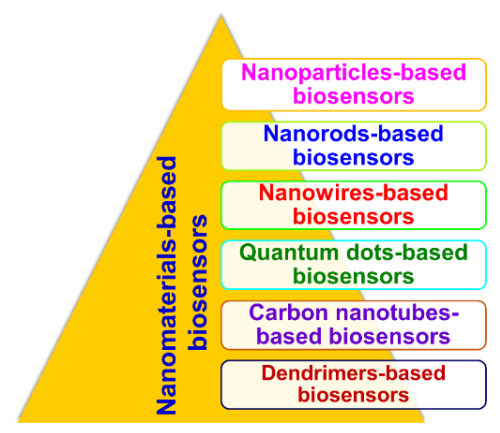
Figure 11. Types of nanomaterials-based biosensors (nanobiosensors).
The basic working principle of nanobiosensors is along the same lines of conventional macro- and micro-counterparts, but they are constructed using nanoscale components for signal or data transformation [32]. Nanobiosensors have an edge over their conventional macro- and micro-counterparts because of their multidisciplinary applications due to dimensionality. Nanobiosensors are instrumental in the field of nanotechnology for (a) monitoring physical and chemical phenomena in regions difficult to reach, (b) detecting biochemicals in cellular organelles and medical diagnosis, (c) measuring nanoscopic particles in industrial areas and the environment, and (d) detecting ultra-low concentrations of potentially harmful substances [32]. Based on the classification of the NMs, their involvement in the enhancement of biosensing mechanisms has been broadly investigated. For instance, NPs-based biosensors include all sensors that employ metallic NPs as enhancers of biochemical signals. Similarly, nanotube-based biosensors, if they involve CNTs, are used as enhancers of reaction specificity and efficiency, while biosensors using NWs as charge transport and carriers are termed as NW biosensors. Likewise, QD-based sensors employ QDs as contrast agents for improving optical responses.
2.1. Nanoparticle-Based Biosensors
NPs have been widely used in various biomedical applications, like in the development of biosensors for health diagnosis, imaging, drug delivery, and therapy, owing to their unique properties. Because of their small size and shape, their physical and chemical properties are strongly influenced by the binding of target biomolecules [33]. These properties of NPs enable them to be exploited for various bioanalytical applications. They are considered suitable for electrode modification in which they increase the sensitivity and specificity of electrochemical catalysis [34]. Moreover, catalytic active NMs, such as transition metal oxides, have been developed as nanoenzymes, which allow providing catalysis of biochemical reactions on biosensors. The NPs include metal and noble metal NPs, such as gold (Au), silver (Ag), platinum (Pt), palladium (Pd), cobalt (Co), iron (Fe), and copper (Cu), and metal oxide NPs (ZnO, TiO2, SnO2 and MnO2), which exhibit excellent optical, electronic, magnetic, chemical, mechanical, and catalytic properties. NP biosensing performance is tailored by coating with various matrices, such as metal oxides, silica network, polymers, graphene, fibers, and dendrimers [35].
Gold NPs, which fall into the class of noble metal NPs, are extensively investigated and used owing to their unique optical, electronic, and physicochemical properties. They are widely used in biomedical research because of the following advantages: Simple synthesis techniques, easier fabrication procedures, greater chemical stability, biocompatibility, vast electrochemical potential range, high catalytic activity, and their nanocomposite forms [36][37]. Wu et al. demonstrated gold NP-based electrochemical sensors for sensitive detection of uranyl in natural water. The developed sensor determined uranyl in the range of 2.4 to 480 µg L−1, and a detection limit of 0.3 µg L−1 was obtained by anodic stripping voltammetry [38]. Luo et al. established a novel “turn–on” fluorescent sensor for detecting Pb2+, based on graphene quantum dots (GQDs) and gold nanoparticles (AuNPs). The designed sensor showed an extremely broad detection range of Pb2+ from 50 nm to 4 µm, with a detection limit of 16.7 nm [39]. Ghasemi et al. demonstrated a novel non-enzymatic glucose sensor based on gold-nickel bimetallic NPs doped aluminosilicate framework prepared from agro-waste material that exhibited a wide linear range for glucose (1–1900 µM) and low limit of detection (0.063 µM) [40].
Silver nanoparticles (Ag NPs) have gained much research interest in biomedical applications due to excellent surface-enhanced Raman scattering (SERS), biocompatibility, high conductivity, amplified electrochemical signals, and catalytic activity [41][42][43]. Rivero et al. demonstrated an optical fiber sensor based on both localized surface plasmon resonance (LSPR) and lossy-mode resonance (LMR) using Ag NPs. The devices showed high sensitivity (0.943 nm per RH %), a large dynamic range (42.4 nm for RH changes between 25% and 70%), and a fast response time (476 ms and 447 ms for rise and fall, respectively). This device can be used to monitor human breathing [44]. Mehdinia et al. developed a multi-functional colorimetric probe for Fe2+, H2O2, and glucose detection based on the Fenton reaction and biosynthesized AgNPs. The low detection limit for Fe2+ was 0.54 µM, for H2O2 was 0.032 µM, and for glucose was 0.29 µM [45].
Over the past decade, owing to their unique electronic and electro-catalytic characteristics, platinum nanoparticles (Pt NPs) have gained much interest in the field of electrochemical biosensors for biomedical applications. The electron transfer process in Pt NPs is affected by material composition, surface reactive environment, crystalline plane, and orientation [43]. Liu et al. fabricated a new Pt NP/a-IGZO-based ammonia sensor showing high sensing response (SR) of 1467 (at 1000 ppm NH3/air, 250 °C and exhibiting fast sensing speed [46]. Dharuman et al. developed a graphitic carbon nitride modified with Pt and zinc oxide NPs for non-enzymatic glucose sensing with a wide linear detection range of 250 μM to 110 mM. It can be reusable four times in whole blood and eight times in blood serum [47].
Palladium nanoparticles (Pd NPs) are another fascinating NP for biomedical applications because of their high catalytic and sensing activities. In addition, palladium (Pd) is much more abundant than Au and Pt metals making it employable in various sensors for sensing applications in a cost-effective way. Pd NPs with controllable size and shape exhibit high electro-catalytic and sensing characteristics for different chemical and biological analytes [45][48]. Ye et al. prepared a Pd/Co-NCNTs exhibited excellent electrocatalytic ability for hydrazine oxidation and showed a high sensitivity of 343.909 μA mM−1 with a low detection limit of 0.007 μM for hydrazine [49]. Swihart et al. developed a unique 3D Pd-decorated crumple reduced graphene oxide ball (Pd-CGB) nanocomposite for hydrogen (H2) detection in air at room temperature. The sensitivity of the sensor is measured for the H2 concentrations (0.0025–2%) with response value, response time, and recovery time of 14.8%, 73 s, and 126 s, respectively, at 2% H2 [50]. Afzali et al. developed a novel sensor based on Pd/CNF/[M3OA]+[NTF2]− modified CPE through a sensitive square-wave voltammetric procedure for the determination of the anticancer drug pemetrexed. A linear concentration was detected in the range of 1.00–35.0 nM with a detection limit of 0.33 nM by square wave voltammetry (SWV) technique [51].
Copper (Cu) has gathered much research interest as a unique sensing material owing to its excellent electrical conductivity, stability, electrocatalytic properties, and low cost compared to noble metals. Recently, Huang et al. prepared and studied the performance of electrochemical glucose sensors based on copper nanoparticles (Cu NPs) loaded on a flexible graphite sheet. The developed sensor exhibited a low detection limit of 1.05 µmol L−1 and high sensitivity of 7254.1 μA mM−1 cm−2, with R2 = 0.9961 from 0.1 to 3.4 mmol L−1 and 3804.5 μA mM−1 cm−2 (R2 = 0.9995) from 3.4 to 5.6 mmol L−1. Cu NPs also exhibited excellent anti-interference properties against sodium chloride, acetaminophenol, ascorbic acid, dopamine, and uric acid, with good reproducibility [52]. Roushani et al. developed a novel sensor for analytical detection of H2O2 based on the incorporation of CuNPs onto an MWCNTs/IL/Chit/Rutin nanocomposite film. The electrochemical performance of the sensor for detecting H2O2 was investigated by cyclic voltammetry and chronoamperometry techniques. The response to H2O2 was linear, in the range of 0.35 μM to 2500 μM, with a detection limit of 0.11 μM [53]. Zhao et al. fabricated a Cu/rGO decorated buckypaper electrode for glucose detection. The constructed device exhibited a linear range of 0.1–2 mM, with a detection limit of 11 µM [54].
Metal Oxide-Based Nanoparticles
Over the last decade, metal oxide-based nanomaterials have been vividly employed in various fields, such as electrochemistry, magnetism, catalysis, and sensors, because of their broad range of electrical, chemical, and physical properties. These oxide-based materials are used as an effective electrocatalyst for sensing various analytes in the field of biology and biomedicine because of their strong electrocatalytic activity, low cost, and high organic capture ability. The most often employed metal oxide nanoparticles include copper oxide (CuO), nickel oxide (NiO), iron oxide (Fe2O3), cobalt oxide (Co3O4), manganese oxide (MnO2), zinc oxide (ZnO), titanium oxide (TiO2), tin oxide (SnO2), cadmium oxide (CdO), molybdenum oxide (MoO3), and cerium oxide (CeO2) [55].
Nickel oxide-based nanoparticles (NiO NPs) exhibit superior electrical, magnetic, optical, thermal, catalytic, and mechanical properties. NiO-based nanostructures have been used as catalysts, thermistors, sensors, and additives, for gases and ceramics. NiO NPs are also p-type semiconductors with a direct bandgap of (3.56 eV) that can exhibit super-paramagnetic, as well as superanti-ferromagnetic properties, depending on their size and oxidation states [56]. Recently, Recently, Duan et al. fabricated high performance FET glucose biosensors based on bimetallic Ni/Cu metal organic frame works. The fabricated device exhibited a low detection limit of 0.51 µM with a linear range of 1 µM–20 mM [57]. Kamyabi et al. fabricated novel electroluminescence (ECL) glucose biosensors based on immobilized glucose oxidase in the cavity of nickel foam modified with NiO NPs. The proposed ECL biosensor showed superior performance toward glucose in 0.1 M phosphate buffer solution (pH 7.4) with a wide linear range (2.7 × 10−9 to 4.5 × 10−3 M) and a low detection limit (5.0 × 10−10 M) [58].
Cobalt oxide-based nanoparticles (Co3O4 NPS) have attracted considerable interest due to their exceptional physical, magnetic, optical, electronic, and chemical properties. They possess promising applications, such as catalysts, solar selective absorber, gas sensors, lithium-ion batteries, supercapacitors, and pigment for glasses, and ceramics, photocatalysis, magnetic material, and chemical sensors. It is a p-type semiconductor with a direct optical band gap of 1.66 and 2.19 eV [59]. Chu et al. developed a screen-printed glutamate biosensor chip using porous Co3O4 nanocubic crystals. The developed biosensor chip exhibited a high sensitivity of 20.12 μA mM−1 cm−2, as well as a wide linear range from 10 to 600 μM and a detection limit of 10 μM [60]. Wazir et al. developed a potentiometric urea biosensor fabricated on glass filter paper through the immobilization of urease enzyme onto cobalt oxide- chitosan nanocomposite. The sensitivity was measured over the concentration range between 1 × 10−4 and 8 × 10−2 M of the urea electrolyte solution, revealing that the fabricated biosensor exhibited good sensing ability with a linear slope curve of 45 mV/decade [61]. Ge et al. constructed a Co3O4-Au polyhedron-based photoelectrochemical (PEC) biosensor for detecting miRNA-141 detection with a linear range of 1 pM to 50 nM, and a detection limit of 0.2 pM [62].
Iron oxide (Fe2O3) and manganese oxide (MnO2)-based NPs are considered the best known magnetic NMs because of their bioanalytical applications and higher electron transfer rates. They are also considered to be promising materials for electrochemical biosensors [63][64]. Phan et al. demonstrated the possibility of using the magneto-reactance effect of a soft ferromagnetic amorphous ribbon with a nanohole-patterned surface to develop a highly sensitive magnetic biosensor for detection and quantification of anticancer drugs tagged to super-paramagnetic NPs [65]. Zhang et al. developed a fast and highly specific LF-NMR biosensor that can directly detect Salmonella, without sample pretreatment, with a detection limit of 2.6 × 104 CFU mL−1 [66]. Stankovic et al. developed a disposable biosensor based on graphene nanoribbons supported with MnO2 NPs. The sensor displayed a detection limit of 0.05 mmol L−1 and a high sensitivity of 56.32 μA mmol−1cm−2 [67].
Other metal oxide-based NPs, such as ZnO, TiO2, SnO2, and MoO3, have recently gained much attention. ZnO based nanoparticles possess good electron transfer rate and thermal/chemical stability, oxidation resistance, biocompatibility, and high conductivity. ZnO is an n-type semiconductor with a wide bandgap energy of 3.37 eV [68][69]. Hjiri et al. prepared a carbon monoxide sensor using ZnO NPs synthesized by the sol-gel technique. The developed gas sensor exhibited a response of 74% toward 80 ppm of CO gas with a response/recovery time of 21 and 70 s, respectively, at 250 °C and high stability with time [70]. SnO2-based NPs have also been used for detecting toxic gases and pesticide sensing applications [71][72]. TiO2-based NPs can be used in electrochemical sensors for medical, biomedical, and pharmaceutical applications. Tereshchenko et al. introduced a novel and simple optical immunosensor to determine Salmonella typhimurium based on TiO2 NPs deposited on a glass substrate with a sensitivity in the range of 103–105 cL mL−1 [73]. Recently, Ravikumar et al. reported on rapid and facile method for detecting H2O2 in chemical reactions using molybdenum oxide (MoO3) NPs [74].
2.2. Quantum Dot-Based Biosensors
QDs are inorganic nanocrystals (NCs), which belong to 0D NMs displaying unique optical properties of broad excitation, narrow size-tunable emission spectra, high photochemical stability, and negligible photo-bleaching [75]. They have been widely used, mainly as alternatives to fluorophores, for developing optical biosensors to detect organic compounds, pharmaceutical analytes, and biomolecules, such as nucleic acids, proteins, amino acids, enzymes, carbohydrates, and neurotransmitters [76]. They have also been employed for the in vivo detection of cancer. They are ideal candidates for multiplexed optical bioanalysis due to their ultra-high sensitivity, high specificity, cost-effectiveness, miniaturized size, size-dependent emission wavelength, and rapid analyte detection [77]. Cui et al. proposed a simple and efficient electrochemical sensor for Cu (II) based on GQDs and graphene. The combination of GQDs and graphene-enhanced the performance, with a low detection limit of 1.34 nM in a wide linear range of 0.015 μM to 8.775 μM for Cu (II) [78]. Packirisamy et al. demonstrated the fabrication and application of fluorescent turn-on biosensors for ultrasensitive detection of small cell lung cancer biomarkers using biofunctionalized GQDs as the energy donor and AuNPs as energy acceptor. The fluorescent biosensor exhibited a fast response time (16 min), and broader linear detection range (0.1 pg mL−1 to 1000 ng mL−1), and low detection limit of 0.09 pg mL−1 [79]. Xiao et al. demonstrated CdTe/CdS/ZnS core/shell/shell QDs-based fluorescent biosensors for the determination of L-ascorbic acid. The concentration was detected in the linear range of 8.0 × 10−9 to 1.0 × 10−7 M with a detection limit of 1.8 × 10−9 M [80]. Sun et al. described a “turn-on” magnetic fluorescent biosensor based on GQDs, Fe3O4, and molybdenum disulfide (MoS2) nanosheets for detecting EpCAM in the linear range between 2 and 64 nM with a detection limit of 1.19 nM. It is used for rapid, efficient, and sensitive separation and detection of circulating tumor cells (CTCs) [81].
2.3. Nanowire-Based Biosensors
NWs provide favorable conditions for creating robust, sensitive, and selective electrical detectors of biological binding events. The NWs exhibit highly reproducible optical and electrical characteristics. Current flow in any 1D systems, such as NWs and NTs is extremely sensitive to minor perturbations because, in such systems, flow is extremely close to the surface [82]. The diameter of the NWs are comparable to the biological and chemical species that are being sensed. They offer excellent transduction generating signals, which ultimately interface to macroscopic instruments. The combination of tunable conducting properties of semiconducting NWs and the ability to bind analytes on their surface yields a direct, label-free electrical readout [83]. NWs-based sensors operate on the principle of ion-selective FETs and rely on the interaction of external charges with carriers in the nearby semiconductor, which results in enhanced sensitivity at low ionic strength. Park et al. fabricated fiber optic sensor using ZnO NWs and AuNPs for highly sensitive plasmonic biosensing [84]. Priolo et al. demonstrated label-free and PCR-free silicon NWs-based optical biosensor for direct genome detection. They exhibited a detection limit of 2 copies per reaction for the synthetic genome and 20 copies per reaction for the genome extracted from human blood [85]. Nuzaihan et al. prepared a silicon NW-based biosensor with novel molecular gate control for electrical detection of Dengue virus (DENV) DNA. The developed sensor had a low detection limit of 2.0 fM concentration with high sensitivity of 45.0 μA M−1 [86].
2.4. Nanorods-Based Biosensors
Nanorods are often used as simple electrochemical modifiers providing a highly specific process. They are usually prepared from gold, graphene, manganese, zinc, or iron oxide, or the combination of these materials [87][88]. The detection of nucleic acid or basic biochemical markers, such as glucose and hydrogen peroxide, are their most common applications. Liu et al. constructed a new CDs/Au NR assembly-based FRET sensor for detecting lead ions. A linear range from 0 to 155 μM, with a detection limit of 0.05 μM [89]. Zhu et al. demonstrated a ZnO NRs-based FET biosensor for continuous glucose monitoring using AC frequency mixing. The fabricated sensor achieved a high sensitivity of 1.6 mA (μM−1 cm−2) with a concentration detection limit of 1 μM, and exhibited an excellent long-term stability on continuous monitoring for 38 h [90]. Sun et al. used GNRs and graphene oxide (GO) to enhance the sensitivity of a wavelength modulation SPR biosensor to detect bovine IgG. The developed biosensor based on GNRs/GO sensing had detected bovine IgG in the concentration range of 0.075–40.0 µg m L−1 [91]. Hahn et al. fabricated a vertically grown ZnO NRs-based FET biosensor to detect phosphate with high sensitivity (80.57 μA mM−1 cm−2) in a wide linear range (0.1 µM–7.0 mM) [92].
2.5. Carbon Nanotubes-Based Biosensors
CNTs are exciting 1D NMs and are the most extensively investigated nanotubes class of materials in biosensors, diagnostics, tissue engineering, cell tracking and labeling, drug delivery, and biomolecules. They are hollow cylindrical tubes composed of one, two, or several concentric graphite layers capped by fullerenic hemispheres, which are referred to as single-, double-, and multi-walled CNTs, respectively. They have unique structures, excellent electrical and mechanical properties, high thermal conductivity, high chemical stability, remarkable electrocatalytic activity, minimal surface fouling, low over-voltage, and high aspect ratio (surface-to-volume) [93][94][95]. Because of their high surface-to-volume ratio and novel electron transport properties, the electronic conductance of theses nanostructures is strongly influenced by minor surface perturbations, such as those associated with the binding of macromolecules. CNT-based biosensors and diagnostics have been employed for the highly sensitive detection of analytes in healthcare, industries, environmental monitoring, and food quality analysis. They have been predominantly used in electrochemical sensing, for glucose monitoring, but also for detecting fructose, galactose, neurotransmitters, neurochemicals, amino acids, immunoglobulin, albumin, streptavidin, insulin, human chorionic gonadotropin, C-reactive protein, cancer biomarkers, cells, microorganisms, DNA, and other biomolecules. Multi-wall-carbon nanotubes (MWCNTs) are represented in all applications of nanotubes in biosensing. Such 1D nanomaterials provide real-time and sensitive label-free bioelectronic detection and massive redundancy in nanosensor arrays [75]. Cui et al. developed a wearable-based amperometric biosensor painted onto gloves as a new sensing platform used to determine lactate [96]. Janssen et al. demonstrated a CNT-based biosensor to sense a standard protein, bovine serum albumin (BSA), as a proof-of-concept. The developed sensor had a detection limit of 2.89 ng mL−1 [97]. Tang et al. fabricated a single-walled carbon nanotube (SWNT)-based DNA sensors and described the sensing mechanism. This work demonstrated clear experimental evidence on SWNT-DNA binding on DNA functionality, which paved a path for the future design of SWNT biocomplexes for applications in biotechnology and DNA-based nanotube manipulation techniques [98]. Hong et al. constructed metallic floating electrode-based DNA sensors with controllable responses. They showed the enhancement in the sensor response on increasing the number of floating electrodes [99]. Park et al. demonstrated a CNT-based biosensor system-on-a-chip for detecting a neurotransmitter. Here, CNT-based sensors were integrated with CMOS chips, which is useful in various biomedical applications, such as sensing components in LoC (lab-on-a-chip) systems for neuronal culture [100].
2.6. Dendrimer-Based Biosensors
Dendrimers are nanometer-scale 3D macromolecules in the size of an average protein-, and are hyper-branched, mono-dispersed, and star-shaped, with a high density of surface functional groups. The shape of dendrimers provides a vast surface area for the conjugation of biologically active molecules. They are composed of three distinct components: The core, the interior dendron, and the exterior surface with terminal functional groups [101][102]. They have been used extensively in various biosensors, diagnostics, and drug delivery based on electrochemistry, fluorescence, SERC, impedimentary, and SPR. Dendrimer-based biosensors increase analytical sensitivity, stability, and reproducibility but reduce no specific interactions [103][104][105]. Bakar et al. detected dengue using a PAMAM dendrimer integrated tapered optical fiber sensor. The resolution and detection limit of the sensor were 19.53 nM−1 and 1 pM, respectively, in the concentration range of 0.1 pM to 1 µM [106]. Fen et al. developed an SPR sensor based on self-assembled monolayer/reduced graphene oxide-polyamidoamine dendrimer (SAM/NH2rGO/PAMAM) thin films to detect DENV-2 E-proteins. Their SPR sensor exhibited a detection limit of 0.08 pM DENV-2E-proteins in the range of 0.08 pM–0.5 pM [107]. Table 2 represents the list of various nanomaterials employed in the development of biosensors.
Table 2. Represents the list of various nanomaterials employed in the development of biosensors.
| Nanomaterial | Analyte | Transducer | Linear Range | Detection Limit | References |
|---|---|---|---|---|---|
| Au NBPs | Aflatoxin B1 (AFB1) Aflatoxin B1 (AFB1) |
SPR Impedimetric |
0.1–500 nM 0.1–25 nM |
0.4 nM 0.1 nM |
[108] |
| Au NPs | Uranyl | Electrochemical | 2.4–480 µg L−1 | 0.3 µg L−1 | [38] |
| Au NPs | Pb2+ | Fluorescent | 50 nm–4 µm | 16.7 nm | [39] |
| Au/CdS QDs/TNTs | Cholesterol H2O2 |
Electrochemical Electrochemical |
0.024–1.2 mM 18.73–355.87 µm |
0.012 µM 0.06 µM |
[109] |
| Au NPs | E.coli | Electrochemical | 10–106 CFU mL−1 | 15 CFU mL−1 | [110] |
| Au NP-MoS2-rGO | Carcinoembryonic antigen (CEA) | SAW | 36.58 ng mL−1 | 0.084 ng mL−1 | [111] |
| Au/rGO | miENA-122 | Electrochemical | 10 µm–10 pm | 1.73 pM | [112] |
| Au NPs/TiO2 | H2O2 | Electrochemical | 65–1600 µm | 5 µm | [113] |
| Ag NPs | H2O2 Glucose Fe2+ |
Colorimetric | 0.05–7.5 µm 1.5–3.0 µm 1–90 µm |
0.032 µm 0.29 µm 0.54 µm |
[45] |
| Ag/Pd NPs | Ractopamine Clenbuterol Salbutamol |
Electrochemical | 0.01–100 ng mL−1 0.01–100 ng mL−1 0.01–100 ng mL−1 |
1.52 pg mL−1 1.44 pg mL−1 1.38 pg mL−1 |
[114] |
| Ag@CQDs-rGO | Dopamine | Electrochemical | 0.1–300 µm | 0.59 nm | [115] |
| Ag NP-MWNT | Glucose | Electrochemical | 0.025–1.0 mM | 0.01 mM | [116] |
| Ag NPs | Mucin 1 | Electro-chemiluminescence | 1.135 fg mL−1–0.1135 ng mL−1 | 0.37 fg mL−1 | [117] |
| Pt NPs | Adrenaline | Voltammetric | 9.99 × 10−1–2.13 × 10−4 mol L−1 | 2.93 × 10−4 mol L−1 | [118] |
| Pt NPs/RGO-CS-Fc | H2O2 | Electrochemical | 2.0 × 10−8 M–3.0 × 10−8 M | 20 nm | [119] |
| Pt-Fe3O4@C | Sarcosine | Amperometric | 0.5–60 µm | 0.43 µm | [120] |
| Pt NFs/PANi | Urea | Cyclic Voltammetry | 20 mM | 10 µm | [121] |
| Pt@CeO2 NM | Dopamine | Electrochemical | 2–180 nM | 0.71 nM | [122] |
| Pd/Co-NCNT | Hydrazin | Electrochemical | 0.05–406.045 µm | 0.007 µm | [49] |
| Pd/CNF/[M3OA]+[NTF2]− | H2 | 1.00–35.0 nM | 0.33 nM | [51] | |
| Cu NPs/Rutin/MWCNTs/IL/Chit/GCE | H2O2 | Cyclic Voltammetry | 0.35–2500 µM | 0.11 µm | [53] |
| Cu/rGO-BP | Glucose | Electrochemical | 0.1–2 mM | 11 µm | [54] |
| Cu2O@CeO2-Au | PSA | Amperometric | 0.03 pg mL−1 | 0.0001–100.0 ng mL−1 | [123] |
| Ni/Cu MOF | Glucose | FET | 1 µM–20 mM | 0.51 µM | [57] |
| NiO/PANINS | Glucose | Amperometric | 1–3000 µM | 0.06 µM | [124] |
| NiO@Au | Lactic acid | Electrochemical | 100.0 µM–0.5 M | 11.6 µM | [125] |
| Co3O4 NCs | Glutamate | Electrochemical chip | 10–600 µM | 10 µM | [60] |
| Co3O4-Au | miRNA-141 | Photo-electricalchemical | 1 pM–50 nM | 0.2 pM | [62] |
| MnO-Mn3O4@rGO | H2O2 | Impedimetric | 0.004–17 mM | 0.1 µM | [126] |
| MnO2 NFs | Salmonella | Impedimetric | 3.0 × 101–3.0 × 106 | 19 CFU mL−1 | [127] |
| Fe2O3/NiO/Mn2O3 NPs | Folic acid | Electrochemical | 0.1 nM-0.01 mM | 96.89 ± 4.85 pM | [128] |
| ZnO-rGO | Dopamine | Cyclic Voltammetric | 0.1–1500 pM | 8.75 ± 0.64 pM | [129] |
| ZnO NRs | Phosphate | FET | 0.1 µM–7.0 mM | 0.5 mM | [92] |
| ZnO NFs | Amyloid | Optical | 2–20 µL | 2.76 µg | [130] |
| Ca/Al-ZnO NPs | CO2 | Semiconductor | 0.25–5 RH% | 200 ppm | [131] |
| Cr doped SnO2 NPs | Riboflavin | Voltammetric | 0.2 × 10−6–1.0 × 10−4 M | 107 nM | [132] |
| TiO2/APTES | glucose | Impedimetric | 50–1000 µmol | 24 µmol | [133] |
| TiO2 NTs | Asulam | photoelectrochemical | 0.02–2.0 ng mL−1 | 4.1 pg mL−1 | [134] |
| MoO3@RGO | Breast cancer | Electrochemical | 0.001–500 ng mL−1 | 0.001 ng mL−1 | [135] |
| Graphene QDs | Cu2+ | Electrochemical | 0.015–8.775 µM | 1.34 nM | [78] |
| Graphene QDs | Lung cancer+ | Fluorescence | 0.1 pg mL−1–1000 ng mL−1 | 0.09 pg mL−1 | [79] |
| CdTe/CdS//ZnS core/shell/shell QDs | l-ascorbic acid | Fluorescence | 8.0 × 10−9–1.0 × 10−7 M | 1.8 × 10−9 M | [80] |
| NSET amptamer@Fe3O4@GOD and MoS2 | Tumor cell(EpCAM) | Magnetic fluorescence | 2–64 nM | 1.19 nM | [81] |
| Au NPs@PDA@CuInZnS QDs | P53 gene | Electrochemiluminescenece | 0.1–15 nmol L−1 | 0.03 nmol L−1 | [136] |
| CaM/SiNW-FETs | Protein | FET | 10−8–10−6 M | 7 nM | [137] |
| Si NWs | Dengue virus | FET | 1 µM–10 fM | 2.0 fM | [86] |
| ZnO NRs | Phosphate | FET | 0.1 µM–7.0 mM | 0.5 mM | [92] |
| G/Au NR/PT | HPV DNA | Electrochemical | 1.0 × 10−13–1.0 × 10−10 m L−1 | 4.03 × 10−14 m L−1 | [138] |
| Graphene-Au NRs | NADHEthanol | AmperometricVoltammetric | 20–160 µM5–377 µM | 6 µM1.5 µM | [139] |
| LAC-CNTs-SPCE | Para-cresol | Electrochemical | 0.2–25 ppm | 0.05 ppm | [140] |
| Co3O4-CNT/TiO2 | Glucose | Photoelectrochemical | 0–4 mM | 0.16 µM | [141] |
| CNT thin-film transistor (TFT) | DNA | Thin film transistor (TFT) | 1.6 × 10−4–5 µmol L−1 | 0.88 µg L−1 | [142] |
| GQDs-MWCNTs | Dopamine | Electrochemical | 0.005–100.0 µM | 0.87 nM | [143] |
| CNT/Au NPs | Choline | Amperometric | 0.05–0.8 mM | 15 µM | [144] |
| PAMAM dendrimer | DENV 2E | Optical fiber | 0.1 pM–1 µM | 19.53 nm nM−1 | [106] |
| SAM/NH2rGO/PAMAM | DENV 2E | SPR | 0.08 pM–0.5 pM | 0.08 pM | [107] |
This entry is adapted from the peer-reviewed paper 10.3390/s21041109
References
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to biosensors. Essays Biochem. 2016, 60, 1–8.
- Heineman, W.R.; Jensen, W.B. Leland C. Clark Jr. (1918–2005). Biosens. Bioelectron. 2006, 21, 1403–1404.
- Clark, L.C.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45.
- Updike, S.J.; Hicks, G.P. The enzyme electrode. Nature 1967, 214, 986–988.
- Guilbault, G.G.; Montalvo, J.G., Jr. Urea-specific enzyme electrode. J. Am. Chem. Soc. 1969, 91, 2164–2165.
- Guilbault, G.G.; Lubrano, G.J. An enzyme electrode for the amperometric determination of glucose. Anal. Chim. Acta 1973, 64, 439–455.
- Mosbach, K.; Danielsson, B. An enzyme thermistor. Biochim. Biophys. Acta. 1974, 364, 140–145.
- Lübbers, D.W.; Opitz, N. The pCO2-/pO2-optode: A new probe for measurement of pCO2 or pO in fluids and gases (authors transl). Z. Naturforsch C Biosci. 1975, 30, 532–533.
- Clemens, A.H.; Chang, P.H.; Myers, R.W. Le développement d’un système automatique d’infusion d’insuline controle par la glycemie, son système de dosage du glucose et ses algorithmes de controle. Journ. Annu. Diabétol. Hotel Dieu 1976, 269–278.
- Clemens, A.H.; Chang, P.H.; Myers, R.W. The development of biostator, a glucose controller insulin infusion system (GCIIS). Horm. Metab. Res. Suppl. 1977, 7, 22–23.
- Geyssant, A.; Dormois, D.; Barthelemy, J.C.; Lacour, J.R. Lactate determination with the lactate analyser LA 640: A critical study. Scand. J. Clin. Lab. Investig. 1985, 45, 145–149.
- Cass, A.E.; Davis, G.; Francis, G.D.; Hill, H.A.; Aston, W.J.; Higgins, I.J.; Plotkin, E.V.; Scott, L.D.; Turner, A.P. Ferrocene-mediated enzyme electrode for amperometric determination of glucose. Anal. Chem. 1984, 56, 667–671.
- Liedberg, W.; Nylander, C.; Lundstrm, I. Surface plasmon resonance for gas detection and biosensing. Sens. Actuators A Phys. 1983, 4, 299–304.
- Cremer, M. Über die Ursache der elektromotorischen Eigenschaften der Gewebe, zugleich ein Beitrag zur Lehre von den polyphasischen Elektrolytketten. Z. Biol. 1906, 47, 562–608.
- Sörensen, S.P.L. Enzymstudien. II. Mitteilung. Über die Messung und die Bedeutung der Wasserstoffionenkoncentration bei enzymatischen Prozessen [Enzyme studies. 2nd Report. On the measurement and the importance of hydrogen ion concentration during enzymatic processes]. Biochem. Z. 1909, 21, 131–304. (In German)
- Griffin, E.G.; Nelson, J.M. The influence of certain substances on the activity of invertase. J. Am. Chem. Soc. 1916, 38, 722–730.
- Nelson, J.M.; Griffin, E.G. Adsorption of invertase. J. Am. Chem. Soc. 1916, 38, 1109–1115.
- Hughes, W.S. The potential difference between glass and electrolytes in contact with the glass. J. Am. Chem. Soc. 1922, 44, 2860–2867.
- Bergveld, P. Development of an ion-sensitive solid-state device for neurophysiological measurements. IEEE Trans. Biomed. Eng. 1970, 17, 70–71.
- Newman, J.D.; Turner, A.P.F. Home blood glucose biosensors: A commercial perspective. Biosens. Bioelectron. 2005, 20, 2435–2453.
- D’Orazio, P. Biosensors in clinical chemistry. Clin. Chim. Acta 2003, 334, 41–69.
- Suzuki, S.; Takahashi, F.; Satoh, I.; Sonobe, N. Ethanol and lactic acid sensors using electrodes coated with dehydrogenase–collagen membranes. Bull. Chem. Soc. Jap. 1975, 48, 3246–3249.
- Yoo, E.-H.; Lee, S.-Y. Glucose biosensors: An overview of use in clinical practice. Sensors 2010, 10, 4558–4576.
- Schultz, J.S. Oxygen Sensor of Plasma Constituents. U.S. Patent No. 4,344,438A, 17 August 1982.
- Roederer, J.E.; Bastiaans, G.J. Microgravimetric immunoassay with piezoelectric crystals. Anal. Chem. 1983, 55, 2333–2336.
- Mun’delanji, C.V.; Tamiya, E. Nanobiosensors and nanobioanalyses: A Review. In Nanobiosensors and Nanobioanalyses, 1st ed.; Mun’delanji, C.V., Kerman, K., Hsing, I.M., Tamiya, E., Eds.; Springer: Tokyo, Japan, 2015; pp. 3–20.
- Poncharal, P.; Wang, Z.L.; Ugarte, D.; De Heer, W.A. Electrostatic deflections and electromechanical resonances of carbon nanotubes. Science 1999, 283, 1513.
- Gribi, S.; De Dunilac, S.B.; Ghezzi, D.; Lacour, S.P. A microfabricated nerve-on-a-chip platform for rapid assessment of neural conduction in explanted peripheral nerve fibers. Nat. Commun. 2018, 9, 4403.
- Theavenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Biosens. Bioelectron. 2001, 16, 121–131.
- Dincer, C.; Bruch, R.; Costa-Rama, E.; Fernández-Abedul, M.T.; Merkoçi, A.; Manz, A.; Urban, G.A.; Güder, F. Disposable Sensors in Diagnostics, Food, and Environmental Monitoring. Adv. Mater. 2019, 31, 1806739.
- Turner, A.P.F. Biosensors: Sense and sensibility. Chem. Soc. Rev. 2013, 42, 3184–3196.
- Karim, R.A.; Reda, Y.; Fattah, A.A. Review—Nanostructured materials-based nanosensors. J. Electrochem. Soc. 2020, 167, 037554.
- Van den Berg, B.; Wain, R.; Dobson, C.M.; Ellis, R.J. Macromolecular crowding perturbs protein refolding kinetics: Implications for protein folding inside the cell. EMBO J. 2000, 19, 3870–3875.
- Martinkova, P.; Kostelnik, A.; Valek, T.; Pohanka, M. Main streams in the construction of biosensors and their applications. Int. J. Electrochem. Sci. 2017, 12, 7386–7403.
- Maduraiveeran, G.; Sasidharan, M.; Ganesan, V. Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosens. Bioelectron. 2018, 103, 113–129.
- Li, Y.; Schluesener, H.J.; Xu, S. Gold nanoparticle-based biosensors. Gold Bull. 2010, 43, 29–41.
- Vidotti, M.; Carvalhal, R.F.; Mendes, R.K.; Ferreira, D.C.M.; Kubota, L.T. Biosensors based on gold nanostructures. J. Braz. Chem. Soc. 2011, 22, 3–20.
- Shi, S.; Wu, H.; Zhang, L.; Wang, S.; Xiong, P.; Qin, Z.; Chu, M.; Liao, J. Gold nanoparticles based electrochemical sensor for sensitive detection of uranyl in natural water. J. Electroanal. Chem. 2021, 880, 114884.
- Niu, X.F.; Zhong, Y.; Chen, R.; Wang, F.; Liu, Y.; Luo, D. A “turn-on” fluorescence sensor for Pb2+ detection based on graphene quantum dots and gold nanoparticles. Sens. Actuators B Chem. 2018, 255, 1577–1581.
- Amiripour, F.P.; Ghasemi, S.; Azizi, S.N. A novel non-enzymatic glucose sensor based on gold-nickel bimetallic nanoparticles doped aluminosilicate framework prepared from agro-waste material. Appl. Surf. Sci. 2021, 537, 147827.
- El-Dessouky, R.; Georges, M.; Azzazy, H.M.E. Silver nanostructures: Properties, synthesis, and biosensor applications. In Functional Nanoparticles for Bioanalysis, Nanomedicine, and Bioelectronic Devices; ACS Symposium series, 1112; Hepel, M., Zhong, C.-J., Eds.; ACS: Washington, DC, USA, 2012; Volume 1, pp. 359–404.
- Loiseau, A.; Asila, V.; Aullen, G.B.; Lam, M.; Salmain, M.; Boujday, S. Silver-based plasmonic nanoparticicles for and their uses in biosensing. Biosensors 2019, 9, 78.
- Malekzad, H.; Zangabad, P.S.; Mirshekari, H.; Karimi, M.; Hamblin, M.R. Noble metal nanoparticles in biosensors: Recent studies and applications. Nanotech. Rev. 2017, 6, 301–329.
- Rivero, P.J.; Urrutia, A.; Goicoechea, J.; Arregui, F.J. Optical fiber humidity sensors based on localized surface plasmon resonance (LSPR) and lossy-mode resonance (LMR) in overlays loaded with silver nanoparticles. Sens. Actuators B Chem. 2012, 173, 244–249.
- Basiri, S.; Mehdinia, A.; Jabbari, A. A sensitive triple colorimetric sensor based on plasmonic response quenching of green synthesized silver nanoparticles for determination of Fe2+, hydrogen peroxide, and glucose. Colloids Surf. A Physicochem. Eng. Asp. 2018, 545, 138–146.
- Chen, P.L.; Liu, I.P.; Chen, W.C.; Niu, J.S.; Liu, W.C. Study of a platinum nanoparticle (Pt NP)/amorphous In-Ga-Zn-O (A-IGZO) thin-film-based ammonia gas sensor. Sens. Actuators B Chem. 2020, 322, 128592.
- Imaran, H.; Vaishali, K.; Francy, S.A.; Manikandan, P.N.; Dharuman, V. Platinum and zinc oxide modified carbon nitride electrode as non-enzymatic highly selective and reusable electrochemical diabetic sensor in human blood. Bioelectrochemistry 2021, 137, 107645.
- Phan, T.T.V.; Huynh, T.C.; Manivasagan, P.; Mondal, S.; Oh, J. An up-to-date review on biomedical applications of palladium nanoparticles. Nanomater 2020, 10, 66.
- Zhang, Y.; Huang, B.; Ye, J.; Ye, J. A sensitive and selective amperometric hydrazine sensor based on palladium nanoparticles loaded on cobalt-wrapped nitrogen-doped carbon nanotubes. J. Eelctroanal. Chem. 2017, 801, 215–223.
- Mohammadi, M.M.; Kumar, A.; Liu, J.; Liu, Y.; Thundat, T.; Swihart, M.T. Hydrogen sensing at room temperature using flame-synthesized palladium-decorated crumpled reduced graphene oxide nanocomposites. ACS Sens. 2020, 5, 2344–2350.
- Afzali, M.; Mostafavi, A.; Nekooie, R.; Jahromi, Z. A novel voltammetric sensor based on palladium nanoparticles/carbon nanofibers/ionic liquid modified carbon paste electrode for sensitive determination of anti-cancer drug pemetrexed. J. Mol. Liq. 2019, 282, 456–465.
- Li, J.H.; Tang, J.X.; Wei, L.; He, S.J.; Ma, L.Q.; Shen, W.C.; Kang, F.Y.; Huang, Z.H. Preparation and performance of electrochemical glucose sensors based on copper nanoparticles loaded on flexible graphite sheet. New Carbon Mater. 2020, 35, 410–419.
- Roushani, M.; Dizajdizi, B.Z. Development of nonenzymatic hydrogen peroxide sensor based on catalytic properties of copper nanoparticles/Rutin/MWCNTs/IL/Chit. Catal. Commun. 2015, 69, 133–137.
- Zhu, T.; Wang, X.; Chang, W.; Zhang, Y.; Maruyama, T.; Luo, L.; Zhao, X. Green fabrication of Cu/rGO decorated SWCNT buckypaper as a flexible electrode for glucose detection. Mater. Sci. Eng. C 2021, 120, 111757.
- Shi, X.; Gu, W.; Li, B.; Chen, N.; Zhao, K.; Xian, Y. Enzymatic biosensors based on the use of metal oxide nanoparticles. Microchim. Acta 2014, 181, 1–22.
- Hashem, M.; Saion, E.; Al-Hada, N.M.; Kamari, H.M.; Shaari, A.H.; Talib, Z.A.; Paiman, S.B.; Kamarudeen, M.A. Fabrication and characterization of semiconductor nickel oxide (NiO) nanoparticles manufactured using a facile thermal treatment. Results Phys. 2016, 6, 1024–1030.
- Wang, B.; Luo, Y.; Gao, L.; Liu, B.; Duan, G. High-performance field-effect transistor glucose biosensors based on bimetallic Ni/Cu metal-organic frameworks. Biosens. Bioelectron. 2021, 171, 112736.
- Kamyabi, M.A.; Moharramnezhad, M. A highly sensitive ECL platform based on GOD and NiO nanoparticle decorated nickel foam for determination of glucose in serum samples. Anal. Methods 2020, 12, 1670–1678.
- Bhargava, R.; Khan, S.; Ahmad, N.; Ansari, M.M.N. Investigation of structural, optical and electrical properties of Co3O4 nanoparticles. AIP Conf. Proc. 2018, 1953, 030034.
- Hu, F.; Liu, T.; Pang, J.; Chu, Z.; Jin, W. Facile preparation of porous Co3O4 nanocubes for directly screen-printing an ultrasensitive glutamate biosensor microchip. Sens. Actuators B Chem. 2020, 306, 127587.
- Ali, A.; Israr-Qadir, M.; Wazir, Z.; Tufail, M.; Ibupoto, Z.H.; Jamil-Rana, S.; Atif, M.; Khan, S.A.; Willande, M. Cobalt oxide magnetic nanoparticles–chitosan nanocomposite based electrochemical urea biosensor. Indian J. Phys. 2014, 89, 331–336.
- Zhao, J.; Fu, C.; Huang, C.; Zhang, S.; Wang, F.; Zhang, Y.; Zhang, L.; Ge, S.; Yu, J. Co3O4-Au polyhedron mimic peroxidase- and cascade enzyme-assisted cycling process-based photoelectrochemical biosensor for monitoring of miRNA-141. Chem. Eng. J. 2021, 406, 126892.
- Santos, T.A.P.R. Sensors and biosensors based on magnetic nanoparticles. TrAC Trends Anal. Chem. 2014, 62, 28–36.
- Haun, J.B.; Yoon, T.J.; Lee, H.; Weissleder, R. Magnetic nanoparticle biosensors. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 291–304.
- Devakota, J.; Wingo, J.; Mai, T.T.T.; Nguyen, X.P.; Huong, N.T.; Mukherjee, P.; Srikanth, H.; Phan, M.H. A highly sensitive magnetic biosensor for detection and quantification of anticancer drugs tagged to superparamagnetic nanoparticles. J. Appl. Phys. 2014, 115, 503.
- Li, T.; Jin, L.; Feng, K.; Yang, T.; Yue, X.; Wu, B.; Ding, S.; Liang, X.; Huang, G.; Zhang, J. A novel low-field NMR biosensor based on dendritic superparamagnetic iron oxide nanoparticles for the rapid detection of Salmonella in milk. LWT 2020, 133, 110149.
- Vukojevic, V.; Djurdjic, S.; Ognjanovic, M.; Fabian, M.; Samphao, A.; Kalcher, K.; Stankovic, D.M. Enzymatic glucose biosensor based on manganese dioxide nanoparticles decorated on graphene nanoribbons. J. Electroanal. Chem. 2018, 823, 610–616.
- Xu, C.X.; Yang, C.; Gu, B.X.; Fang, S.J. Nanostructured ZnO for biosensing applications. Chin. Sci. Bull. 2013, 58, 2563–2566.
- Bhati, V.S.; Hojamberdiev, M.; Kumar, M. Enhanced sensing performance of ZnO nanostructures-based gas sensors: A review. Energy Rep. 2020, 6, 46–62.
- Hjiri, M.; Bahanan, F.; Aida, M.S.; El Mir, L.; Neri, G. High performance CO gas sensor based on ZnO nanoparticles. J. Inorg. Organomet. Polym. 2020, 30, 4063–4071.
- Borhaninia, A.; Nikfarjam, A.; Salehifar, N. Gas sensing properties of SnO2 nanoparticles mixed with gold nanoparticles. Trans. Nonferrous Met. Soc. 2017, 27, 1777–1784.
- Kolmakov, V.; Zhang, Y.; Cheng, G.; Moskovits, M. Detection of CO and O2 using tin oxide nanowire sensors. Adv. Mater. 2003, 15, 997–1000.
- Viter, R.; Tereshchenko, A.; Smyntyna, V.; Ogorodniichuk, J.; Starodub, N.; Yakimova, R.; Khranovskyy, V.; Ramanavicius, A. Toward development of optical biosensors based on photoluminescence of TiO2 nanoparticles for the detection of Salmonella. Sens. Actuators B Chem. 2017, 252, 95–102.
- Rashmi, B.N.; Harlapur, S.F.; Ravikumar, C.R.; Avinash, B.; Gurushantha, K.; Divakara, M.B.; Santosh, M.S.; Veena, K. MoO3 nanoparticles based electrodes as novel electrochemical sensors for the detection of H2O2. Mater. Today Proc. 2020.
- Pandit, S.; Dassgupta, D.; Dewan, N.; Ahmed, P. Nanotechnology-based biosensors and its application. Pharm. Innov. J. 2016, 5, 18–25.
- Vashist, S.K.; Venkatesh, A.G.; Mitsakakis, K.; Czilwik, G.; Roth, G.; Von Stetten, F.; Zengerle, R. Nanotechnology-based biosensors and diagnostics: Technology push versus industrial/healthcare requirements. Bionanoscience 2012, 2, 115–126.
- Ma, F.; Li, C.C.; Zhang, C.Y. Development of quantum dot-based biosensors: Principles and applications. J. Mater. Chem. B 2018, 6, 6173–6190.
- Wang, Y.; Zhao, S.; Li, M.; Li, W.; Zhao, Y.; Qi, J.; Cui, X. Graphene quantum dots decorated graphene as an enhanced sensing platform for sensitive and selective detection of copper(II). J. Electroanal. Chem. 2017, 797, 113–120.
- Kalkal, A.; Pradhan, R.; Kadian, S.; Manik, G.; Packirisamy, G. Biofunctionalized graphene quantum dots based fluorescent biosensor toward efficient detection of small cell lung cancer. ACS Appl. Bio Mater. 2020, 3, 4922–4932.
- Huang, S.; Zhu, F.; Xiao, Q.; Su, W.; Sheng, J.; Huang, C.; Hu, B. A CdTe/CdS/ZnS core/shell/shell QDs-based “off–on” fluorescent biosensor for sensitive and specific determination of l-ascorbic acid. RSC Adv. 2014, 4, 46751–46761.
- Cui, F.; Ji, J.; Sun, J.; Wang, J.; Wang, H.; Zhang, Y.; Ding, H.; Lu, Y.; Xu, D.; Sun, X. A novel magnetic fluorescent biosensor based on graphene quantum dots for rapid, efficient, and sensitive separation and detection of circulating tumor cells. Anal. Bioanal. Chem. 2019, 411, 985–995.
- Ramanathan, K.; Bangar, M.A.; Yun, M.; Chen, W.; Myung, N.V.; Mulchandani, A. Bioaffinity sensing using biologically functionalized conducting polymer nanowire. J. Am. Chem. Soc. 2005, 127, 496–497.
- Patolsky, F.; Zheng, G.; Lieber, C.M. Nanowire-based biosensors. Anal. Chem. 2006, 78, 4260–4269.
- Kim, H.M.; Park, J.H.; Lee, S.K. Fiber optic sensor based on ZnO nanowires decorated by Au nanoparticles for improved plasmonic biosensor. Sci. Rep. 2019, 9, 15605.
- Leonardi, A.A.; Faro, M.J.L.; Petralia, S.; Fazio, B.; Musumeci, P.; Conoci, S.; Irreara, A.; Priolo, F. Ultrasensitive label- and PCR-free genome detection based on cooperative hybridization of silicon nanowires optical biosensors. ACS Sens. 2018, 3, 1690–1697.
- Nuzaihan, M.N.M.; Hashim, U.; Md Arsad, M.K.; Kasjoo, S.R.; Rahaman, S.F.A.; Rusilnda, A.R.; Fathil, M.F.M.; Adzhri, R.; Shahimin, M.M. Electrical detection of dengue virus (DENV) DNA oligomer using silicon nanowire biosensor with novel molecular gate control. Bisens. Bioelectron. 2016, 83, 106–114.
- Cao, J.; Sun, T.; Grattan, K.T.V. Gold nanorod-based localized surface plasmon resonance biosensors: A review. Sens. Actuators B Chem. 2014, 195, 332–351.
- Ibupoto, Z.H.; Ali, S.M.U.; Khun, L.; Chey, C.O.; Nur, O.; Willander, M. ZnO nanorods based enzymatic biosensor for selective determination of penicillin. Biosensors 2011, 1, 153–163.
- Liu, G.; Feng, D.Q.; Qian, Y.; Wang, W.; Zhu, J.J. Construction of FRET biosensor for off-on detection of lead ions based on carbon dots and gold nanorods. Talanta 2019, 201, 90–95.
- Zong, X.; Zhu, R. ZnO nanorod-based FET biosensor for continuous glucose monitoring. Sens. Actuator B Chem. 2018, 255, 2448–2453.
- Zhang, H.; Song, D.; Gao, S.; Zhang, H.; Zhang, J.; Sun, Y. Enhanced wavelength modulation SPR biosensor based on gold nanorods for immunoglobulin detection. Talanta 2013, 115, 857–862.
- Ahmad, R.; Ahn, M.S.; Hahn, Y.B. ZnO nanorods array based field-effect transistor biosensor for phosphate detection. J. Colloid Interface Sci. 2017, 498, 292–297.
- Singh, R.P. Prospects of nanobiomaterials for biosensing. Int. J. Electrochem. 2011, 2011, 125487.
- Simon, J.; Flahaut, E.; Golzio, M. Overview of carbon nanotubes for biomedical applications. Materials 2019, 12, 624.
- Sireesha, M.; Babu, J.V.; Kiran, A.S.K.; Ramakrishna, S. A review on carbon nanotubes in biosensor devices and their applications in medicine. Nanocomposites 2018, 4, 36–57.
- Luo, X.; Shi, W.; Yu, H.; Xie, Z.; Li, K.; Cui, Y. Wearable carbon nanotube-based biosensors on gloves for lactate. Sensors 2018, 18, 3398.
- Janssen, J.; Lambeta, M.; White, P.; Byagowi, A. Carbon nanotube-based electrochemical biosensor for label-free protein detection. Biosensors 2019, 9, 144.
- Xiaowu Tang, X.; Bansaruntip, S.; Nakayama, N.; Yenilmez, E.; Chang, Y.L.; Wang, Q. Carbon nanotube DNA sensor and sensing mechanism. Nano Lett. 2006, 6, 1632–1636.
- Kim, B.; Lee, J.; Namgung, S.; Kim, J.; Park, J.Y.; Lee, M.S.; Hong, S. DNA sensors based on CNT-FET with floating electrodes. Sens. Actuators B Chem. 2012, 169, 182–187.
- Lee, B.Y.; Seo, S.M.; Lee, D.J.; Lee, M.; Lee, J.; Cheon, J.H.; Cho, E.; Lee, H.; Chung, I.Y.; Park, Y.J.; et al. Biosensor system-on-a-chip including CMOS-based signal processing circuits and 64 carbon nanotube-based sensors for the detection of a neurotransmitter. Lab Chip 2010, 10, 894–898.
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Hanifehpour, Y.; Koshki, K.N.; Asl, R.P. Dendrimers: Synthesis, applications, and properties. Nanoscale Res. Lett. 2014, 9, 247.
- Zheng, Y.; Li, S.; Weng, Z.; Gao, C. Hyperbranched polymers: Advances for synthesis to applications. Chem. Soc. Rev. 2015, 44, 4091–4130.
- Yanez, C.S.; Rodriguez, C.C. Dendrimers: Amazing platform for bioactive molecule delivery system. Materials 2020, 13, 570.
- Satija, J.; Sai, V.V.R.; Muherji, S. Dendrimers in biosensors: Concept and applications. J. Mater. Chem. 2011, 21, 14367–14386.
- Caminade, A.M.; Turrin, C.O. Dendrimers for drug delivery. J. Mater. Chem. B 2014, 2, 4055–4066.
- Kamil, Y.M.; Al-Rekabi, S.H.; Yaacob, M.H.; Syahir, A.; Chee, H.Y.; Mahid, M.A.; Bakar, M.H.A. Detection of dengue using PAMAM dendrimer integrated tapered optical fiber sensor. Sci. Rep. 2019, 9, 13483.
- Omar, N.A.S.; Fen, Y.W.; Abdullah, J.; Kamil, Y.M.; Ebtisyam, W.M.; Daniyal, M.M.; Sadrohosseini, A.R.; Mahdi, M.A. Sensitive detection of dengue virus type 2 E-proteins signals using self-assembled monolayers/reduced graphene oxide-PAMAM dendrimer thin film-SPR optical sensor. Sci. Rep. 2020, 10, 2374.
- De Castro, A.C.; Alves, L.M.; Siquieroli, A.C.; Madurro, J.M.; Brito-Madurro, A.G. Label-free electrochemical immunosensor for detection of oncomarker CA125 in serum. Microchem. J. 2020, 155, 104746.
- Khaliq, N.; Rasheed, M.A.; Khan, M.; Maqbool, M.; Ahmad, M.; Karim, S.; Nisar, A.; Schmuki, P.; Cho, S.O.; Ali, G. Voltage-Switchable Biosensor with Gold Nanoparticles on TiO2 Nanotubes Decorated with CdS Quantum Dots for the Detection of Cholesterol and H2O2. ACS Appl. Mater. Interfaces 2021, 13, 3653–3668.
- Vu, Q.K.; Tran, Q.H.; Vu, N.P.; Anh, Y.-L.; Dang, T.T.L.; Tonezzer, M.; Ngyyen, T.H.H. A label-free electrochemical biosensor based on screen-printed electrodes modified with gold nanoparticles for quick detection of bacterial pathogens. Mater. Today Commun. 2020, 101726.
- Jandas, P.J.; Luo, J.; Prabakaran, K.; Chen, F.; Fu, Y.Q. Highly stable, love-mode surface acoustic wave biosensor using Au nanoparticle-MoS2-rGO nano-cluster doped polyimide nanocomposite for the selective detection of carcinoembryonic antigen. Mater. Chem. Phys. 2020, 246, 122800.
- Kasturi, S.; Eom, Y.; Torati, S.R.; Kim, C.G. Highly sensitive electrochemical biosensor based on naturally reduced rGO/Au nanocomposite for the detection of miRNA-122 biomarker. J. Ind. Eng. Chem. 2021, 93, 186–195.
- Liu, X.; Zhang, J.; Liu, S.; Zhang, Q.; Liu, X.; Wong, D.K.Y. Gold Nanoparticle Encapsulated-Tubular TiO2 Nanocluster as a Scaffold for Development of Thiolated Enzyme Biosensors. Anal. Chem. 2013, 85, 4350–4356.
- Wang, H.; Zhang, Y.; Du, B.; Ma, H.; Wu, D.; Wei, Q. A silver–palladium alloy nanoparticle-based electrochemical biosensor for simultaneous detection of ractopamine, clenbuterol and salbutamol. Biosens. Bioelectron. 2013, 49, 14–19.
- Han, G.; Cai, J.; Liu, C.; Ren, J.; Wang, X.; Yang, J.; Wang, X. Highly sensitive electrochemical sensor based on xylan-based nanocomposite for dopamine detection. Appl. Surf. Sci. 2021, 541, 148566.
- Chen, L.; Xie, H.; Li, J. Electrochemical glucose biosensor based on silver nanoparticles/multiwalled carbon nanotubes modified electrode. J. Solid State Electrochem. 2012, 16, 3323–3329.
- Wei, U.-P.; Zhang, Y.-W.; Mao, C.-J. A silver nanoparticle-assisted signal amplification electrochemiluminescence biosensor for highly sensitive detection of mucin 1. J. Mater. Chem. B 2020, 8, 2471–2475.
- Brondani, D.; Scheeren, C.W.; Dupont, J.; Vieira, I.C. Biosensor based on platinum nanoparticles dispersed in ionic liquid and laccase for determination of adrenaline. Sens. Actuators B 2009, 140, 252–259.
- Bai, Z.; Li, G.; Liang, J.; Su, J.; Zhang, Y.; Chen, H.; Huang, Y.; Sui, W.; Zhao, Y. Non-enzymatic electrochemical biosensor based on Pt NPs/RGO-CS-Fc nano-hybrids for the detection of hydrogen peroxide in living cells. Biosens. Bioelectron. 2016, 82, 185–194.
- Yang, Q.; Li, N.; Li, Q.; Chen, S.; Wang, H.-L.; Yang, H. Amperometric sarcosine biosensor based on hollow magnetic Pt–Fe3O4@C nanospheres. Anal. Chim. Acta 2019, 1078, 161–167.
- Jia, W.; Su, L.; Lei, Y. Pt nanoflower/polyaniline composite nanofibers based urea biosensor. Biosens. Bioelectron. 2011, 30, 158–164.
- Fu, C.; Sun, Y.; Huang, C.; Wang, F.; Li, N.; Zhang, L.; Ge, S.; Yu, J. Ultrasensitive sandwich-like electrochemical biosensor based on core-shell 2 as signal tags and double molecular recognition for cerebral dopamine detection. Tlanta 2019, 223, 121719.
- Li, F.; Li, Y.; Feng, J.; Dong, Y.; Wang, P.; Chen, L.; Chen, Z.; Liu, H.; Wei, Q. Ultrasensitive amperometric immunosensor for PSA detection based on Cu22-Au nanocomposites as integrated triple signal amplification strategy. Bisensor. Bioelectron. 2017, 87, 630–637.
- Kailasa, S.; Rani, B.G.; Reddy, M.S.B.; Jayarambabu, N.; Munindra, P.; Sharma, S.; Rao, K.V. NiO nanoparticles -decorated conductive polyaniline nanosheets for amperometric glucose biosensor. Mater. Chem. Phys. 2020, 242, 122524.
- Maduraiveeran, G.; Chen, A. Design of an enzyme-mimicking nanocomposite for the sensitive electrochemical detection of lactic acid in human serum and urine. Electrochim. Acta 2021, 368, 137612.
- Li, Y.; Tang, L.; Deng, D.; He, H.; Yan, X.; Wang, J.; Luo, L. Hetero-structured MnO-Mn3O4@rGO composites: Synthesis and nonenzymatic detection of H2O2. Mater. Sci. Eng. C 2021, 118, 111443.
- Xue, L.; Guo, R.; Huang, F.; Qi, W.; Liu, Y.; Cai, G.; Lin, J. An impedance biosensor based on magnetic nanobead net and MnO2 nanoflowers for rapid and sensitive detection of foodborne bacteria. Biosens. Bioelectron. 2021, 173, 112800.
- Alam, M.M.; Rahman, M.M.; Uddin, M.T.; Asiri, A.M.; Uddin, J.; Islam, M.A. Fabrication of enzyme-less folic acid sensor probe based on facile ternary doped Fe2O3/NiO/Mn2O3 nanoparticles. Curr. Res. Biotechnol. 2020, 2, 176–186.
- Verma, S.; Arya, P.; Singh, A.; Kaswan, J.; Shukla, A.; Kushwaha, H.R.; Gupta, S.; Singh, S.P. ZnO-rGO nanocomposite based bioelectrode for sensitive and ultrafast detection of dopamine in human serum. Biosens. Bioelectron. 2020, 165, 112347.
- Akthar, N.; Metkar, S.K.; Girigoswami, A.; Girigoswami, K. ZnO nanoflower based sensitive nano-biosensor for amyloid detection. Mater. Sci. Eng. C 2017, 78, 960–968.
- Dhahri, R.; Leonardi, S.G.; Hjiri, M.; El Mir, L.; Bonavita, E.; Donato, N.; Iannazzo, D.; Neri, G. Enhanced performance of novel calcium/aluminum co-doped zinc oxide for CO2 sensors. Sens. Actuators B Chem. 2017, 239, 36–44.
- Lavanya, N.; Radhakrishna, S.; Sekar, C.; Navaneethan, M.; Hayakawa, Y. Fabrication of Cr doped SnO2 nanoparticles based biosensor for the selective determination of riboflavin in pharmaceuticals. Analyst 2013, 138, 2061–2067.
- Ognjanovic, M.; Stankovic, V.; Knezevic, S.; Antic, B.; Djuric, S.V.; Stankovic, D.M. TiO2/APTES cross-linked to carboxylic graphene based impedimetric glucose biosensor. Microchem. J. 2020, 158, 105150.
- Tian, J.; Li, Y.; Dong, J.; Huang, M.; Lu, J. Photoelectrochemical TiO2 nanotube arrays biosensor for asulam determination based on in-situ generation of quantum dots. Biosens. Bioelectron. 2018, 110, 1–7.
- Augustinbe, S.; Kumar, P.; Malhotra, B.D. Amine-functionalized MoO3@RGO nanohybrids-based biosensor for breast cancer detection. ACS Appl. Bio Mater. 2019, 2, 5366–5378.
- Liu, Y.; Chen, X.; Ma, Q. A novel amplified electrochemiluminescence biosensor based on Au @CuInZnS QDs nanocomposites for ultrasensitive detection of p53 gene. Biosens. Bioselectron. 2018, 117, 240–245.
- Lin, T.W.; Hsieh, P.J.; Lin, C.L.; Fang, Y.Y.; Yang, J.X.; Tsai, C.C.; Chiang, P.L.; Pan, C.Y.; Chen, Y.T. Label-free detection of protein-protein interactions using a calmodulin-modified nanowire transistor. Proc. Natl. Acad. Sci. USA 2010, 107, 1047–1052.
- Huang, H.; Bai, W.; Dong, C.; Guo, R.; Liu, Z. An ultrasensitive electrochemical DNA biosensor based on graphene/Au nanorod/polythionine for human papillomavirus DNA detection. Biosens. Bioelectron. 2015, 68, 442–446.
- Li, L.; Lu, H.; Deng, L. A sensitive NADH and ethanol biosensor based on graphene–Au nanorods nanocomposites. Talanta 2013, 113, 1–6.
- Zhao, K.; Veksha, A.; Ge, L.; Lisak, G. Near real-time analysis of para-cresol in wastewater with a laccase-carbon nanotube-based biosensor. Chemosphere 2020, 128699.
- Cakiroglu, B.; Ozacar, M. A self-powered photoelectrochemical glucose biosensor based on supercapacitor Co3O4-CNT hybrid on TiO2. Biosens. Bioelectron. 2018, 119, 34–41.
- Li, W.; Gao, Y.; Zhang, J.; Wang, X.; Yin, F.; Li, Z.; Zhang, M. Universal DNA detection realized by peptide based carbon nanotube biosensors. Nanoscale Adv. 2020, 2, 717–723.
- Huang, Q.; Lin, X.; Tong, L.; Tong, Q.X. Graphene Quantum Dots/Multiwalled Carbon Nanotubes Composite-Based Electrochemical Sensor for Detecting Dopamine Release from Living Cells. ACS Sustain. Chem. Eng. 2020, 8, 1644–1650.
- Wu, B.; Ou, Z.; Ju, X.; Hou, S. Carbon Nanotubes/Gold Nanoparticles Composite Film for the Construction of a Novel Amperometric Choline Biosensor. J. Nanomater. 2011, 2011, 1464919.
