Biofertilizers are a promising alternative to chemical fertilizers and gaining importance for attaining sustainable agriculture.
- biofertilizers
- microorganisms
- soil fertility
- crop productivity
- sustainable agriculture
- chemical fertilizers
1. Introduction
To help meet the escalating demand for food arising from the continuous expansion of the world’s population, different crop nourishment strategies are being explored by farmers. According to FAO’s estimates, the demand for agricultural products will increase to 60% by 2030 [1]. Enhancing the production while keeping the environment safe is one of the major challenges in the 21st century [2]. Fertilizers have been used extensively to increase crop production from arable land. Increasing use of chemical fertilizers in agriculture may make a country self-sufficient in food production, but chemicals have an adverse impact both on the environment and living organisms. In addition, the chemical fertilizers are expensive, affect the soil, reduce its water-holding capacity and fertility, cause imbalance in the soil nutrients, and result in unacceptable levels of water pollution [3]. On the other hand, biofertilizers are eco-friendly, cost-effective, non-toxic, and easy to apply; they help maintain soil structure and biodiversity of the agricultural land. Thus, they serve as a good substitute for chemical fertilizers [4][5].
Biofertilizers, also called microbial inoculants, are organic products containing specific microorganisms, which are derived from plant roots and root zones. They have been shown to improve the growth and yield of the plant by 10–40% [6]. These bioinoculants colonize the rhizosphere and the interior of the plant, promoting plant growth when applied to the seed, plant surface, or the soil [7]. They not only improve soil fertility and crop productivity by adding nutrients to the soil, but also protect the plant from pests and diseases. They have been shown to enhance the growth of the root system, extend its life, degrade the harmful materials, increase the survival of seedlings, and reduce the time to flowering [8]. Another beneficial aspect is that after continuous use of biofertilizers for 3–4 years, there is no need for their application, as parental inocula are sufficient for growth and multiplication [9].
There are 17 essential elements required for proper growth and development of the plant. Among them, nitrogen (N), phosphorous (P), and potassium (K) are needed in relatively large quantities [10]. Several microorganisms are commonly used as biofertilizers including nitrogen-fixing soil bacteria and cyanobacteria, phosphate-solubilizing bacteria, used along with the combination of molds and fungi [11]. Similarly, phytohormone producing bacteria are also used in biofertilizer formulation. They provide the growth-promoting substances like indole acetic acid (IAA), amino acids, and vitamins to the plant and improve the productivity and fertility of the soil while maintaining the crop yield [12].
2. Types of Biofertilizers
Biofertilizers are grouped into different types on the basis of their functions and mode of action. The commonly used biofertilizers are nitrogen fixer (N-fixer), potassium solubilizer (K-solubilizer), phosphorus solubilizer (P-solubilizer), and plant growth promoting rhizobacteria (PGPR) [13]. In one gram of fertile soil, up to 1010 bacteria can be present, with a live weight of 2000 kg/ha [14]. Soil bacteria could be cocci (sphere, 0.5 µm), bacilli (rod, 0.5–0.3 µm), or spiral shaped (1–100 µm). The presence of bacteria in the soil depends upon the physical and chemical properties of the soil, organic matter, and phosphorus contents, as well as cultural activities. However, nutrient fixation and plant growth enhancement by bacteria are key components for achieving sustainable agriculture goals in the future. Microbes also facilitate various nutrient cycles in the ecosystem. Table 1 contains a summary of the classification of the biofertilizers based on the type of microbe used and mode of action, with suitable examples.
Table 1. Classification of biofertilizers, mechanism of action, and their examples.
| Biofertilizers | Mechanism | Groups | Examples | References |
|---|---|---|---|---|
| Nitrogen fixing | Increase soil nitrogen content by fixing atmospheric N and make it available to the plants | Free-living | Azotobacter, Anabaena, Clostridium, Aulosira Bejerinkia, Nostoc, Klebsiella, Stigonema, Desulfovibrio, Rhodospirillum, and Rhodopseudomonas | [15] |
| Symbiotic | Rhizobium, Frankia, Anabaena azollae, and Trichodesmium | |||
| Associative symbiotic | Azospirillum spp., Herbaspirillum spp., Alcaligenes, Enterobacter, Azoarcus spp. Acetobacter diazotrophicus | |||
| Phosphorus solubilizing | Solubilize the insoluble forms of P in the soil into soluble forms by secreting organic acids and lowering soil pH to dissolve bound phosphates | Bacteria | Bacillus circulans, B subtilis, Pseudomonas striata, Penicillium spp. B. polymyxa Microccocus Agrobacterium, Aereobacter and Flavobacterium | [16] |
| Fungi | Penicillum spp., Aspergillus awamori and Trichoderma | |||
| Phosphorus mobilizing | Transfer phosphorus from the soil to the root cortex. These are broad spectrum bio-fertilizers. | Mycorrhiza | Arbuscular mycorrhiza, Glomus spp., Gigaspora spp., Acaulospora spp., Scutellospora spp., and Sclerocystis spp. | [17] |
| Potassium solubilizing | Solubilize potassium (silicates) by producing organic acids that decompose silicates and help in the removal of metal ions and make it available to plants. | Bacteria | Bacillus. mucilaginosus, B. circulanscan, B. edaphicus, and Arthrobacter spp. | [18] |
| Fungi | Aspergillus niger. | |||
| Potassium mobilizing | They mobilize the inaccessible forms of potassium in the soil. | Bacteria | Bacillus spp. | [19] |
| Fungi | Aspergillus niger. | |||
| Micronutrient | Oxidizing sulfur to sulfates which are usable by plants. | Sulfur oxidizing | Thiobacillus spp. | [20] |
| Solubilize the zinc by proton, chelated ligands, acidification, and by oxidoreductive systems. | Zinc solubilizing | Mycorhiza Pseudomonas spp., and Bacillus spp. | [21] | |
| Plant growth Promoting | Produce hormones that promote root growth, improve nutrient availability, and improve crop yield | Plant growth promoting rhizobacteria | Pseudomonas spp. Agrobacterium, Pseudomonas fluorescens, Arthrobacter, Erwinia, Bacillus, Rhizobium, Enterobacter, Streptomyces, and Xanthomonas | [22] |
2.1. Nitrogen Fixing Biofertilizers
Nitrogen is the most limiting nutritional factor for plant growth [23]. The atmosphere contains about 80% of the nitrogen in free state, but most of the plants cannot utilize atmospheric nitrogen. A specialized group of microbes are required to fix this nitrogen and make it available to the plant. These microorganisms are known as biological nitrogen fixers (BNFs). They transform the inert N2 into plant-usable organic form [24]. Nitrogen fixation can provide 300–400 kg N/ha/yr and increase the crop yield by 10–50%. In plants, up to 25% of total nitrogen comes from N-fixation. The roots of plants release substances into the soil, which support colonization and nitrogen fixation by bacteria in the rhizosphere of plants. They can efficiently substitute for chemical fertilizers to a varied extent, thus reducing the chemical load from the environment. A rough approximation of such substitution is shown in Table 2. They are grouped into free-living bacteria (Azotobacter and Azospirillium), blue-green algae, and symbionts, such as Rhizobium, Frankia, and Azolla. The N2-fixing bacteria associated with legumes include Rhizobium, Mesorhizobium, Azorhizobium, Bradyrhizobium, Sinorhizobium, and Allorhizobium and those with non-legumes include Achromobacter, Alcaligenes, Arthrobacter, Acetobacter, Azomonas, Beijerinckia, Clostridium, Bacillus, Enterobacter, Erwinia, Desulfovibrio, Derxia, Corynebacterium, Campylobacter, Herbaspirillum, Klebsiella, Lignobacter, Mycobacterium, Rhodospirillum, Rhodo-pseudomonas, Xanthobacter, Mycobacterium, and Methylosinus [25]. Although many genera are isolated from the rhizosphere, mainly members of Azospirillum and Azotobacter genera have been widely tested to increase yield of legumes and cereals under field condition [26]. The main N2-fixing bacteria are described below.
Table 2. Substitution of nitrogen by biofertilizers. Modified: [27].
| Biofertilizers | Substitutes/Ha/Year |
|---|---|
| Rhizobium | 50–100 kg N |
| Azolla | 9.2–18.4 kg N |
| Azospirillum | 27.6 kg N (maize) |
| Blue Green Algae | 24.8–29.9 kg N |
| Frankia | 89.7 kg N |
2.1.1. The Rhizobium
Rhizobium belongs to the bacterial family Rhizobiaceae and is the best example of symbiotic nitrogen fixation. It can fix N2 in legumes as well as in non-legume crops. Rhizobium has been shown to fix up to 300 kg N/ha/year in different legume crops [28]. The bacteria infect the legume root and form nodules, within which they reduce molecular nitrogen to ammonia, which is utilized by the plant to produce proteins, vitamins, and other nitrogen-containing compounds. Thus, these root nodules act as factories of ammonia production [29]. Rhizobium species improve the growth of non-legumes by inducing changes in root morphology and growth physiology. The Rhizobium application increased crop growth by improving plant height, seed germination, leaf chlorophyll, and N content [30]. Seed inoculation of rice with different strains of Rhizobium at graded levels of N increased straw yield by 4% to 19% and rice grain yield by 8% to 22% [31]. Rhizobium, Bradyshzodium, Sinorhizobium, Azorhizobium, and Mesorhizobium are collectively called rhizobia. They can act directly by fixing nitrogen or influencing plant hormones or indirectly by decreasing the inhibitory effects of pathogens [32]. Rhizobium is commonly used in agronomic practices to ensure adequate nitrogen (approximately 80% of biologically fixed N comes from symbiosis) and have potential to replace chemical N fertilizers [33]. Rhizobium maintains the soil fertility along with higher crop yields [34]. Datta et al. isolated Rhizobium strains and concluded that growth and yield parameters were significantly enhanced by Rhizobium application in comparison to the control [35].
2.1.2. Azotobacter
Azotobacter is a free-living, nitrogen-fixing diazotrophic bacterium; it plays an important role in the nitrogen cycle because of its various metabolic functions [36]. Azotobacter has the ability to produce vitamins like thiamine and riboflavin [37]. It belongs to the family Azotobacteriaceae and is used as a biofertilizer for all non-leguminous plants, especially rice, cotton, vegetables, sugarcane, sweet potato, and sweet sorghum. Dutta and Singh (2002) reported a significant increase in seed yield in rapeseed and mustard following Azotobacter inoculation. It fixes almost 30 kg/N/year; it is mainly commercialized for sugarcane crop, as it increases the cane yield by 25–50 tons/hectares and sugar content by 10–15%. Azotobacter is present in both alkaline and acidic soils. A. chroococcum is the most prevalent species found in the soil, but other species like A. vinelandii, A. insignis, A. beijerinckii, and A. macrocytogenes are also found [38]. Eklund et al. (2013) demonstrated that the presence of A. chroococcum in the rhizosphere of cucumber and tomato was correlated with increased growth and germination of seedlings [39][40]. Another study showed inoculation with A. chroococcum caused a significantly increase in plant growth compared to control [41]. Azotobacter also produces antifungal compounds and antibiotics that inhibit the growth of several pathogenic fungi in the root zone and help prevent seedling mortality [42][43]. The major limiting factor for the proliferation of Azotobacter is the presence of reduced amount of organic matter in the soils; consequently, the rhizoplane lacks Azotobacter cells [31][44].
2.1.3. Azospirillum
This bacterium is also essential, as it fixes a considerable amount of nitrogen in the soil. It is associated with the rhizospheric region and fixes up to 20–40 kg N/ha in non-leguminous plants, such as cereals, millets, oilseeds, cotton, and sorghum [45]. It mostly forms a symbiotic association with plants. Several studies have shown the potential of Azospirillum for crop improvement [46][47][48][49]. Azospirillum-inoculated wheat seedlings developed good water status; fresh weight was higher from inoculated seeds than from non-inoculated seeds. Somers et al. (2005) showed that A. brasilense could synthesize phenyl acetic acid (PAA), an auxin-like molecule with anti-microbial activity. It is demonstrated that the co-inoculation of A. lipoferum and B. megaterium provided balanced nitrogen and phosphorus nutrition to the plant and produced a higher yield than inoculation with only Azospirillum [50].
2.1.4. Anabaena Azollae
It is a symbiotic bacterium and used to fix the atmospheric nitrogen, mostly in rice [51][52]. It is always associated with the free-floating fern known as Azolla. Azolla leaves contain 4–5% nitrogen (on dry weight basis) and 0.2–0.4% (on wet weight basis), quickly decompose, and provide the nitrogen to the plant. The Azolla-Anabaena system contributes 1.1 kg N/ha/day; one crop of Azolla provided 20–40 kg N/ha to the rice crop in about 20–25 days [53]. Azolla is used as a biofertilizer in many countries, such as Vietnam, China, Thailand, and the Philippines [54][55][56]. Another benefit of using this biofertilizer is its metal tolerance ability; thus, can be applied to the heavy metal-polluted areas [57].
2.1.5. Blue-Green Algae (Cyanobacteria)
Nitrogen-fixing cyanobacteria are the most widespread N2 fixers on the earth. Cyanobacteria or blue green algae are a diverse group of prokaryotes consisting of Nostoc, Anabaena, Oscillatoria, Aulosira, and Lyngbya [58]. They play an important role in nourishing the soil with nitrogen and supply vitamin B complex and growth-promoting substances like auxins, indole acetic acid, and gibberellic acid, which accelerate plant growth. They fix 20–30 Kg/N/ha in submerged rice fields and increase crop yield by 10–15% when applied at 10 Kg/ha. Reportedly, N availability to plants was increased by the application of cyanobacteria in agriculture, particularly in the rice fields [59][60]. Cyanobacteria have been shown to enhance seed germination, shoot and root growth, and yield of wheat and rice. In rice fields, blue-green algae can fix 25–30 kg N/ha/season [61]. In a study, the effect of the exudates of the cyanobacterial strains were tested on the growth parameters of Sorghum durra and Helianthus annuus. Shoot length was increased to about 120–242% as compared to control with various other positive effects [62]. Strains showed potential for releasing bioactive compounds and enhanced the plant growth and yield. In another study, rice inoculation with cyanobacteria isolated from rice field showed the positive effects simultaneously on rice plant and soil properties [63]. Application of cyanobacteria as biofertilizers is useful for economically weak farmers who are unable to invest in costly chemical fertilizers. Cyanobacterial biofertilizers can be used for a variety of biomes and environments (terrestrial, rain, or desert) [64][65].
2.2. Phosphate Solubilizing and Mobilizing Biofertilizers
Plant contains about 0.2% of phosphorus on a dry weight basis, and it is an essential nutrient for plant growth and development. Compared to other macro nutrients, phosphorus is so far the least mobile nutrient available to plants under most soil conditions. Microorganisms are needed to convert the insoluble forms of phosphate to the soluble forms [66][67]. Several bacteria and a few fungi species are involved in the phosphate solubilizing process [68]. The phosphate-solubilizing bacteria (PSB) convert the insoluble phosphate, such as HPO4 and H2PO4, into the soluble form by using different mechanisms, including the production of organic acids, chelation, and ion exchange reactions. Among the microbial populations, phosphate-solubilizing bacteria account for 1–50%, whereas fungi account for only 0.1–0.5% of phosphate-solubilizing activities [69]. The PSB can release metabolites, such as organic acids, having hydroxyl (gluconic) and carboxyl (ketogluconic) groups that chelate the cation bound to the phosphate and convert it to the soluble form, which is utilized by the plants. The secreted acids also reduce the pH of the soil and dissolve the bound phosphate to make it available to the plants [20]. Along with the organic method, microorganisms also use the proton-extrusion mechanism to solubilize the phosphate [70][71]. The PSB provide the phosphate as well as other trace elements, such as Fe and Zn, ultimately enhancing the plant growth. They also synthesize the enzyme that kills the pathogens, thus protecting the plant from diseases. Strains from bacterial genera Pseudomonas, Bacillus, Rhizobium, and Enterobacter, along with Penicillium and Aspergillus fungi, are well-known phosphate solubilizers [72]. The application of Pseudomonas fuorescens strain in acidic soils of Cameroon significantly improved the shoot length, grain yield, plant dry weight, and seed phosphorus content in maize [73]. In a recent study, phosphorus solubilizer Aspergillus niger was evaluated for its efficiency as biofertilizer; it significantly increased the plant height, fruit size, leaf length/width, and number of fruits per plant when compared with untreated plants. However, plants co-inoculated with both phosphorus solubilizing (A. niger) and the N fixing Azotobacter showed more improved performance than those treated with each biofertilizer alone [74].
Phosphate-mobilizing microbes can mobilize the immobile forms of phosphorous [75]. They transfer and mobilize the insoluble phosphate from soil layers to the root cortex. Arbuscular mycorrhiza is an example of phosphate-mobilizing fungi, in which fungi penetrate the roots and increase the surface area of roots, stimulate metabolic processes, and absorb the nutrients into the roots. Reportedly, phosphorus-solubilizing bacteria (PSB) sometimes act as phosphate mobilizers [17]. Under optimum condition, they have potential to solubilize/mobilize about 30–50 kg P2O5/ha, due to which crop yield could increase by 10–20% [76].
2.2.1. Mycorrhiza
It is a symbiotic association between the host plant and a certain group of fungi. It is arguably the most important symbiosis on earth [77][78]. This association provides the essential nutrients to the plant, mostly phosphorous and growth hormones, which promote plant growth. They also increase the surface area of roots to increase the absorption of nutrients from the soil and provide resistance to plants against plant pathogens [79]. The hyphae of fungi absorb the insoluble phosphorus and convert it into the solubilized form, which is taken up by the plant and, in return, the plant provides shelter and other nutrients to the fungi. These fungi are ubiquitous in geographical distribution and are associated with all crops, except Brasicacea [13].
2.2.2. Endomycorrhiza or VAM Fungi
Vesicular arbuscular mycorrhiza (VAM) is the symbiotic association between certain phycomycetous fungi and angiosperm roots. These symbiotic soil fungi colonize the roots of approximately 80% of plant families [80][81]. They enhance the transfer of nutrients from the soil into the root system via specialized structures known as vesicles and arbuscules. This association provides many benefits to the plant. The fungal hyphae enhance the uptake of phosphorous and other nutrients as well as increase the root and shoot length. They also help the plant to uptake a large amount of water from the roots. VAM can potentially increase plant tolerance to various biotic and abiotic stresses and could replace the fertilizer requirements of trees and reduce the needs of current levels of chemical fertilizers [82][83][84]. VAM fungi could contribute to more than twofold increased acquisition of the less mobile nutrients like P, S, Ca, Mg, Zn, and Cu from the rhizosphere [85]. Six genera of fungi have been shown to form mycorrhizal associations: Glomus, Acaulospora, Gigaspora, Sclerocystis, Entrophospora, and Scutellospora [86]. Co-inoculation treatment of VAM fungi, Glomus fasciculatum with Bradyrhizobium sp. + Pseudomonas striata or Penicillium variable, significantly increased the nutrient uptake and plant yield [87]. In addition, VAM fungi enhance the uptake of nutrients by secreting the enzymes and organic acids. An increased concentration of K was found in mycorrhizal plants in comparison to non-mycorrhizal plants [27].
2.3. Potassium Solubilizing and Mobilizing Biofertilizers
Potassium (K) is the second most abundant and important plant nutrient after nitrogen and phosphorus. Although K is an abundant element in the soil, only 1–2% is available to plants, whereas the rest is present as mineral K that cannot be taken up by plants. Therefore, a continuous K replenishment of soil solution is required [70][88]. It plays a vital role in the growth and development of plants. If not supplied in adequate quantity, the plants will grow slowly, have poorly developed roots, and produce small seeds and low yields [89][90]. It has been reported that a wide range of bacterial and fungal strains use various mechanisms, including the production of acids, chelation, acidolysis, complexolysis, and exchange reactions to solubilize the insoluble K into soluble forms [18][91][92]. Examples of potassium-solubilizing biofertilizer include Bacillus spp. and Aspergillus niger. Arthrobacter spp., Cladosporium, and Sphingomonas aminobacter with varying potential for K solubilization. B. edaphicus and B. mucilaginosus are known to improve solubilization as well as mobilization. B. mucilaginosus, when inoculated in soil, improved the oil content and groundnut biomass by 35.4% and 25%, respectively, along with enhanced K and P availability [93]. Recently, a study has shown that a potassium-solubilizing strain Bacillus pseudomycoides enhanced K uptake in tea plants in the mica waste-treated soil by increasing potassium availability [94]. Another strain Bacillus cereus significantly increased the plant height, shoot dry weight, and branches number by about 15%, 26%, and 27%, respectively, compared to the control [95]. Some fungi like Aspergillus spp., and Penicillium spp. also have potential to solubilize and mobilize K from organic and inorganic sources [96]. Thus, role of K solubilizers is significant for ensuring the regular supply of K to crop plants. These also exert positive impact on the availability of other essential nutrients to the soil, and thus play an important role in maintaining soil sustainability [97].
2.4. Sulfur Oxidizing Biofertilizers
Sulfur as a micronutrient is also required by the plants. It has been reported that sulfur plays a key role in improving certain biological and physical properties of the soil. Sulfur is famous for soil buffering from high pH values. Previous studies have shown that sulfur also promotes the efficiency of nitrogenous and phosphorus fertilizers and increases the efficiency of crops to uptake micronutrients [98]. An example of sulfur-oxidizing microbe is Thiobacillus spp.; Thiobacillus thioparous and T. thioxidans can oxidize sulfur to plant usable sulfates that help in nourishment of plants [99][100]. A recent study has shown that inoculation of Thiobacillus along with elemental sulfur increases the oxidation of elemental sulfur, resulting in increased nutrients availability in soil and consequently increased plant growth [101]. Sulfur compounds, especially in reduced form, significantly pollute the environment. Sulfur oxidizing bacteria also play significant roles in environmental protection by biological elimination of sulfur pollution [102].
2.5. Zinc Solubilizing Biofertilizers
Zinc is one of the essential micronutrients required at relatively small concentrations (5–100 mg/kg) in tissues for the growth and reproduction of plants. Zinc deficiency is very common in soil that results from the increased application of fertilizers in an imbalanced manner, intensive agriculture, and poor soil health. It is estimated that by 2025, zinc deficiency may increase from 42% to 63% if the contributing reasons are neglected. Zinc is involved in the synthesis of growth hormones. Zinc deficiency in plants leads to retarded shoot growth, reduced membrane integrity and reduced leaf size, chlorosis, and increased susceptibility to light, heat, and fungal infections and affects grain yield, root development, pollen formation, and water uptake and transport [21][103]. Zinc deficiency can lead to yellowing of leaves and stunted growth in wheat. Consuming zinc-deficient wheat can lead to zinc deficiency in humans as well [21]. Zinc deficiency is considered the fifth most important human-related death in less developed countries. Therefore, addressing Zn deficiency in agriculture is getting top priority among other minor nutrients [104][105][106]. Microbial inoculants have been identified to solubilize the complex form of zinc in soil [107]. Mycorrhiza, Saccharomyces spp., and several genera of rhizobacteria such as Pseudomonas spp. and Bacillus spp. are reported to increase Zn availability in soil. These microbes solubilize the zinc by chelated ligands and oxidoreductive systems [21][108]. These bacteria also produce phytochromes, antibiotics, vitamins, and antifungal substances, and help the plant in many aspects [109]. In a study, rice plants inoculated with a suitable combination of Zn solubilizing bacterial strains (Burkholderia spp. and Acinetobacter spp.) increased the growth attributes and rice yield and were found more efficient in acquiring Zn from the soil as compared to non-inoculated plants [110]. Biofertilizers containing Zn solubilizing bacteria have been reported to boost up the maize production [111].
2.6. Plant Growth Promoting Rhizobacteria (PGPR)
A group of free-living rhizosphere bacteria that colonize plant roots and exert a beneficial effect on plant growth are referred to as PGPR [112]. They act as biofertilizers by promoting growth and development of plants, facilitating biotic and abiotic stress tolerance, and helping in the mineralization of the soil by decomposing organic matter. Inoculation of PGPR imparts various beneficial effects to the plant. They increase the tolerance of plant to drought [113][114][115][116], salinity [117][118], and biotic stress [119][120]. They enhance the seed germination [121][122] and soil fertility [123][124] and promote growth by producing phytohormones including Auxins, IAA, ethylene, gibberellin, etc. [125][126][127]. They can modulate plant secondary metabolites and bioremediation of heavy metals and pollutants [128][129][130][131][132]. PGPR includes member of several genera, e.g., Agrobacterium, Arthrobacter, Alcaligenes, Azotobacter, Acinetobacter, Actinoplanes. Bacillus, Frankia, Pseudomonas, Rhizobium, Micrococcus Streptomyces, Xanthomonas, Enterobacter, Cellulomonas, Serratia, Flavobacterium, Thiobacillus, etc. [133]. The detailed contribution of PGPR to plant growth promotion and their modes of action has been included in several reviews [134][135][136][137][138][139][140][141].
3. The Potential and Suitability of Biofertilizers for Crops
Biofertilizers play an important role in improving soil fertility and enhancing crop yield [142]. When applied to the soil, they participate in nutrient cycling and improve the soil structure and crop productivity [143][144]. The uniqueness of microbes and their capabilities in environmental and cultural conditions have made them potential candidates in the agriculture field for resolving food related issues [145]. The use of potential biofertilizers will not merely play a key role in efficiency and sustainability of soil, but also conserve the environment by reducing the adverse impact of agricultural practices and improving the food quality as well [146]. Biofertilizers solubilize the key nutrients and make them available to the plants. They contribute to the development of root hairs and consequently improve water uptake by plants [147][148]. They produce phytohormones and improve the soil fertility, consequently improving the growth and development of plants without detrimental side-effects [149]. A list of beneficial traits that these microbes impart to the plants can be seen in Figure 1. Researchers are continuously working on the development of biofertilizers to increase their application in agricultural industry for sustainable ecosystem and holistic well-being.
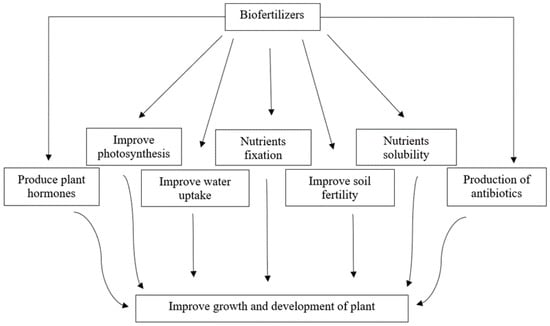
Figure 1. Potential role of microbes as biofertilizers in the growth and development of plant.
Different crops need different biofertilizers for better results. Table 3 is explaining the best biofertilizers for the different crops. Later scientists observed that specific combination of biofertilizers gives better results as compared to inoculation of single/individual fertilizer. For field-grown maize, inoculation with Azotobacter and Azospirillum significantly increased the grain yield, and total dry weight was increased up to 115% [150]. Similarly, it is reported that inoculation of rice seedlings with Azospirillum spp. and Azotobacter spp. successfully substituted inorganic nitrogen fertilizer and increased rice yield from 2–3 t·ha−1 to 3.9 to 6.4 t·ha−1 [151]. Another research has tested the impact of rice root inoculation on the yield under different nitrogen fertility levels. Surprisingly, the significant yield was observed at the lowest level of inorganic N fertilization [152]. Using phosphorus solubilizing bacteria as biofertilizers could increase the sugarcane yield up to 50%, thus saving 50% of costly phosphate fertilizer [153].
Table 3. Biofertilizers for the specific crops.
| Biofertilizer | Function | Crops | References |
|---|---|---|---|
| Rhizobium (symbiotic) | Fixes 200–300 kg N/ha/year | Pea, pulses legumes, cow pea, green gram, black gram, groundnut, soyabean, berseem, wheat, jowar, bajra, maize | [27][154] |
| Increases yield up to 10–30% | |||
| Maintain soil fertility | |||
| Azotobacter | Supplies 20–40 kg N/ha/year | Mustard, sunflower, banana, sugarcane, grapes, papaya, watermelon, tomato, chilly, lady finger, coconut | [5][155][156] |
| Promote growth substances such as vitamins, IAA, gibberellic acids. | |||
| Increase yield up to 10–15% | |||
| Maintain soil fertility | |||
| Azospirillum | Fixes 20–160 kg N/ha /year | Rice, sugarcane, millet, wheat, sorghum, bajra | [85][157] |
| Increase water and mineral uptake | |||
| Production of plant hormones | |||
| Enhance root growth | |||
| Increase crop yield | |||
| Blue-green algae | Fixes 20–40 kg N/ha/year | Rice | [59] |
| Promote growth substances such as vitamins, IAA, Gibberelic acids | |||
| Azolla | Fixes 30–60 kg N/ha/year | Rice | [158] |
| Used as green manure | |||
| Arbuscular mycorrhizal fungi (symbiotic) | Increase root absorption area for nutrient access | Soybean, wheat, and corn | [159] |
| Fixes phosphate | |||
| Increase crop yield | |||
| Pseudomonas | Production of siderophores and plant hormones | Potato, reddish, sugar beat | [160] |
| Fixes phosphate | |||
| Increase crop yield | |||
| Bacillus spp. | Solubilize the phosphate and fix the nitrogen in soil | Many vegetables and fruits | [161][162] |
| Synthesis of growth hormones | |||
| Production of antibiotics | |||
| Increase crop yield |
Chickpea is one of the major pulse crops grown in many countries worldwide. Single and combined inoculation of bacterial and fungal strains (Bacillus sp. RM−2 and Aspergillus niger S−36) significantly enhanced many growth parameters of chickpea over the control. However, dual inoculation of bacterial and fungal species was found more effective comparatively than their respective single inoculations [163]. The findings of Mohammadi et al. [164] showed that biofertilizers significantly enhanced the nutrient uptake of chickpea. The effects of soil fertility on chlorophyll, nutrients, grain yield, and yield components of chickpea seed are shown briefly in Table 4. Combined application of PSB and Trichoderma produced the highest leaf P content (0.33%) and grain P content (279 mg 100 g−1). The production of acids by Bacillus spp. under P-limited conditions may increase the solubility of phosphorus. The same study also revealed that chickpeas inoculated with biofertilizer (PSB+ Trichoderma) possess significantly increased grain protein content (15.06%). A meta-analysis study has confirmed that the combined application of N fixers and P solubilizers significantly increases the yield as compared to single inoculation. This indicates the synergies between both fertilizers instead of competition [165]. Another study tested the inoculation of soil with two cyanobacterial species (Nostoc entophytum and Oscillatoria angustissima) as biofertilizers for pea plants that significantly enhanced the growth, germination percentage, and photosynthetic pigment fraction. However, the most effective results were noted with inoculation of one species and a half dose of chemical fertilizer, which allowed saving of 50% of chemical fertilizers [166]. Another group also tested the efficiency of biofertilizers, and they concluded that inoculation of the wheat plant with biofertilizers (Azotobacter, Yeast, and Azotobacter + Yeast) resulted in significantly higher values of most of the growth and yield parameters. They noted that mixed inoculums were found better than single inoculums. [167].
Table 4. The effect of soil fertility on chlorophyll, nutrients, grain yield, and yield components of chickpea seed. Modified: Mohammadi et al. [164].
| Biofertilizers | Chlorophyll | N | P | K |
| mg/100 gm | ||||
| PSB | 43.41 b | 2269 b | 273.5 b | 1201.1 b |
| Trichoderma | 43.35 b | 2295 b | 266.2 c | 1176.3 c |
| PSB + fungi | 44.12 a | 2315 a | 299.8 a | 1232.1 a |
| Control | 43.22 b | 2167 c | 264.9 c | 1199.8 b |
| Biofertilizers | Grain Yield (kg/ha) | Pod Number Per Plant | Fertile Pod Per Plant | |
| PSB | 1756.1 c | 39.72 b | 25.84 c | |
| Trichoderma | 1866.2 b | 40.79 b | 27.41 b | |
| PSB + fungi | 2560.3 b | 57.66 a | 35.07 a | |
| Control | 1310.7 d | 30.83 c | 20.73 d | |
Mean values in each column with the same superscript(s) do not differ significantly (p = 0.05).
Habibi et al. (2011) strongly suggested that using combined strains in biofertilizers plus a half dose of chemical or organic fertilizers could significantly increase oil and grain yield in medicinal pumpkin [168]. They showed that biofertilizers improved the efficiency of traditional chemical fertilizers, ultimately reducing the use of expensive chemical fertilizers and reducing environmental pollution. Another study reported that the integration of biofertilizers with chemical fertilizers produced maximum rice yield [169]. They combined nitrogen, phosphorus, and potassium chemical fertilizers with biofertilizers (Azospirillum, Azotobacter, and Azolla) and obtained the highest grain yield and straw yield (3.57 and 4.32 t/ha, respectively) of rice. Then they grew peanut crop on the residual soil and found the highest pod yield. This revealed that biofertilizers applied to one crop can have beneficial effects on the next crop too.
The use of biofilm is also getting popular in biofertilizer technology. This technique was tested on wheat crops in which a biofilm prepared from Anabaena torulosa was used as a matrix for Azotobacter, Pseudomonas, Serratia, and Mesorhizobium, and it resulted in 40–50% increased nitrogen fixation [170]. Biofertilizers have been shown to enhance plant tolerance to environmental stresses. The study has shown that inoculation of plants with arbuscular mycorrhiza fungi enhances the plant growth under salt stress [171]. A study demonstrated that Psuedomonas spp. exert a positive effect on the growth and germination of seeds under water stress. [172]. Another study has shown that the inoculation of mycorrhiza increased the photosynthetic efficiency of rice plant under saline and drought conditions [173]. Biofertilizers are also useful to protect the crops from the hazardous effects of heavy metals. A study revealed that the use of biofertilizers was found effective in moderating cadmium toxicity in the soil for sunflower and maize cultivation [174].
4. Biofertilizers: A Hero or a Villain in the Field
Biofertilizers are very helpful in getting a high yield of crops. They can convert the insoluble form of nutrients to the soluble form, making the soil rich and suitable for the proper growth of plants. The biofertilizers come as an alternative to chemical fertilizers. Chemical fertilizers release the chemicals that are damaging to our soil and environment. Biofertilizers contain the natural component, which does not harm the plant. In fact, they protect the plant from other diseases, fungal attack, and free pollutants [8]. Biofertilizers also protect the plants from strict conditions like drought, alkalinity, etc. Biofertilizers are of lower cost than the chemical fertilizers, but these biofertilizers sometimes deceive the farmer. Regarding the specific type of biofertilizers used for specific crops, the choice should be correct. The knowledge of biofertilizer composition is crucial to exploit the synergistic action of various microbes. The biological and chemical interaction of biofertilizers with crop and soil is very complex. These interactions are highly affected by moisture, pH, temperature, and other environmental variables, which ultimately affect the efficacy of biofertilizers. That is why a good understanding of plant and microbe interrelationships is mandatory [144]. If the conditions are not right for the microbes to multiply and do their work, their populations are likely to peter out. In other words, the user would have wasted time and money on a product that was not suitable according to the soil conditions. Thus, great care should be taken in choosing the biofertilizers to achieve the maximum results. There is a great need to overcome the biofertilizer production, market level, and resources constraints that include improper handling of strains, lack of farmers awareness, limited investment for production units, etc. Biofertilizers being living organisms required proper facilities for handling, transportation, and storage [13].
5. Potential of Biofertilizers in Agriculture Market
The market of biofertilizers is continuously expanding due to rising awareness of biofertilizers towards the growing economy. The market of biofertilizers was valued about $440 million in 2012 globally and is expected to grow at the rate of 10% per annum [175]. Rhizobia is famous for being used as a biofertilizer, constituting 79% of world demand, while phosphate mobilizing biofertilizers constitute about 15% [176]. Manufacturing companies and regulatory government authorities are the main stakeholders of the biofertilizers market. There are many companies in the market ensuring safe production and distribution of biofertilizers. However, there are still some countries like in Africa and Asia that are suffering from hunger and malnutrition but cannot access the latest agricultural technologies. The strategy of using biofertilizers can play a significant role in this direction, as these fertilizers can be easily produced by small companies and can be used in small agricultural lands. Azospirillum is an excellent example of this in America; it can dramatically increase plant growth. They selected promising Azospirillum strains by rigorous testing in the field and, after suitable formulations, were directed for production and commercialization. Nowadays, more than 100 products of Azospirillum strains are commercially available, which aimed to enhance crop yield mainly in wheat, maize, and soybean in South America [177]. Similarly, 167 million hectares area and one lakh hectares area are cultivated as organic farming using biofertilizers in China and India, respectively [178].
6. Limitations in the production of biofertilizers
Though biofertilizers have proven their worth in agriculture with promising results over the past 50 years, desired success is still to be achieved. Several constraints limit the application of this technology at large scale. Some of the possible reasons including competition of bioinoculant and natural flora of soil for niche, poor soil characteristics, presence of environmental and soil pollutants and extreme climatic conditions, unavailability of appropriate strain and suitable carrier material, unavailability of skilled and experienced staff in production unit, unavailability of sufficient funds and equipment from government and private bodies, lack of storage and transport facilities, lack of awareness among farmers, marketing constraints like unavailability of a suitable strain at a suitable place at the right time, lack of regulations and standards for production, and lack of promotion network. A list of possible constraints with recommendations that need to be taken care can be seen in Table 5. Practically, all of these factors determine the potential success of biofertilizers.
Table 5. Constraints/limitations in the production and commercialization of biofertilizers.
|
Constraints/Limitations |
Recommendations |
|
|
Technical constraints |
Unavailability of suitable carrier material |
Identification and selection of appropriate economic carrier to maintain shelf life and effectiveness of biofertilizers. |
|
Skilled staff should be hire and manpower should be trained via proper training. |
||
|
Lack of skilled staff in production units |
Frequent monitoring of the biofertilizer production units for quality assurance |
|
|
Marketing constraints |
Lack of regulation and standards for biofertilizer |
Necessary legislation for monitoring biofertilizers should be done by government. |
|
Limited transportation and storage facilities |
||
|
Poor and incomplete labeling of biofertilizer products |
Proper labeling of biofertilizers should be done (giving genus name, viable count, and expiry date, etc.) |
|
|
Lack of promotion network and publicity among the end users. |
Wide publicity of biofertilizers should be done through scientific training, fairs, exhibitions, or media. |
|
|
Biological constraints |
Unavailability of appropriate strain Tendency of strain to mutate during fermentation. |
Continuous efforts for identification of strains and screening for their efficiency across the type of soil, agro-climatic conditions, and farming situations is recommended. |
|
Nonavailability of right inoculant at the right place at the right time |
Standardization of biofertilizer dose in a particular crop and soil. |
|
|
Understanding on strain effectiveness should be strengthened through extensive research in this field |
||
|
Field-level constraints |
Soil conditions like acidity, presence of salts and toxic elements in the soil, and extreme climatic conditions may make the results of biofertilizers inconsistent. |
It is needed to strengthen the research and technologies to reduce effects and to counteract stated soil and environmental conditions. |
|
Inadequate awareness among the farming community about bio-inoculants |
A strong training and awareness program may be initiated to aware and motivate farmers. |
|
|
Financial constraints |
Nonavailability of sufficient funds and equipment from government and private bodies. |
Use of high-tech equipment is required for quality products. |
|
Government should provide funding and loans for development of production units. |
||
7. Conclusion and Prospects
Biofertilizers are a good approach to increasing crop productivity. In recent years, the biofertilizers are used to provide the essential nutrients to the plant and significantly increase its yield. These are eco-friendly, cost effective, provides the natural environment to the plant, boost the defense system of the plant, and protect the plant from drought, acidity, and other strict conditions. The advantages of biofertilizers exceed its usage from the other harmful chemical fertilizers. In this review, the most important microorganisms used as biofertilizers are explained along with their mechanism of action. It is also observed that the inoculation of two different types of biofertilizers increases the yield of crops more significantly than the single biofertilizer or solo chemical fertilizer inoculation. The increasing demand for biofertilizers reflects an eco-friendly and sustainable agriculture system in the future. However, knowledge about soil properties, field environment, and host specificity of strains is mandatory for the successful production and application of biofertilizers. Recent advances in the field of molecular biology, biotechnology, genetic engineering, microbial taxonomy, and nanotechnology have played a significant role in the production of biofertilizers with improved efficiency, higher competitive ability, and multiple functionalities. Biofertilizers can maintain crop productivity with low environmental impact and can be an effective substitute for chemical fertilizers. Research efforts are still required in this field to explore and identify soil-specific strains, to gain further insights into biofertilizer composition, and to improve the existing strains using biotechnological methods.
This entry is adapted from the peer-reviewed paper 10.3390/su13041868
References
- Mia, M.B.; Shamsuddin, Z. Rhizobium as a crop enhancer and biofertilizer for increased cereal production. Afr. J. Biotechnol. 2010, 9, 6001–6009.
- Berg, G. Plant–microbe interactions promoting plant growth and health: Perspectives for controlled use of microorganisms in agriculture. Appl. Microbiol. Biotechnol. 2009, 84, 11–18.
- Sprent, J.I.; Sprent, P. Nitrogen fixing organisms: Pure and applied aspects. Nitrogen Fixing Org. 1990, 19, 288.
- Deepali, G.K.; Gangwar, K. Biofertilizers: An Ecofriendly Way to Replace Chemical Fertilizers. 2010. Available online: (accessed on 7 February 2021).
- Thomas, L.; Singh, I. Microbial Biofertilizers: Types and Applications. In Biofertilizers for Sustainable Agriculture and Environment; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–19.
- Kawalekar, J.S. Role of biofertilizers and biopesticides for sustainable agriculture. J. Bio. Innov. 2013, 2, 73–78.
- Raghuwanshi, R. Opportunities and challenges to sustainable agriculture in India. Nebio 2012, 3, 78–86.
- Youssef, M.; Eissa, M. Biofertilizers and their role in management of plant parasitic nematodes. A review. J. Biotechnol. Pharm. Res. 2014, 5, 1–6.
- Bumandalai, O.; Tserennadmid, R. Effect of Chlorella vulgaris as a biofertilizer on germination of tomato and cucumber seeds. Int. J. Aquat. Biol. 2019, 7, 95–99.
- Mishra, P.; Dash, D. Rejuvenation of biofertilizer for sustainable agriculture and economic development. Consilience 2014, 41–61.
- Umesha, S.; Singh, P.K.; Singh, R.P. Microbial biotechnology and sustainable agriculture. In Biotechnology for Sustainable Agriculture; Elsevier: Amsterdam, The Netherlands, 2018; pp. 185–205.
- Parikh, S.J.; James, B.R. Soil: The foundation of agriculture. Nat. Educ. Knowl. 2012, 3, 2.
- Mahdi, S.S.; Hassan, G.; Samoon, S.; Rather, H.; Dar, S.A.; Zehra, B. Bio-fertilizers in organic agriculture. J. Phytol. 2010, 2, 42–54.
- Raynaud, X.; Nunan, N. Spatial ecology of bacteria at the microscale in soil. PLoS ONE 2014, 9, e87217.
- Choudhury, A.; Kennedy, I. Prospects and potentials for systems of biological nitrogen fixation in sustainable rice production. Biol. Fertil. Soils 2004, 39, 219–227.
- Board, N. The Complete Technology Book on Bio-Fertilizer and Organic Farming; National Institute of Industrial Research: Delhi, India, 2004.
- Chang, C.-H.; Yang, S.-S. Thermo-tolerant phosphate-solubilizing microbes for multi-functional biofertilizer preparation. Bioresour. Technol. 2009, 100, 1648–1658.
- Etesami, H.; Emami, S.; Alikhani, H.A. Potassium solubilizing bacteria (KSB): Mechanisms, promotion of plant growth, and future prospects A review. J. Soil Sci. Plant Nutr. 2017, 17, 897–911.
- Jha, Y. Potassium mobilizing bacteria: Enhance potassium intake in paddy to regulates membrane permeability and accumulate carbohydrates under salinity stress. Braz. J. Biol. Sci. 2017, 4, 333–344.
- Itelima, J.; Bang, W.; Onyimba, I.; Oj, E. A review: Biofertilizer; a key player in enhancing soil fertility and crop productivity. J. Microbiol. Biotechnol. Rep. 2018, 2, 22–28.
- Kamran, S.; Shahid, I.; Baig, D.N.; Rizwan, M.; Malik, K.A.; Mehnaz, S. Contribution of zinc solubilizing bacteria in growth promotion and zinc content of wheat. Front. Microbiol. 2017, 8, 2593.
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1473.
- Gupta, G.; Panwar, J.; Akhtar, M.S.; Jha, P.N. Endophytic nitrogen-fixing bacteria as biofertilizer. In Sustainable Agriculture Reviews; Springer: Berlin/Heidelberg, Germany, 2012; pp. 183–221.
- Reed, S.C.; Cleveland, C.C.; Townsend, A.R. Functional ecology of free-living nitrogen fixation: A contemporary perspective. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 489–512.
- Meena, V.S.; Mishra, P.K.; Bisht, J.K.; Pattanayak, A. Agriculturally Important Microbes for Sustainable Agriculture: Volume 2: Applications in Crop Production and Protection; Springer: Berlin/Heidelberg, Germany, 2017.
- Bhat, T.A.; Ahmad, L.; Ganai, M.A.; Khan, O. Nitrogen fixing biofertilizers; mechanism and growth promotion: A review. J. Pure Appl. Microbiol. 2015, 9, 1675–1690.
- Brahmaprakash, G.; Sahu, P.K. Biofertilizers for sustainability. J. Indian Inst. Sci. 2012, 92, 37–62.
- Pindi, P.K. Liquid Microbial Consortium- A Potential Tool for Sustainable Soil Health. J. Fertil. Pestic. 2012, 3, 124.
- Flores-Félix, J.D.; Menéndez, E.; Rivera, L.P.; Marcos-García, M.; Martínez-Hidalgo, P.; Mateos, P.F.; Martínez-Molina, E.; Velázquez, M.d.l.E.; García-Fraile, P.; Rivas, R. Use of Rhizobium leguminosarum as a potential biofertilizer for Lactuca sativa and Daucus carota crops. J. Plant Nutr. Soil Sci. 2013, 176, 876–882.
- Sara, S.; Morad, M.; Reza, C.M. Effects of seed inoculation by Rhizobium strains on chlorophyll content and protein percentage in common bean cultivars (Phaseolus vulgaris L.). Int. J. Biosci. 2013, 3, 1–8.
- Sammauria, R.; Kumawat, S.; Kumawat, P.; Singh, J.; Jatwa, T.K. Microbial inoculants: Potential tool for sustainability of agricultural production systems. Arch. Microbiol. 2020, 202, 677–693.
- Mabrouk, Y.; Hemissi, I.; Salem, I.B.; Mejri, S.; Saidi, M.; Belhadj, O. Potential of rhizobia in improving nitrogen fixation and yields of legumes. Symbiosis 2018, 107–122.
- Rubio-Canalejas, A.; Celador-Lera, L.; Cruz-González, X.; Menéndez, E.; Rivas, R. Rhizobium as potential biofertilizer of Eruca Sativa. In Biological Nitrogen Fixation and Beneficial Plant-Microbe Interaction; Springer: Berlin/Heidelberg, Germany, 2016; pp. 213–220.
- Arora, N.K.; Verma, M.; Mishra, J. Rhizobial bioformulations: Past, present and future. In Rhizotrophs: Plant Growth Promotion to Bioremediation; Springer: Berlin/Heidelberg, Germany, 2017; pp. 69–99.
- Datta, A.; Singh, R.K.; Tabassum, S. Isolation, characterization and growth of Rhizobium strains under optimum conditions for effective biofertilizer production. Int. J. Pharm. Sci. Rev. Res. 2015, 32, 199–208.
- Sahoo, R.K.; Ansari, M.W.; Dangar, T.K.; Mohanty, S.; Tuteja, N. Phenotypic and molecular characterisation of efficient nitrogen-fixing Azotobacter strains from rice fields for crop improvement. Protoplasma 2014, 251, 511–523.
- Revillas, J.; Rodelas, B.; Pozo, C.; Martínez-Toledo, M.; González-López, J. Production of B-group vitamins by two Azotobacter strains with phenolic compounds as sole carbon source under diazotrophic and adiazotrophic conditions. J. Appl. Microbiol. 2000, 89, 486–493.
- Kizilkaya, R. Nitrogen fixation capacity of Azotobacter spp. strains isolated from soils in different ecosystems and relationship between them and the microbiological properties of soils. J. Environ. Biol. 2009, 30, 73–82.
- Wani, S.A.; Chand, S.; Ali, T. Potential use of Azotobacter chroococcum in crop production: An overview. Curr. Agric. Res. J. 2013, 1, 35–38.
- Eklund, E. Secondary effects of some Pseudomonads in the rhizosphere of peat grown cucumber plant. Suppl. Acta Agric. Scand. 1970, 3, 613.
- Romero-Perdomo, F.; Abril, J.; Camelo, M.; Moreno-Galván, A.; Pastrana, I.; Rojas-Tapias, D.; Bonilla, R. Azotobacter chroococcum as a potentially useful bacterial biofertilizer for cotton (Gossypium hirsutum): Effect in reducing N fertilization. Rev. Argent. Microbiol. 2017, 49, 377–383.
- Bhosale, H.; Kadam, T.; Bobade, A. Identification and production of zotobacter vinelandii and its antifungal activit against Fusarium o sporum. J. Environ. Biol. 2013, 34, 177–182.
- Wani, S.A.; Chand, S.; Wani, M.A.; Ramzan, M.; Hakeem, K.R. Azotobacter chroococcum–A potential biofertilizer in agriculture: An overview. J. Soil Sci. Agric. Environ. Prospect. 2016, 333–348.
- Menendez, E.; Garcia-Fraile, P. Plant probiotic bacteria: Solutions to feed the world. AIMS Microbiol. 2017, 3, 502.
- Isawa, T.; Yasuda, M.; Awazaki, H.; Minamisawa, K.; Shinozaki, S.; Nakashita, H. Azospirillum sp. strain B510 enhances rice growth and yield. Microbes Environ. 2009.
- Skonieski, F.R.; Viégas, J.; Martin, T.N.; Nörnberg, J.L.; Meinerz, G.R.; Tonin, T.J.; Bernhard, P.; Frata, M.T. Effect of seed inoculation with Azospirillum brasilense and nitrogen fertilization rates on maize plant yield and silage quality. J. Rev. Bras. Zootec. 2017, 46, 722–730.
- Leite, R.d.C.; dos Santos, J.G.; Silva, E.L.; Alves, C.R.; Hungria, M.; Leite, R.d.C.; dos Santos, A.C. Productivity increase, reduction of nitrogen fertiliser use and drought-stress mitigation by inoculation of Marandu grass (Urochloa brizantha) with Azospirillum brasilense. J. Crop. Pasture Sci. 2019, 70, 61–67.
- Galindo, F.S.; Teixeira Filho, M.C.M.; Buzetti, S.; Rodrigues, W.L.; Fernandes, G.C.; Boleta, E.H.M.; Neto, M.B.; de Azambuja Pereira, M.R.; Rosa, P.A.L.; Pereira, Í.T. Influence of Azospirillum brasilense associated with silicon and nitrogen fertilization on macronutrient contents in corn. Open Agric. 2020, 5, 126–137.
- Oliveira, I.J.; Fontes, J.R.A.; Pereira, B.F.F.; Muniz, A.W. Inoculation with Azospirillum brasiliense increases maize yield. Chem. Biol. Technol. Agric. 2018, 5, 1–9.
- El-Komy, H. Coimmobilization of Azospirillum lipoferum and Bacillus megaterium for successful phosphorus and nitrogen nutrition of wheat plants. Food Technol. Biotechnol. 2005, 43, 19–27.
- Yadav, R.; Abraham, G.; Singh, Y.; Singh, P. Advancements in the utilization of Azolla-Anabaena system in relation to sustainable agricultural practices. Proc. Indian Natl. Sci. Acad. 2014, 80, 301–316.
- Bocchi, S.; Malgioglio, A. Azolla-Anabaena as a biofertilizer for rice paddy fields in the Po Valley, a temperate rice area in Northern Italy. Int. J. Agron. 2010, 2010, 1–5.
- Setiawati, M.R.; Damayani, M.; Herdiyantoro, D.; Suryatmana, P.; Anggraini, D.; Khumairah, F.H. The application dosage of Azolla pinnata in fresh and powder form as organic fertilizer on soil chemical properties, growth and yield of rice plant. AIP Conf. Proc. 2018, 1927, 030017.
- Fan, C. The Biological Nitrogen Fixation Systems Adopted in Rice Paddy Fields in China. In The Nitrogen Fixation and Its Research in China; Springer: Berlin/Heidelberg, Germany, 1992; pp. 423–437.
- Qiu, Y.-L.; Yu, J. Azolla—A model organism for plant genomic studies. J. Genom. Proteom. Bioinform. 2003, 1, 15–25.
- Tekle-Haimanot, A.; Doku, E. Comparison of Azolla mexicana and N and P fertilization on paddy taro (Colocasia esculenta) yield. Trop. Agric. Lond. Trinidad 1995, 72, 70.
- Akhtar, M.; Sarwar, N.; Ashraf, A.; Ejaz, A.; Ali, S.; Rizwan, M.; Science, S. Beneficial role of Azolla sp. in paddy soils and their use as bioremediators in polluted aqueous environments: Implications and future perspectives. Arch. Agron. 2020, 1–14.
- Sharma, N.K.; Tiwari, S.P.; Tripathi, K.; Rai, A.K. Sustainability and cyanobacteria (blue-green algae): Facts and challenges. J. Appl. Phycol. 2011, 23, 1059–1081.
- Singh, J.S.; Kumar, A.; Rai, A.N.; Singh, D.P. Cyanobacteria: A precious bio-resource in agriculture, ecosystem, and environmental sustainability. Front. Microbiol. 2016, 7, 529.
- Mishra, U.; Pabbi, S. Cyanobacteria: A potential biofertilizer for rice. Resonance 2004, 9, 6–10.
- Venkataraman, G.S. Blue-Green Algae for Rice Production: A Manual for Its Promotion; Food & Agriculture Org: Rome, Italy, 1981.
- Essa, A.M.; Ibrahim, W.M.; Mahmud, R.M.; ElKassim, N.A. Potential impact of cyanobacterial exudates on seed germination and antioxidant enzymes of crop plant seedlings. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 1010–1024.
- Hasan, M.A. Cyanobacteria from Rice Field and Comparative Study of Their Performances as Biofertilizer on Rice Plants. J. Glob. Biosci. 2020, 9, 8078–8087.
- Bothe, H.; Schmitz, O.; Yates, M.G.; Newton, W. Nitrogen fixation and hydrogen metabolism in cyanobacteria. Microbiol. Mol. Biol. Rev. 2010, 74, 529–551.
- Chittora, D.; Meena, M.; Barupal, T.; Swapnil, P.; Sharma, K. Cyanobacteria as a source of biofertilizers for sustainable agriculture. Biochem. Biophys. Rep. 2020, 22, 100737.
- Kalayu, G. Phosphate solubilizing microorganisms: Promising approach as biofertilizers. Int. J. Agron. 2019, 1–7.
- Prabhu, N.; Borkar, S.; Garg, S. Phosphate solubilization by microorganisms: Overview, mechanisms, applications and advances. Adv. Biol. Sci. Res. 2019, 161–176.
- Antoun, H. Beneficial microorganisms for the sustainable use of phosphates in agriculture. Procedia Eng. 2012, 46, 62–67.
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2013, 2, 587.
- Park, K.H.; Lee, C.Y.; Son, H.J. Mechanism of insoluble phosphate solubilization by Pseudomonas fluorescens RAF15 isolated from ginseng rhizosphere and its plant growth-promoting activities. Lett. Appl. Microbiol. 2009, 49, 222–228.
- Patel, D.; Goswami, D. Phosphorus Solubilization and Mobilization: Mechanisms, Current Developments, and Future Challenge. In Advances in Plant Microbiome and Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–20.
- Anand, K.; Kumari, B.; Mallick, M. Phosphate solubilizing microbes: An effective and alternative approach as biofertilizers. J. Pharm. Pharm. Sci. 2016, 8, 37–40.
- Henri, F.; Laurette, N.N.; Annette, D.; John, Q.; Wolfgang, M.; Franccedil, E.; Dieudonne, N. Solubilization of inorganic phosphates and plant growth promotion by strains of Pseudomonas fluorescens isolated from acidic soils of Cameroon. Afr. J. Microbiol. Res. 2008, 2, 171–178.
- Din, M.; Nelofer, R.; Salman, M.; Khan, F.H.; Khan, A.; Ahmad, M.; Jalil, F.; Din, J.U.; Khan, M. Production of nitrogen fixing Azotobacter (SR-4) and phosphorus solubilizing Aspergillus niger and their evaluation on Lagenaria siceraria and Abelmoschus esculentus. Biotechnol. Rep. 2019, 22, e00323.
- Suthar, H.; Hingurao, K.; Vaghashiya, J.; Parmar, J. Fermentation: A process for biofertilizer production. In Microorganisms for Green Revolution; Springer: Berlin/Heidelberg, Germany, 2017; pp. 229–252.
- Asoegwu, C.R.; Awuchi, C.G.; Nelson, K.; Orji, C.G.; Nwosu, O.U.; Egbufor, U.C.; Awuchi, C.G. A Review on the Role of Biofertilizers In Reducing Soil Pollution and Increasing Soil Nutrients. Himal. J. Agric. 2020, 1, 34–38.
- Bücking, H.; Liepold, E.; Ambilwade, P. The role of the mycorrhizal symbiosis in nutrient uptake of plants and the regulatory mechanisms underlying these transport processes. Plant Sci. 2012, 4, 108–132.
- DeLuca, T.; Pingree, M.; Gao, S. Assessing soil biological health in forest soils. In Developments in Soil Science; Elsevier: Amsterdam, The Netherlands, 2019; Volume 36, pp. 397–426.
- Dwivedi, S.; Sangeeta, G.R.; Gopal, R. Role of mycorrhizae as biofertilizer and bioprotectant. Int. J. Pharm. Bio. Sci. 2015, 6, 1014–1026.
- Douds, D.D.; Gadkar, V.; Adholeya, A. Mass production of VAM fungus biofertilizer. In Mycorrhizal Biology; Springer: Berlin/Heidelberg, Germany, 2000; pp. 197–215.
- Sadhana, B. Arbuscular Mycorrhizal Fungi (AMF) as a biofertilizer-a review. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 384–400.
- Abbasi, H.; Akhtar, A.; Sharf, R. Vesicular arbuscular mycorrhizal (VAM) fungi: A tool for sustainable agriculture. Am. J. Plant Nutr. Fertil. Technol. 2015, 5, 40–49.
- Rai, S.; Shukla, N. Biofertilizer: An Alternative of Synthetic Fertilizers. J. Plant Arch. 2020, 20, 1374–1379.
- Vala, Y.; Desai, N.B. Biofertilizers: An Approach towards Saving Fertilizers. 2020. Available online: (accessed on 8 February 2021).
- Pathak, D.; Kumar, M.; Rani, K. Biofertilizer application in horticultural crops. In Microorganisms for Green Revolution; Springer: Berlin/Heidelberg, Germany, 2017; pp. 215–227.
- Sullia, S. Use of vesicular-arbuscular mycorrhiza (VAM) as biofertilizer for horticultural plants in developing countries. In Horticulture—New Technologies and Applications; Springer: Berlin/Heidelberg, Germany, 1991; pp. 49–53.
- Khan, M.S.; Zaidi, A.; Wani, P.A. Role of phosphate-solubilizing microorganisms in sustainable agriculture—A review. Agron. Sustain. Dev. 2007, 27, 29–43.
- Meena, V.S.; Maurya, B.; Verma, J.P. Does a rhizospheric microorganism enhance K+ availability in agricultural soils? Microbiol. Res. 2014, 169, 337–347.
- Prajapati, K.; Modi, H. The importance of potassium in plant growth-a review. Indian J. Plant Sci. 2012, 1, 177–186.
- Williams, L.; Pittman, J. Cell Biology of Metals and Nutrients, Plant Cell Monographs; Springer: Berlin, Germany, 2010; pp. 95–117.
- Sindhu, S.; Parmar, P.; Phour, M.; Sehrawat, A. Potassium-solubilizing microorganisms (KSMs) and its effect on plant growth improvement. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2016; pp. 171–185.
- Ahmad, M.; Nadeem, S.M.; Naveed, M.; Zahir, Z.A. Potassium-solubilizing bacteria and their application in agriculture. Potassium Solubilizing Microorg. Sustain. Agric. 2016, 293–313.
- Sugumaran, P.; Janarthanam, B. Solubilization of potassium containing minerals by bacteria and their effect on plant growth. World J. Agric. Sci. 2007, 3, 350–355.
- Pramanik, P.; Goswami, A.; Ghosh, S.; Kalita, C. An indigenous strain of potassium-solubilizing bacteria Bacillus pseudomycoides enhanced potassium uptake in tea plants by increasing potassium availability in the mica waste-treated soil of North-east India. J. Appl. Microbiol. 2019, 126, 215–222.
- Ali, A.M.; Awad, M.Y.; Hegab, S.A.; Gawad, A.M.A.E.; Eissa, M.A. Effect of potassium solubilizing bacteria (Bacillus cereus) on growth and yield of potato. J. Plant Nutr. 2021, 44, 411–420.
- Xiafang, S.; Weiyi, H. Mechanism of potassium release from feldspar affected by the sprain Nbt of silicate bacterium. Acta Pedol. Sin. 2002, 39, 863–871.
- Bahadur, I.; Maurya, B.R.; Kumar, A.; Meena, V.S.; Raghuwanshi, R. Towards the soil sustainability and potassium-solubilizing microorganisms. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2016; pp. 255–266.
- El-Halfawi, M.; Ibrahim, S.; Kandil, H.; Niculiţă, M.; Rusu, C. Influence of elemental sulphur, organic matter, sulfur oxidizing bacteria and cabronite alone or in combination on cowpea plants and the used soil. Factori Procese Pedogenetice Zona Temperatã 9 S. nouã 2010, 13–29.
- Riaz, U.; Mehdi, S.M.; Iqbal, S.; Khalid, H.I.; Qadir, A.A.; Anum, W.; Ahmad, M.; Murtaza, G. Bio-fertilizers: Eco-Friendly approach for plant and soil environment. In Bioremediation and Biotechnology; Springer: Berlin/Heidelberg, Germany, 2020; pp. 189–213.
- Vidyalakshmi, R.; Paranthaman, R.; Bhakyaraj, R. Sulphur Oxidizing Bacteria and Pulse Nutrition- A Review. World J. Agric. Sci. 2009, 5, 270–278.
- Pourbabaee, A.A.; Koohbori Dinekaboodi, S.; Seyed Hosseini, H.M.; Alikhani, H.A.; Emami, S. Potential application of selected sulfur-oxidizing bacteria and different sources of sulfur in plant growth promotion under different moisture conditions. Commun. Soil Sci. Plant Anal. 2020, 51, 735–745.
- Pokorna, D.; Zabranska, J. Sulfur-oxidizing bacteria in environmental technology. Biotechnol. Adv. 2015, 33, 1246–1259.
- Tavallali, V.; Rahemi, M.; Eshghi, S.; Kholdebarin, B.; Ramezanian, A. Zinc alleviates salt stress and increases antioxidant enzyme activity in the leaves of pistachio (Pistacia vera L. ‘Badami’) seedlings. Turk. J. Agric. 2010, 34, 349–359.
- Graham, R.D. Micronutrient deficiencies in crops and their global significance. In Micronutrient Deficiencies in Global Crop Production; Springer: Berlin/Heidelberg, Germany, 2008; pp. 41–61.
- Kumar, A.; Dewangan, S.; Lawate, P.; Bahadur, I.; Prajapati, S. Zinc-Solubilizing Bacteria: A Boon for Sustainable Agriculture. In Plant Growth Promoting Rhizobacteria for Sustainable Stress Management; Springer: Berlin/Heidelberg, Germany, 2019; pp. 139–155.
- Hussain, A.; Zahir, Z.A.; Asghar, H.N.; Ahmad, M.; Jamil, M.; Naveed, M.; Akhtar, M.F.U.Z. Zinc solubilizing bacteria for zinc biofortification in cereals: A step toward sustainable nutritional security. In Role of Rhizospheric Microbes in Soil; Springer: Berlin/Heidelberg, Germany, 2018; pp. 203–227.
- Naz, I.; Ahmad, H.; Khokhar, S.N.; Khan, K.; Shah, A.H. Impact of zinc solubilizing bacteria on zinc contents of wheat. Am. Eurasian J. Agric. Environ. Sci. 2016, 16, 449–454.
- Raj, S. Bio-fertilizers for micronutrients. Biofertil. Newsl. 2007. Available online: (accessed on 8 February 2021).
- Goteti, P.K.; Emmanuel, L.D.A.; Desai, S.; Shaik, M.H.A. Prospective zinc solubilising bacteria for enhanced nutrient uptake and growth promotion in maize (Zea mays L.). Int. J. Microbiol. 2013, 2013, 1–7.
- Vaid, S.K.; Kumar, B.; Sharma, A.; Shukla, A.; Srivastava, P. Effect of Zn solubilizing bacteria on growth promotion and Zn nutrition of rice. J. Soil Sci. Plant Nutr. 2014, 14, 889–910.
- Hussain, A.; Zahir, Z.A.; Ditta, A.; Tahir, M.U.; Ahmad, M.; Mumtaz, M.Z.; Hayat, K.; Hussain, S. Production and implication of bio-activated organic fertilizer enriched with zinc-solubilizing bacteria to boost up maize (Zea mays L.) production and biofortification under two cropping seasons. Agronomy 2020, 10, 39.
- Beneduzi, A.; Ambrosini, A.; Passaglia, L.M. Plant growth-promoting rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genet. Mol. Biol. 2012, 35, 1044–1051.
- Timmusk, S.; Abd El-Daim, I.A.; Copolovici, L.; Tanilas, T.; Kännaste, A.; Behers, L.; Nevo, E.; Seisenbaeva, G.; Stenström, E.; Niinemets, Ü. Drought-tolerance of wheat improved by rhizosphere bacteria from harsh environments: Enhanced biomass production and reduced emissions of stress volatiles. PLoS ONE 2014, 9, e96086.
- Vurukonda, S.S.K.P.; Vardharajula, S.; Shrivastava, M.; SkZ, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. J. Microbiol. Res. 2016, 184, 13–24.
- Niu, X.; Song, L.; Xiao, Y.; Ge, W. Drought-tolerant plant growth-promoting rhizobacteria associated with foxtail millet in a semi-arid agroecosystem and their potential in alleviating drought stress. J. Front. Microbiol. 2018, 8, 2580.
- Ilyas, N.; Mumtaz, K.; Akhtar, N.; Yasmin, H.; Sayyed, R.; Khan, W.; Enshasy, H.A.E.; Dailin, D.J.; Elsayed, E.A.; Ali, Z. Exopolysaccharides Producing Bacteria for the Amelioration of Drought Stress in Wheat. J. Sustain. 2020, 12, 8876.
- Mayak, S.; Tirosh, T.; Glick, B.R. Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol. Biochem. 2004, 42, 565–572.
- Bharti, N.; Yadav, D.; Barnawal, D.; Maji, D.; Kalra, A. Exiguobacterium oxidotolerans, a halotolerant plant growth promoting rhizobacteria, improves yield and content of secondary metabolites in Bacopa monnieri (L.) Pennell under primary and secondary salt stress. J. World J. Microbiol. Biotechnol. 2013, 29, 379–387.
- de Vasconcellos, R.L.F.; Cardoso, E.J.B.N. Rhizospheric streptomycetes as potential biocontrol agents of Fusarium and Armillaria pine rot and as PGPR for Pinus taeda. J. Biocontrol 2009, 54, 807–816.
- Verma, P.; Yadav, A.N.; Khannam, K.S.; Kumar, S.; Saxena, A.K.; Suman, A. Molecular diversity and multifarious plant growth promoting attributes of Bacilli associated with wheat (Triticum aestivum L.) rhizosphere from six diverse agro-ecological zones of India. J. Basic Microbiol. 2016, 56, 44–58.
- Almaghrabi, O.A.; Abdelmoneim, T.; Albishri, H.M.; Moussa, T.A. Enhancement of maize growth using some plant growth promoting rhizobacteria (PGPR) under laboratory conditions. J. Life Sci. J. 2014, 11, 764–772.
- Nezarat, S.; Gholami, A. Screening plant growth promoting rhizobacteria for improving seed germination, seedling growth and yield of maize. J. Pak. J. Biol. Sci. 2009, 12, 26.
- Islam, S.; Akanda, A.M.; Prova, A.; Islam, M.T.; Hossain, M.M. Isolation and identification of plant growth promoting rhizobacteria from cucumber rhizosphere and their effect on plant growth promotion and disease suppression. J. Front. Microbiol. 2016, 6, 1360.
- Jang, J.H.; Woo, S.Y.; Kim, S.H.; Khaine, I.; Kwak, M.J.; Lee, H.K.; Lee, T.Y.; Lee, W.Y. Effects of increased soil fertility and plant growth-promoting rhizobacteria inoculation on biomass yield, energy value, and physiological response of poplar in short-rotation coppices in a reclaimed tideland: A case study in the Saemangeum area of Korea. J. Biomass 2017, 107, 29–38.
- Kumari, B.; Mallick, M.; Hora, A. Plant growth-promoting rhizobacteria (PGPR): Their potential for development of sustainable agriculture. Bioexploit. Sustain. Agric. 2016, 1–19.
- Barnawal, D.; Bharti, N.; Pandey, S.S.; Pandey, A.; Chanotiya, C.S.; Kalra, A. Plant growth-promoting rhizobacteria enhance wheat salt and drought stress tolerance by altering endogenous phytohormone levels and TaCTR1/TaDREB2 expression. J. Physiol. Plant. 2017, 161, 502–514.
- Tahir, H.A.; Gu, Q.; Wu, H.; Raza, W.; Hanif, A.; Wu, L.; Colman, M.V.; Gao, X. Plant growth promotion by volatile organic compounds produced by Bacillus subtilis SYST2. J. Front. Microbiol. 2017, 8, 171.
- Sayyed, R.; Patel, P.; Shaikh, S. Plant growth promotion and root colonization by EPS producing Enterobacter sp. RZS5 under heavy metal contaminated soil. Indian J. Exp. Biol. 2015, 53, 116–123.
- Ordookhani, K.; Sharafzadeh, S.; Zare, M. Influence of PGPR on growth, essential oil and nutrients uptake of sweet basil. J. Adv. Environ. Biol. 2011, 5, 672–677.
- Pandey, S.; Ghosh, P.K.; Ghosh, S.; De, T.K.; Maiti, T.K. Role of heavy metal resistant Ochrobactrum sp. and Bacillus spp. strains in bioremediation of a rice cultivar and their PGPR like activities. J. Microbiol. 2013, 51, 11–17.
- Khan, N.; Bano, A. Role of plant growth promoting rhizobacteria and Ag-nano particle in the bioremediation of heavy metals and maize growth under municipal wastewater irrigation. Int. J. Phytoremediation 2016, 18, 211–221.
- Patel, P.; Shaikh, S.; Sayyed, R. Dynamism of PGPR in bioremediation and plant growth promotion in heavy metal contaminated soil. Indian J. Exp. Biol. 2016, 54, 286–290.
- Yadav, A.; Verma, P.; Singh, B.; Chauahan, V. Plant growth promoting bacteria: Biodiversity and multifunctional attributes for sustainable agriculture. Adv. Biotechnol. Microbiol. 2017, 5, 1–16.
- Dutta, S.; Podile, A.R. Plant growth promoting rhizobacteria (PGPR): The bugs to debug the root zone. J. Crit. Rev. Microbiol. 2010, 36, 232–244.
- Ahemad, M.; Kibret, M. Mechanisms and applications of plant growth promoting rhizobacteria: Current perspective. J. King Saud Univ. Sci. 2014, 26, 1–20.
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Nasrulhaq Boyce, A. Role of plant growth promoting rhizobacteria in agricultural sustainability—A review. Molecules 2016, 21, 573.
- Parray, J.A.; Jan, S.; Kamili, A.N.; Qadri, R.A.; Egamberdieva, D.; Ahmad, P. Current perspectives on plant growth-promoting rhizobacteria. J. Plant Growth Regul. 2016, 35, 877–902.
- Swarnalakshmi, K.; Yadav, V.; Tyagi, D.; Dhar, D.W.; Kannepalli, A.; Kumar, S. Significance of Plant Growth Promoting Rhizobacteria in Grain Legumes: Growth Promotion and Crop Production. Plants 2020, 9, 1596.
- Gouda, S.; Kerry, R.G.; Das, G.; Paramithiotis, S.; Shin, H.-S.; Patra, J.K. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. J. Microbiol. Res. 2018, 206, 131–140.
- Oleńska, E.; Małek, W.; Wójcik, M.; Swiecicka, I.; Thijs, S.; Vangronsveld, J. Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions: A methodical review. Sci. Total Environ. 2020, 743, 140682.
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012.
- Yadav, K.K.; Sarkar, S. Biofertilizers, impact on soil fertility and crop productivity under sustainable agriculture. Environ. Ecol. 2019, 37, 89–93.
- Mishra, D.; Rajvir, S.; Mishra, U.; Kumar, S.S. Role of bio-fertilizer in organic agriculture: A review. Res. J. Recent Sci. ISSN 2013, 2277, 2502.
- Malusà, E.; Pinzari, F.; Canfora, L. Efficacy of biofertilizers: Challenges to improve crop production. In Microbial Inoculants in Sustainable Agricultural Productivity; Springer: Berlin/Heidelberg, Germany, 2016; pp. 17–40.
- Naveed, M.; Mehboob, I.; Shaker, M.A.; Hussain, M.B.; Farooq, M. Biofertilizers in Pakistan: Initiatives and limitations. Int. J. Agric. Biol. 2015, 17, 411–420.
- Singh, M.; Dotaniya, M.; Mishra, A.; Dotaniya, C.; Regar, K.; Lata, M. Role of biofertilizers in conservation agriculture. In Conservation Agriculture; Springer: Berlin/Heidelberg, Germany, 2016; pp. 113–134.
- Singh, S.P.; Singh, S.; Dubey, A.N.; Rajput, R.K. Biofertilizers and Plant Growth Regulators as Key Player in Sustainable Agriculture by Enhancing Soil Fertility and Crop Productivity. Environ. Agric. Health 2020. Available online: (accessed on 8 February 2021).
- Weber, N.; Herrmann, I.; Hochholdinger, F.; Ludewig, U.; Neumann, G. PGPR-induced growth stimulation and nutrient acquisition in maize: Do root hairs matter? Sci. Agric. Bohem. 2018, 49, 164–172.
- Wong, W.; Tan, S.; Ge, L.; Chen, X.; Yong, J. The importance of phytohormones and microbes in biofertilizers. In Bacterial Metabolites in Sustainable Agroecosystem; Springer: Berlin/Heidelberg, Germany, 2015; pp. 105–158.
- Gholami, A.; Biyari, A.; Gholipoor, M.; Asadi Rahmani, H. Growth promotion of maize (Zea mays L.) by plant-growth-promoting rhizobacteria under field conditions. Commun. Soil Sci. Plant Anal. 2012, 43, 1263–1272.
- Razie, F.; Anas, I. Effect of Azotobacter and Azospirillum on growth and yield of rice grown on tidal swamp rice field in south Kalimantan. J. Ilmu Tanah Lingkung. 2008, 10, 41–45.
- Banayo, N.P.M.; Cruz, P.C.; Aguilar, E.A.; Badayos, R.B.; Haefele, S.M. Evaluation of biofertilizers in irrigated rice: Effects on grain yield at different fertilizer rates. Agriculture 2012, 2, 73–86.
- Sundara, B.; Natarajan, V.; Hari, K. Influence of phosphorus solubilizing bacteria on the changes in soil available phosphorus and sugarcane and sugar yields. Field Crops Res. 2002, 77, 43–49.
- Zahran, H.H. Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol. Mol. Biol. Rev. 1999, 63, 968–989.
- kumar Maurya, A. Performance of Bio-Fertilizers in Sustainable Agriculture Development. Growth Unorgan. Sect. India 2014. Available online: (accessed on 8 February 2021).
- Jnawali, A.; Ojha, R.; Marahatta, S. Role of Azotobacter in soil fertility and sustainability–A Review. Adv. Plants Agric. Res. 2015, 2, 1–5.
- Okumura, R.S.; Mariano, D.d.C.; Dallacort, R.; Nogueira de Albuquerque, A.; Lobato, A.d.S.; Guedes, E.S.; Neto, C.; Oliveira da Conceicao, H.E.; Alves, G.R. Azospirillum: A new and efficient alternative to biological nitrogen fixation in grasses. J. Food Agric. Environ. 2013, 2, 1142–1146.
- Kollah, B.; Patra, A.K.; Mohanty, S.R. Aquatic microphylla Azolla: A perspective paradigm for sustainable agriculture, environment and global climate change. Environ. Sci. Pollut. Res. 2016, 23, 4358–4369.
- Kobae, Y. Dynamic phosphate uptake in arbuscular mycorrhizal roots under field conditions. Front. Environ. Sci. 2019, 6, 159.
- Kloepper, J.W.; Leong, J.; Teintze, M.; Schroth, M.N. Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature 1980, 286, 885–886.
- Leaungvutiviroj, C.; Ruangphisarn, P.; Hansanimitkul, P.; Shinkawa, H.; Sasaki, K. Development of a new biofertilizer with a high capacity for N2 fixation, phosphate and potassium solubilization and auxin production. Biosci. Biotechnol. Biochem. 2010, 74, 1098–1101.
- Radhakrishnan, R.; Hashem, A.; Abd_Allah, E.F. Bacillus: A biological tool for crop improvement through bio-molecular changes in adverse environments. Front. Physiol. 2017, 8, 667.
- Saxena, J.; Saini, A.; Ravi, I.; Chandra, S.; Garg, V. Consortium of phosphate-solubilizing bacteria and fungi for promotion of growth and yield of chickpea (Cicer arietinum). J. Crop. Improv. 2015, 29, 353–369.
- Mohammadi, K.; Ghalavand, A.; Aghaalikhani, M.; Heidari, G.; Sohrabi, Y. Introducing a sustainable soil fertility system for chickpea (Cicer arietinum L.). Afr. J. Biotechnol. 2011, 10, 6011–6020.
- Hailu, S.; Seyoum, T.; Dechassa, N. Effect of combined application of organic-P and inorganic-N fertilizers on yield of carrot. Afr. J. Biotechnol. 2008, 7, 27–34.
- Osman, M.E.H.; El-Sheekh, M.M.; El-Naggar, A.H.; Gheda, S.F. Effect of two species of cyanobacteria as biofertilizers on some metabolic activities, growth, and yield of pea plant. Biol. Fertil. Soils 2010, 46, 861–875.
- Ahmed, M.; Ahmed, A.G.; Mohamed, M.H.; Tawfik, M. Integrated effect of organic and biofertilizers on wheat productivity in new reclaimed sandy soil. Res. J. Agric. Biol. Sci. 2011, 7, 105–114.
- Habibi, A.; Heidari, G.; Sohrabi, Y.; Badakhshan, H.; Mohammadi, K. Influence of bio, organic and chemical fertilizers on medicinal pumpkin traits. J. Med. Plants Res. 2011, 5, 5590–5597.
- Pattanayak, S.; Rao, D.; Mishra, K. Effect of biofertilizers on yield, nutrient uptake and nitrogen economy of rice-peanut cropping sequence. J. Indian Soc. Soil Sci. 2007, 55, 184–189.
- Khanna, R.; Pawar, J.; Gupta, S.; Verma, H.; Trivedi, H.; Kumar, P.; Kumar, R. Efficiency of biofertilizers in increasing the production potential of cereals and pulses: A. J. Pharmacogn. Phytochem. 2019, 8, 183–188.
- Khatkar, R.; Abraham, T.; Joseph, S.A. Effect of biofertilizers and sulphur levels on growth and yield of blackgram (Vigna mungo L.). Legume Res. Int. J. 2007, 30, 233–234.
- Singh, R.; Yadav, M. Effect of phosphorus and biofertilizers on growth, yield and nutrient uptake of long duration pigeonpea under rainfed condition. J. Food Legumes 2008, 21, 46–48.
- Ruiz-Sánchez, M.; Aroca, R.; Muñoz, Y.; Polón, R.; Ruiz-Lozano, J.M. The arbuscular mycorrhizal symbiosis enhances the photosynthetic efficiency and the antioxidative response of rice plants subjected to drought stress. J. Physiol. 2010, 167, 862–869.
- Gajdos, É.; Lévai, L.; Veres, S.; Kovács, B. Effects of biofertilizers on maize and sunflower seedlings under cadmium stress. Commun. Soil Sci. Plant Anal. 2012, 43, 272–279.
- Owen, D.; Williams, A.P.; Griffith, G.W.; Withers, P.J. Use of commercial bio-inoculants to increase agricultural production through improved phosphrous acquisition. Appl. Soil Ecol. 2015, 86, 41–54.
- Transparency Market Research. Biofertilizers (Nitrogen Fixing, Phosphate Solubilizing and Others) Market for Seed Treatment and Soil Treatment Applications–Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2013–2019; Transparency Market Research: Albany, NY, USA, 2014.
- Cassán, F.; Diaz-Zorita, M. Azospirillum sp. in current agriculture: From the laboratory to the field. Soil Biol. Biochem. 2016, 103, 117–130.
- Sekhar, M.; Riotte, J.; Ruiz, L.; Jouquet, P.; Braun, J.-J. Influences of climate and agriculture on water and biogeochemical cycles: Kabini critical zone observatory. Proc. Indian Natl. Sci. Acad. 2016, 82, 833–846.
