Silk from the silkworm Bombyx mori is well-known for its use in clothing. Silk is also a high-performance biomaterial that is already clinically approved due to its renowned biocompatibility, low immunogenicity and tunable biodegradation (minutes to years)
- silk fibroin
- liquid–liquid phase separation
- Bombyx mori
1. Introduction
More than 1.5 million different medical devices are in use today, and yet the palette of materials approved for human use is extremely small [1]. This places limits on medical progress and has created a strong demand for an orthogonal strategy for the design and discovery of novel materials, including new silks.
“Everyday” silk from the silkworm Bombyx mori is well-known for its use in clothing. However, silk offers solutions to many biological challenges (e.g., housing, protection and on-demand assembly) because it has arisen in nature at least 23 times in independent convergent evolutionary events in a range silkworms, spiders and other organisms, and its ubiquity and widespread use are clear testaments to its biological success [2]. Clinically, silk offers solutions to unmet healthcare challenges and contributes towards a healthy nation. Silk is a high-performance biomaterial that is already clinically approved due to its renowned biocompatibility, low immunogenicity and tunable biodegradation (minutes to years) [3]. Silk degrades into benign products that do not accumulate in the body or the environment [4]. Its unique physical properties (e.g., toughness) support the medical use of silk as a suture material and surgical mesh for load-bearing applications (Sofregen Inc.) [3][5]. Silk’s robust safety record in humans and in ongoing clinical trials makes it a highly attractive material for state-of-the-art medical applications [3]. The spun silk fiber can either be used directly or reverse engineered into liquid silk for processing into stimulus-responsive nanomedicines [6], stabilizers for payloads (drugs, proteins and diagnostics) [7], medical sensors [8], hydrogels for tissue engineering [9] and vehicles for drug and cell delivery [6][10][11].
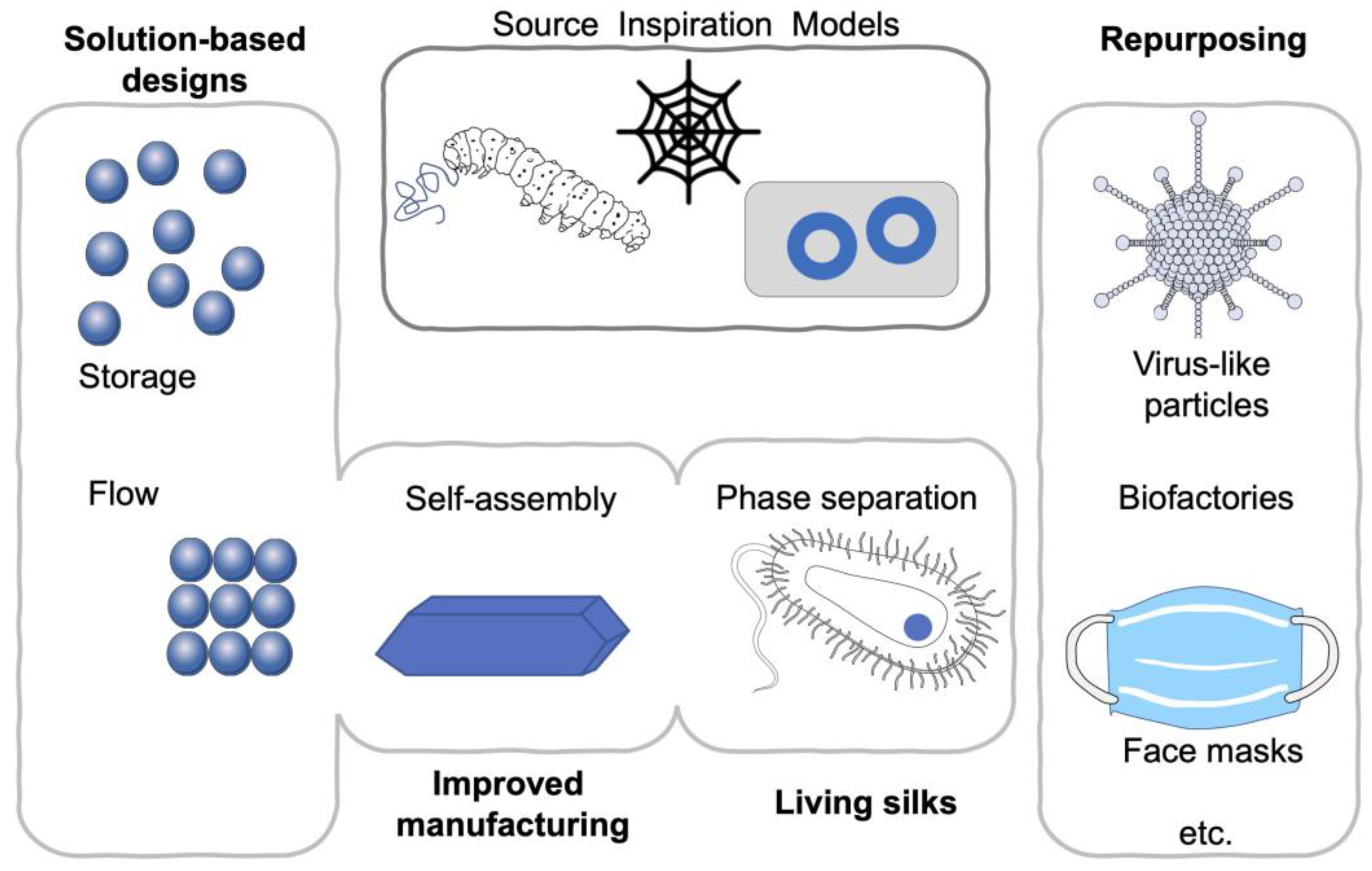
While the biomedical use of silk has spanned several millennia, silk did fall out of fashion with the advent of synthetic manmade fibers, in the erroneous belief that we are able to “beat” nature. However, over the past 20 years, interest has been renewed both in our fundamental understanding of silk and in its biomedical applications [12][13]. Today, more than 13,000 publications on this subject are at our fingertips, and the number continues to grow (PubMed accessed January 2021). At the same time, technological innovations [14] open up new silk processing strategies, uses and applications. A number of timely reviews have covered the biomedical use of silk [3][12], recombinant silks [15] and their applications (e.g., drug delivery [16], tissue engineering [17], additive manufacturing [18] and disease models [19]). For example, an excellent review of silk nanoparticles is included in this special issue on silk-based biomaterials [20]. Silk is also making a marked ingress into the cosmetics and personal care products industry. Here, recombinant spider silk-inspired proteins are often used to ensure vegan product certification (reviewed in Reference [21]). Summary of emerging silk material trends is show in Figure 1.
Figure 1. Summary of emerging silk material trends. Repurposing [22][23][24][25], solution-based designs [26], phase separation [27][28] and flow [29][30][31] impacting manufacturing and creating living materials.
2. Biomedical Applications
The popularity of silk continues to grow, impacting virtually all areas of biomedical research. For example, the precision cutting [32] or patterning of silks has already provided the ability to influence applications, ranging from stem cell biology to the design of rewritable optical storage devices [33]. However, in light of the ongoing Covid-19 pandemic, I have picked one example where silk is having a particular impact. At the start of the Covid-19 pandemic, the shortage of personal protective equipment spurred the demand for homemade face masks, to reduce the transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) via respiratory droplets. Supratik Guha and co-workers examined the potential of common household fabrics, including silk, for their potential use in face mask construction [24]. To measure the filtration efficiencies, this research group used a custom-built system which included a sodium chloride–particle generator to emulate respiratory droplets. The initial publication reported that multiple layers of fabrics improved filtration efficiency, when compared to a single layer [24]. Furthermore, cotton/silk hybrid fabrics were particularly well suited as mask materials. The work attracted global attention. The authors have since published a correction [34], expanded the dataset and responded [25] to letters to the editor [23][35][36].
Subsequent measurements by other researchers from three different laboratories have demonstrated that natural silk has the lowest impedance, compared to cotton or cotton/polyester mixtures with different thread counts per inch (tpi) [23]. For mask performance, impedance is important because it relates directly to the comfort and breathability of the material. Impedance also impacts the degree of protection offered by the mask. As impedance increases, the unfiltered tributary airflow through leaks in the mask also increases, thereby reducing the overall degree of protection. Meticulous measurements indicated that four layers of silk had a 40 to 50% filtration efficiency, which was substantially higher than four layers of 400 tpi cotton (and these data now correct the scientific literature). While cotton failed the NIOSH 42 CFR 84 standard for breathability, silk passed easily. Silk has the added advantage that it minimizes skin irritation. Nonetheless, both N95 and surgical masks outperformed silk or any other materials tested and therefore remain the preferred choice.
3. Conclusion and Outlook
Fashioning facemasks from silk during a global pandemic is a clear testament that the silk fiber continues to support human health in its native form, despite our ability to unspin the silk thread. While the sole focus of this review has been to provide examples of recent advances in fundamental silk science, the application of silk science findings to synthetic polymers, the orthogonal use of silk in living cells and the novel silk processing now available all confirm that the silkworm remains an invaluable asset that goes well beyond its ability to spin the silk thread. Silkworms have evolved to produce large amounts of protein and are thus ideally placed to be genetically re-programmed, allowing B. mori silkworms to also serve as biofactories of tailor-made high-value proteins [37][38]. For example, the use of the B. mori genetic toolbox made possible the expression the SARS-CoV-2 spike protein in silkworms [22], and the use of silkworms to generate virus-like particles is expected to contribute to the SARS-CoV-2 vaccine pipeline.
This entry is adapted from the peer-reviewed paper 10.3390/ma14051160
References
- WHO. Global Atlas of Medical Devices; WHO: Geneva, Switzerland, 2017.
- Sutherland, T.D.; Young, J.H.; Weisman, S.; Hayashi, C.Y.; Merritt, D.J. Insect silk: One name, many materials. Annu. Rev. Entomol. 2010, 55, 171–188.
- Holland, C.; Numata, K.; Rnjak-Kovacina, J.; Seib, F.P. The Biomedical Use of Silk: Past, Present, Future. Adv. Healthc Mater. 2019, 8, e1800465.
- Thurber, A.E.; Omenetto, F.G.; Kaplan, D.L. In vivo bioresponses to silk proteins. Biomaterials 2015, 71, 145–157.
- Jewell, M.; Daunch, W.; Bengtson, B.; Mortarino, E. The development of SERI(R) Surgical Scaffold, an engineered biological scaffold. Ann. N. Y. Acad. Sci. 2015, 1358, 44–55.
- Seib, F.P. Silk nanoparticles—an emerging anticancer nanomedicine. AIMS Bioeng. 2017, 4, 239–258.
- Li, A.B.; Kluge, J.A.; Guziewicz, N.A.; Omenetto, F.G.; Kaplan, D.L. Silk-based stabilization of biomacromolecules. J. Control. Release 2015, 219, 416–430.
- Fan, S.; Zhang, Y.; Huang, X.Y.; Geng, L.; Shao, H.; Hu, X.; Zhang, Y. Silk materials for medical, electronic and optical applications. Sci. China. Tech. Sci. 2019, 62, 903–918.
- Bhattacharjee, P.; Kundu, B.; Naskar, D.; Kim, H.W.; Maiti, T.K.; Bhattacharya, D.; Kundu, S.C. Silk scaffolds in bone tissue engineering: An overview. Acta Biomater. 2017, 63, 1–17.
- Seib, F.P. Reverse-engineered silk hydrogels for cell and drug delivery. Ther. Deliv. 2018, 9, 469–487.
- Xiao, B.; Ma, Y.; Canup, B.S.B.; Tong, X.; Dai, F. Multi-Responsive Silk Fibroin-Based Nanoparticles for Drug Delivery. Front. Chem. 2020, 8, 1010.
- Janani, G.; Kumar, M.; Chouhan, D.; Moses, J.C.; Gangrade, A.; Bhattacharjee, S.; Mandal, B.B. Insight into Silk-Based Biomaterials: From Physicochemical Attributes to Recent Biomedical Applications. ACS Appl. Bio Mater. 2019, 2, 5460–5491.
- Omenetto, F.G.; Kaplan, D.L. New opportunities for an ancient material. Science 2010, 329, 528–531.
- Rockwood, D.N.; Preda, R.C.; Yucel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc 2011, 6, 1612–1631.
- Aigner, T.B.; DeSimone, E.; Scheibel, T. Biomedical Applications of Recombinant Silk-Based Materials. Adv Mater. 2018, 30, e1704636.
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. Silk fibroin as a functional biomaterial for drug and gene delivery. Pharmaceutics 2019, 11, 494.
- Wani, S.U.D.; Gautam, S.P.; Qadrie, Z.L.; Gangadharappa, H.V. Silk fibroin as a natural polymeric based bio-material for tissue engineering and drug delivery systems-A review. Int. J. Biol. Macromol. 2020, 163, 2145–2161.
- Oliveira, J.M. Current and future trends of silk fibroin-based bioinks in 3D printing. J. 3D Print Mater. 2020, 4, 69–79.
- Abbott, R.D.; Kimmerling, E.P.; Cairns, D.M.; Kaplan, D.L. Silk as a Biomaterial to Support Long-Term Three-Dimensional Tissue Cultures. ACS Appl. Mater. Interfaces 2016, 8, 21861–21868.
- Florczak, A.; Grzechowiak, I.; Deptuch, T.; Kucharczyk, K.; Kaminska, A.; Dams-Kozlowska, H. Silk Particles as Carriers of Therapeutic Molecules for Cancer Treatment. MDPI Mater. 2020, 13, 4946.
- Breslauer, D.N. Recombinant Protein Polymers: A Coming Wave of Personal Care Ingredients. ACS Biomater. Sci. Eng. 2020, 6, 5980–5986.
- Fujita, R.; Hino, M.; Ebihara, T.; Nagasato, T.; Masuda, A.; Lee, J.M.; Fujii, T.; Mon, H.; Kakino, K.; Nagai, R.; et al. Efficient production of recombinant SARS-CoV-2 spike protein using the baculovirus-silkworm system. Biochem. Biophys. Res. Commun. 2020, 529, 257–262.
- Hancock, J.N.; Plumley, M.J.; Schilling, K.; Sheets, D.; Wilen, L. Comment on “Aerosol Filtration Efficiency of Common Fabrics Used in Respiratory Cloth Masks”. ACS Nano 2020, 14, 10758–10763.
- Konda, A.; Prakash, A.; Moss, G.A.; Schmoldt, M.; Grant, G.D.; Guha, S. Aerosol Filtration Efficiency of Common Fabrics Used in Respiratory Cloth Masks. ACS Nano 2020, 14, 6339–6347.
- Konda, A.; Prakash, A.; Moss, G.A.; Schmoldt, M.; Grant, G.D.; Guha, S. Response to Letters to the Editor on Aerosol Filtration Efficiency of Common Fabrics Used in Respiratory Cloth Masks: Revised and Expanded Results. ACS Nano 2020, 14, 10764–10770.
- Guo, C.; Li, C.; Vu, H.V.; Hanna, P.; Lechtig, A.; Qiu, Y.; Mu, X.; Ling, S.; Nazarian, A.; Lin, S.J.; et al. Thermoplastic moulding of regenerated silk. Nat. Mater. 2020, 19, 102–108.
- Shen, Y.; Ruggeri, F.S.; Vigolo, D.; Kamada, A.; Qamar, S.; Levin, A.; Iserman, C.; Alberti, S.; George-Hyslop, P.S.; Knowles, T.P.J. Biomolecular condensates undergo a generic shear-mediated liquid-to-solid transition. Nat. Nanotechnol 2020, 15, 841–847.
- Wei, S.P.; Qian, Z.G.; Hu, C.F.; Pan, F.; Chen, M.T.; Lee, S.Y.; Xia, X.X. Formation and functionalization of membraneless compartments in Escherichia coli. Nat. Chem. Biol. 2020, 16, 1143–1148.
- Dunderdale, G.J.; Davidson, S.J.; Ryan, A.J.; Mykhaylyk, O.O. Flow-induced crystallisation of polymers from aqueous solution. Nat. Commun. 2020, 11, 3372.
- Koeppel, A.; Laity, P.R.; Holland, C. The influence of metal ions on native silk rheology. Acta Biomater. 2020, 117, 204–212.
- Schaefer, C.; Laity, P.R.; Holland, C.; McLeish, T.C.B. Silk Protein Solution: A Natural Example of Sticky Reptation. Macromolecules 2020, 53, 2669–2676.
- Pradhan, S.; Ventura, L.; Agostinacchio, F.; Xu, M.; Barbieri, E.; Motta, A.; Pugno, N.M.; Yadavalli, V.K. Biofunctional Silk Kirigami With Engineered Properties. ACS Appl. Mater. Interfaces 2020, 12, 12436–12444.
- Lee, W.; Zhou, Z.; Chen, X.; Qin, N.; Jiang, J.; Liu, K.; Liu, M.; Tao, T.H.; Li, W. A rewritable optical storage medium of silk proteins using near-field nano-optics. Nat. Nanotechnol. 2020, 15, 941–947.
- Konda, A.; Prakash, A.; Moss, G.; Schmoldt, M.; Grant, G.; Guha, S. Correction to Aerosol Filtration Efficiency of Common Fabrics Used in Respiratory Cloth Masks. ACS Nano 2020, 14, 10742–10743.
- Carr, I.A.; Hariharan, P.; Guha, S. Letter to the Editor Regarding Aerosol Filtration Efficiency of Common Fabrics Used in Respiratory Cloth Masks. ACS Nano 2020, 14, 10754–10755.
- Rule, A.; Ramachandran, G.; Koehler, K. Comment on Aerosol Filtration Efficiency of Common Fabrics Used in Respiratory Cloth Masks: Questioning Their Findings. ACS Nano 2020, 14, 10756–10757.
- Park, E.Y.; Maenaka, K. Silkworm Biofactory: Silk to Biology; CRC Press Taylor & Franics Group: Boca Raton, FL, USA, 2019.
- Kato, T.; Kajikawa, M.; Maenaka, K.; Park, E.Y. Silkworm expression system as a platform technology in life science. Appl. Microbiol. Biotechnol. 2010, 85, 459–470.