Bacterial infections are a leading cause of death in both Europe and USA. The use of antibacterial nanomaterials is a very promising approach to combat the microorganisms due to their high specific surface area and intrinsic or chemically incorporated antibacterial action. Graphene, a two dimensional carbon nanomaterial with excellent mechanical, thermal, and electrical properties, and its derivatives, like graphene oxide (GO) and reduced graphene oxide (rGO), are very good candidates for controlling microbial infections. Nonetheless, the mechanisms of antimicrobial action, their cytotoxicity, and other issues continue imprecise. This review offers select examples on the leading advances in the development of antimicrobial nanocomposites incorporating inorganic nanoparticles and graphene or its derivatives, with the goal of providing a improved understanding of the antibacterial properties of graphene-based nanomaterials.
- antibacterial activity
- nanocomposites
- bacterial infection
- antibiotic resistance
- graphene
- graphene oxide
- reduced graphene oxide
While graphene-based materials exhibit antibacterial properties, they have a strong tendency to agglomerate, in particular G and rGO, due to strong van der Waals interactions among the sheets, which condition their antibacterial action. This issue can be prevented via formation of nanocomposites by surface modification with metal ions/oxides NPs. Inorganic NPs can enhance graphene properties due to their high surface area-to-volume ratio, which leads to novel chemical, electrical, mechanical, optical, and electro-optical properties that differ from those of their bulk counterparts. Further, the mixing of G and rGO with metal oxide NPs can improve their dispersibility in polar solvents such as water [10]. Thus, numerous nanocomposites have been developed over recent years that combine the bactericide action of inorganic nanoparticles with the inherent antibacterial property of graphene-based nanomaterials to attain synergistic effects, and the most representative examples will be reviewed in the following sections.
Nanocomposites with Silver Nanoparticles
The antibacterial properties of Ag+ and Ag-based composites have been comprehensively explored [47]. Ag+ can damage the bacterial membranes and also produce ROS via photocatalytic activation. Nonetheless, when Ag nanoparticles interact with bacteria, they agglomerate; hence their effective specific surface area decreases, leading to reduced antibacterial action [48]. To solve this issue, nanocomposites of graphene and Ag nanoparticles were developed [49,50], although the improvements in antibacterial activity were still limited. Therefore, numerous studies have been reported on GO and rGO nanocomposites comprising Ag nanoparticles [51,52,53,54,55,56,57,58,59,60,61,62] (Table 1), showing improved performance compared to GO and Ag nanoparticles alone due to synergistic effects; further, the mixture of both constituents considerably reduces the concentrations required to inhibit all bacteria. Thus, significantly higher antibacterial activity was found for AgNPs-GO composites (1:2 ratio), with an average NP size of 80 nm [52] at a concentration of 200 μg/mL (Table 1), compared to that of GO or AgNPs, attributed to a higher ROS production, hence resulting in stronger oxidative stress and disruption of the cell membrane. Further, the AgNPs attached to GO are more stable and well-dispersed, which also contributes to the higher efficacy. Comparable antibacterial action has been found for similar composites with smaller nanoparticles (ca. 40 nm) for an optimal AgNPs:GO ratio of 1:1 even at concentrations as low as 2.5 μg/mL [58].
| Graphene Material | NP shape/ size (Φ, nm) |
Bacteria Model | Concentration (μg/mL) |
Inhibition (%) |
Ref. |
|---|---|---|---|---|---|
| AgNPs-GO | S/93 | S. enteritidis | 200 | 61 | [51] |
| AgNPs-GO | S/80 | E. coli | 200 | 89 | [52] |
| AgNPs-GO | S/80 | S. epidermidis | 200 | 76 | [52] |
| AgNPs-GO | S/80 | S. aureus | 200 | 81 | [52] |
| AgNPs-GO | S/80 | C. albicans | 200 | 78 | [52] |
| AgNPs-GO | S, QS | E. coli/S. aureus | 100 | 100 | [53] |
| AgNPs-GO | QS/40 | E. coli | 6.4 | 100 | [55] |
| AgNPs-GO | QS/60 | E. coli/S. aureus | 10 | 100 | [56] |
| AgNPs-GO | E/98 | E. coli/S. aureus | 45 | 100 | [57] |
| AgNPs-GO | S/40 | E. coli | 2.5 | 80 | [58] |
| AgNPs-GO | S/40 | S. aureus | 2.5 | 78 | [58] |
| AgNPs-GO | S/3.1 | S. aureus | 64 | 100 | [62] |
| AgNPs-GO | S/3.1 | C. albicans | 64 | 100 | [62] |
| AgNPs-GO | S/3.1 | E. coli/P. aeruginosa | 128 | 100 | [62] |
| AgNPs-rGO | QS/57 | E. coli | 40 | 100 | [54] |
| AgNPs-rGO | S/12 | E. coli | 20 | 100 | [59] |
| Au-rGO | T, S/50 | S. aureus/B. subtilis | 250 | 94 | [67] |
| Au-rGO | T, S/50 | E. coli/P. aeruginosa | 250 | 50 | [67] |
| Au-rGO | T, S | E. coli | 10 | 99 | [68] |
| Cu2ONPs-rGO | C/30 | E. coli | 40 | 70 | [71] |
| Cu2ONPs-rGO | C/30 | S. aureus | 40 | 65 | [71] |
| CuONPs-GO | E/80 | E. coli | 3.0 | 90 | [72] |
| CuONPs-GO | E/80 | S. typhimurium | 3.0 | 99 | [72] |
| TiO2-GO | S/30 | E. coli | 180 | 100 | [77] |
| ZnO-GO | NW/75 | E. coli | 500 | 100 | [84] |
| ZnO-GO | ST/150 | E. coli/P. aeruginosa | 6.5 | 100 | [86] |
| ZnO-GO | ST/150 | S. aureus | 11.5 | 100 | [86] |
| ZnO-GO | ST/150 | B. subtilis | 15.0 | 100 | [86] |
| ZnO-G | S/20 | E. coli | 3.0 | 100 | [89] |
| Ag-Fe2O3-rGO | QS | E. coli | 0.1 | 100 | [93] |
| Fe2O3-GO | S/225 | E. coli | 100 | 97 | [94] |
| MnFe2O4-GO | S/170 | E. coli | 100 | 82 | [95] |
| Ag-CoFe2O4-rGO | QS/75 | E. coli/S. aureus | 12 | 98 | [96] |
| Fe3O4-GO | S/66 | E. coli | 300 | 91 | [97] |
Bacterial cell disruption seems to be the main mechanism against Gram-negative bacteria, while the inhibition of cell division could account for the lysis of Gram-positive ones. AgNPs-GO nanocomposites cause stronger damage towards E. coli membrane compared to S. aureus, as can be observed in Table 1. Thus, the antimicrobial potential of these nanocomposites is also influenced by the thickness of the cell wall of the microorganisms. The wall of Gram-positive contains a thick layer (20–80 nm) of peptidoglycan attached to teichoic acids. In Gram-negative bacteria, the cell wall comprises of a thin (7–8 nm) peptidoglycan layer and an outer membrane. The thicker peptidoglycan layer in Gram-positive bacteria could account for their higher resistance towards the effects of AgNPs-GO. Their bactericide action can be depicted in four stages (Figure 1) [60]: release of Ag+ ions, penetration through the cell membrane, ROS generation, followed by DNA, protein, mitochondrion, lipids and membrane damage, which finally leads to cell death.
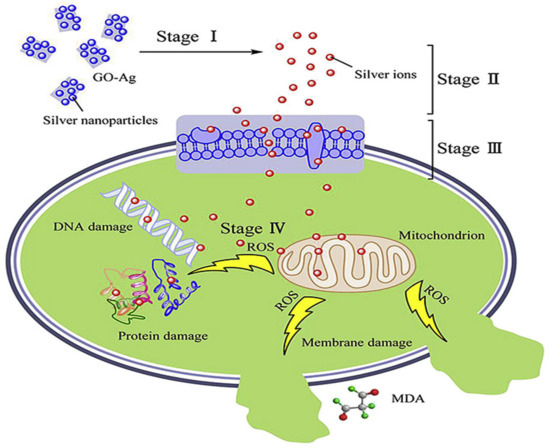
Figure 1. Representation of the antimicrobial action of AgNP-GO nanocomposites. Reproduced from Ref. [60], with permission from Elsevier, 2016.
Lipids constitute a main target during oxidative stress. Free radicals can directly attack the polyunsaturated fatty acids in the bacteria, yeast their membranes, and activate peroxidation of lipids, resulting in a drop in membrane fluidity, which can significantly disrupt membrane-bound proteins. DNA is also a key target. Mechanisms of DNA damage include abstractions and addition reactions by free radicals, leading to carbon-centered sugar radicals and OH- or H-adduct radicals of heterocyclic bases. The sugar moieties creating single- and double-strand breaks in the backbone, adducts of base and sugar groups, and cross-links to other molecules can block replication [63].
It is worthy to mention that AgNPs-rGO nanocomposites as antibacterial agents are more limited due to the tendency of the nanosheets to aggregate via strong van der Waals interactions. While the interaction between G (or rGO) and Ag+ should be mainly via hydrophobic and van der Waals forces, Ag+ nanoparticles have been reported to interact with the negatively charged oxygen-containing functional groups on the GO surface via electrostatic binding [55]. The AgNP-GO nanocomposites showed improved colloidal stability, photo-stability, and antibacterial activities against Gram-negative bacteria compared to AgNPs alone. GO plays a key role in improving the stability of the AgNPs, acting as a platform to prevent their agglomeration. Moreover, composites comprising smaller AgNPs showed better antibacterial activity than those with bigger NPs. This effect of the nanoparticle size has been further corroborated by different authors. Thus, the antibacterial efficacy of AgNP-GO with four NP sizes (10, 30, 50, and 80 nm) against E. coli and S. aureus has been tested, and its was found that the composite with the smallest NP showed the best activity [61]. Similarly, a recent study [62] investigated the bactericide action of AgNP-GO (ratio 1:2.3) manufactured via an environmentally friendly one-step approach, which comprised of spherical NPs with different diameters. The smallest NPs (ca. 3.1 nm) were obtained for the lowest concentrations of the silver precursor and the lowest synthesis temperatures, and led to an exceptional antibacterial action against Gram-negative E. coli and P. aeruginosa, as well as Gram-positive S. aureus and C. albicans (Table 1). Small-sized AgNPs have a larger surface area, resulting in more efficient cell–particle contact.
Nanocomposites with Gold Nanoparticles
The antibacterial activity of gold NPs also depends on their size and shape. Those of smaller dimensions (ca. less than 2 nm) are more likely to penetrate the bacterial cells and cause cell damage, followed by death [64]. Further, triangular-shaped NPs display better activity towards Gram-positive and Gram-negative bacteria than spherical ones [65], since their sharp edges can pierce the membranes of endosomes and translocate to the cytoplasm where they can be retained. The NPs can anchor to the bacterial membrane by electrostatic interaction and disrupt its integrity. They can alter the membrane potential and reduce the ATP levels within the cell, inhibiting the binding of tRNA with ribosomal subunit and thus disturbing translation [66]. Further, Au NPs can produce holes in the cell wall causing leakage of cell contents, and bind with the DNA, hindering transcription. They are also able to generate oxidative via free radical formation: the interaction between small NPs and bacteria probably induces a metabolic imbalance in the bacterial cell, resulting in an increase of ROS production that concluded in bacteria death [64].
Au nanoparticles have also been integrated with GO and rGO [67,68]. These composites also possess higher antibacterial activity than the individual components, and the bacterial cell disruption seems to be induced by the leak of sugars and proteins from the cell membrane when it comes in contact with the nanocomposite [67]. Further, Au nanostructures wrapped by rGO modified with polyethylene glycol (PEG) have been recently applied for the photothermal ablation of bacteria [68], and optimal inhibition of E. coli was attained at high temperatures (56–70 °C), Figure 2. This novel biocompatible process for pathogen ablation opens up a new perspective for treatment of urinary infections.
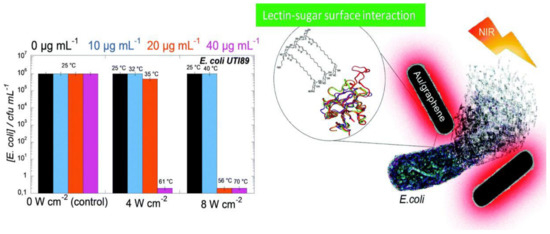
Figure 2. E. coli viability in the presence of rGO–PEG–Au NPs upon irradiation at 4 or 8 W cm−2 for 10 min. Reproduced from Ref. [68], with permission from the Royal Society of Chemistry, 2015.
Nanocomposites with Cooper Oxide Nanoparticles
A few studies have also been devoted to investigating the antibacterial action of Cu [69], Cu2O [70,71] or CuO [72] nanoparticles bound to GO or rGO. At high concentrations, these nanomaterials are toxic to the majority of microorganisms since the redox properties of Cu provoke cellular damage including protein and lipid oxidation. Thus, it is crucial to control the release of Cu2+ ions, which produce ROS species on the bacterial surface. Cu2O can interact with GO and rGO nanosheets via physisorption, electrostatic attraction, or charge-transfer. The nanocomposites have improved bactericidal properties than each individual component, since the graphenic nanosheets can stimulate the Cu2O to generate more ROS [71], and also prevent nanosheet aggregation. Simultaneously, rGO prevents Cu2O from reacting with the environment and releasing Cu2+ rapidly.
In a recent study [71], the antibacterial properties of Cu2O nanoparticles and a rGO-Cu2O nanocomposite were compared as a function of time (Figure 3a,b). When the samples were stored in phosphate buffered saline (PBS) for less than seven days, the nanocomposite showed about 14% higher antibacterial activity against E. coli and S. aureus than the Cu2O nanoparticles, which only produced ROS until the third day, while the nanocomposite systematically produced ROS during this period. For longer periods, the activity of the nanocomposite was considerably higher than that of Cu2O (i.e., 40% and 35% better against E. coli and S. Aureus after one month). The antibacterial mechanism seems to be based on ROS generation (Figure 3d). Upon light illumination, the photoinduced electron would be transferred from the Cu2O to the rGO, which could suppress the recombination of electron-hole pairs. The rGO could accept the photoexcited electrons from Cu2O, enabling improved charge transfer between the bacteria and the rGO-Cu2O nanocomposite [70].
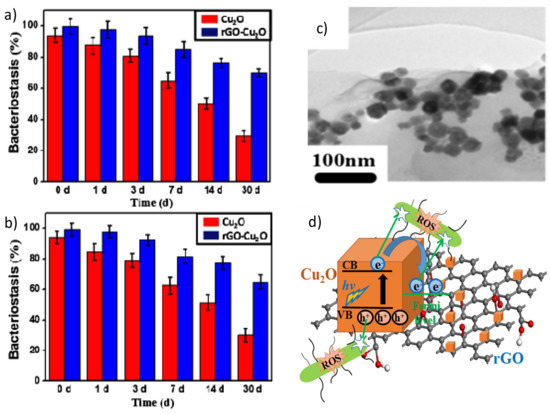
Figure 3. Percentage of bacterial reduction of E. coli (a) and S. aureus (b) in the presence of Cu2O nanoparticles and the Cu2O-rGO nanocomposite after immersion in phosphate buffered saline (PBS) for different days. TEM image of the Cu2O-rGO nanocomposite (c). Schematic representation of the mechanism of ROS production of the Cu2O-rGO nanocomposite (d). Reproduced from Ref. [71], with permission from Elsevier, 2019.
Nanocomposites of CuO nanoparticles and GO have also been developed [72], which were found to be effective antibacterial nanomaterials, strongly inhibiting the proliferation of both E. coli and S. typhimurium bacteria compared to GO or CuO alone. The surface morphology of the nanocomposite significantly differed from that of the pure components (Figure 4), as revealed by scanning electron microscopy (SEM), transmission electron microscopy (TEM), and atomic force microscopy (AFM), and this could account for the improved bactericide action. An inspection of the nanomaterial surface (Figure 4c,e) shows the underlying sheets of GO covered by a high concentration of well-dispersed ellipsoidal NPs, with the short and long axis dimensions being ~80 nm and ~190 nm, respectively. AFM imaging (Figure 4f,g) revealed that the GO and GO-CuO flakes were about 12 and 13 nm in thickness, respectively, indicating that the nanocomposite is composed of a few layers of GO covered by a thin CuO coating. The energy-dispersive X-ray (EDX) mapping confirmed that the material on the modified GO contains cooper and oxygen.
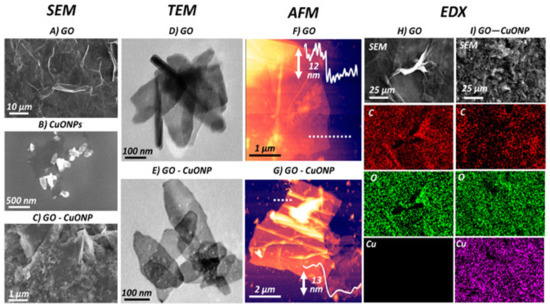
Figure 4. SEM images of (A) GO, (B) CuO nanoparticles, and (C) GO-CuO nanocomposite. (D and E) TEM and (F and G) AFM images of GO and GO-CuO, respectively. Energy-dispersive X-ray (EDX) spectral map for H) GO and I) GO-CuO nanocomposite. Reprinted from Ref. [72], with permission from the American Chemical Society, 2019.
Nanocomposites with Titanium Oxide Nanoparticles
TiO2 is an important semiconducting material due to its chemical inertness, non-toxicity, low cost, excellent chemical/thermal stability, high UV absorption, and strong antibacterial activity against a large variety of microorganisms [73,74,75]. A few works have been recently reported on the photocatalytic antibacterial properties of GO and rGO nanocomposites incorporating TiO2 [76,77,78,79], which provide a green and effective method for the inactivation of various microorganisms by generating ROS. The photocatalytic activity of TiO2 improves after coupling with G derivatives, attributed to the remarkable electron conductivity of the carbon nanomaterial that provides a 2D network reservoir to accept as well as shuttle photogenerated electrons from the semiconductor, which results in the separation and prolonged lifetime of hole-electron pairs [80]. Further, GO can interact with TiO2 via H-bonding and polar forces, while in the case of rGO, the hydrophobic interactions play a dominant role. The antibacterial activity of a GO-TiO2 nanocomposites against E. coli. was investigated, and a complete inactivation was found under light irradiation for 30 min at a concentration of 180 μg/mL [77], Table 1. The inexpensiveness of TiO2 and the easiness of manufacturing magnetic GO–TiO2 make them suitable for water disinfection treatment. In addition, cotton fabrics have been covered with rGO decorated with TiO2 nanoparticles in order to provide self-cleaning characteristics and improve the antimicrobial properties [78], and the nanocomposite coating strongly restricted the growth of E. faecalis and S. aureus. In general, studies indicate higher activity of rGO-TiO2 against Gram-positive bacteria compared to Gram-negative ones. rGO-TiO2 nanocomposites have also been developed for the synergetic degradation of fluoroquinolone in a pulse discharge plasma (PDP) system [79], and the highest removal efficiency (99.4%) was attained at 5 wt% rGO content, which was 23.7% higher than that for TiO2 alone.
Nanocomposites with Zinc Oxide Nanoparticles
ZnO is another semiconductor photocatalyst widely used as an antibacterial agent [81,82]. Its properties depend mainly on its specific surface area and the number of surface sites at which reactions take place with absorbed molecules. The release of Zn2+ has been proposed as one of the principal antibacterial mechanisms of these nanoparticles, together with the penetration and disruption of the bacterial membrane [83]. Nonetheless, as occurs with other nanoparticles, agglomeration hinders efficient antibacterial action. The mixing of GO or rGO with ZnO nanoparticles can prevent their aggregation, leading to nanocomposites with good environmental stability and better antibacterial properties than each of the components alone [84,85,86,87,88,89,90,91]. ZnO-GO nanoparticles have been prepared via a simple one pot reaction [87] in which the GO aided the ZnO dispersion, slowed down the ZnO dissolution and acted as an anchorage point, favoring the direct intimate contact bacteria-ZnO.
The mechanism of photocatalytic inactivation of E. coli by GO-ZnO nanocomposites has also been investigated [88], and it was reported that GO promoted the charge transfer, favoring the bulk production of ROS, hence improving the bacterial inactivation efficiency. ZnO/graphene quantum dot nanocomposites have been synthesized via the hydrothermal method [90] and showed enhanced antibacterial activity on E. coli compared to the individual components, attributed to superior ROS production under UV-irradiation. Further, ZnO nanoparticles on GO [91] were able to inhibit the growth of E. coli, S. typhimurium, B. subtilis, and E. faecalis bacteria (Figure 5), after 24 h of incubation. Excellent antibacterial activity of the nanocomposite can be observed with minimum inhibitory concentrations (MIC) of 6.25 μg/mL for E. coli and S. typhimurium, 12.5 μg/mL for B. subtilis, and 25 μg/mL for E. faecalis, significantly lower than those found for the individual components. This proves that GO-ZnO nanocomposites at low concentration can be effectively used to inhibit the growth of bacteria.
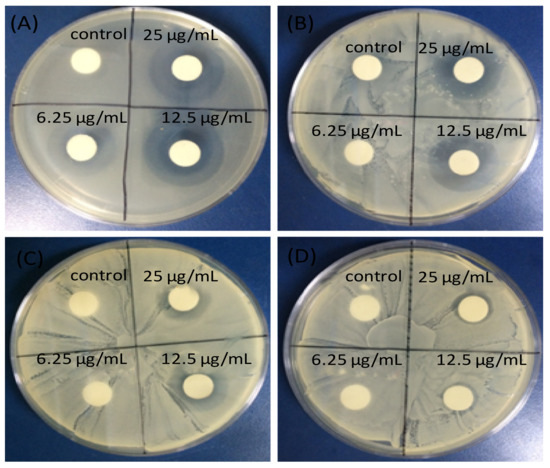
Figure 5. Inhibitory zones of ZnO-GO nanocomposite against (A) Escherichia coli; (B) Salmonella typhimurium; (C) Bacillus subtilis; (D) Enterococcus faecalis. Adapted from Ref. [91], with permission from Dove Medical Press, 2015.
This enhanced behaviour is ascribed to cell membrane disruption due to the release of the Zn2+ ions and the ROS production. Thus, Zn2+ can link to the negatively charged bacterial membrane, causing the protein on the membrane to solidify, thus inhibiting the bacterial proliferation. Besides, electrons can be quickly transferred between the ZnO nanoparticles and the GO, and subsequently absorb surface oxygen to form ROS, resulting in the formation of lipid peroxide, consequently damaging the bacterial membrane.
The nanocomposite synthesis method also influences the antibacterial activity. Those prepared via electrophoretic deposition (EPD) showed better properties than those manufactured via drop-casting [84]. Thus, the EPD method led to effective penetration of the negatively charged GO sheets into ZnO nanowires to form a spider net-like structure, whereas the drop-casting technique caused only the surface coverage of the GO sheets onto the nanowires. The ZnO nanowires alone could just inactivate 58% of E. coli, while both drop-casting and EPD-prepared GO/ZnO nanocomposites displayed significant antibacterial activity; in particular, the EPD one caused 99.5% photoinactivation under visible light irradiation for 1 h at a concentration of 500 μg/mL, Table 1.
Multicomponent Nanocomposites
Several graphene-based multicomponent composites have been prepared by incorporating different nanoparticles with graphene or its derivatives, in order to attain improved antibacterial activity due to synergistic effects. For instance, GO-Ag-TiO2 nanocomposite coatings were synthesized using a hydrothermal process for the control of the food-borne pathogen Campylobacter jejuni. The nanocomposites efficiently inhibited the aggregation and growth of C. jejuni as well as biofilm formation [92]. Different nanocomposites incorporating iron oxide nanoparticles have also been developed [93,94,95,96,97], Table 1. Magnetic GO-MnFe2O4 hybrids at a concentration of 100 μg/mL led to about 82% inhibition on E. coli after only 2 h of contact [95]. Hybrids with polyethylenimine wrapped Ag nanoparticles and Fe2O3 caused 100% inhibition of E. coli at a concentration of only 0.1 μg/mL [93]. A multicomposite of GO, CoFe2O4, and Ag nanoparticles was also manufactured to disinfect water contaminated with E. coli and S. aureus, leading to almost complete inhibition at 12 μg/mL [96]. GO decorated with Ag, TiO2, and ZnO nanostructures has been prepared via ultrasonication and casting, and the antibacterial activity of the nanocomposite was tested against Gram-positive S. aureus and B. anthracoides and Gram-negative E. coli and P. multocida [98]. Figure 6 shows SEM images of GO, Ag, GO-Ag, GO-TiO2-ZnO, and GO-Ag-TiO2-ZnO nanocomposites.
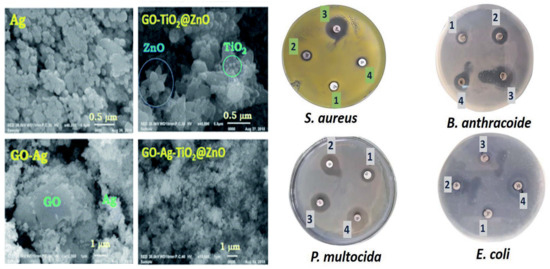
Figure 6. Left: SEM images of Ag, GO–Ag, GO–TiO2@ZnO, and GO–Ag–TiO2@ZnO nanocomposite. Right: Inhibitory zones of GO–Ag–TiO2@ZnO nanocomposite against S. aureus, B. anthracoides and Gram-negative E. coli and P. multocida. Adapted from Ref. [98], with permission from the Royal Society of Chemistry, 2019.
As can be observed, the GO nanosheet was densely packed by the metal oxides, representative of a good combination between the carbon nanomaterial and the inorganic NPs. Thus, the GO nanosheets seem to act as bridges for the metal oxide NPs. Ag NPs have a spherical morphology and are located on the GO surface. The ZnO nanoflower appears over the GO surface, while the spherical TiO2 is deposited onto the ZnO nanoflower. The Ag NPs alone had the lowest inhibitory effect on all tested bacteria. The GO–TiO2-ZnO nanocomposite showed highly suppressing microbial growth against both Gram-positive and Gram-negative bacteria, while the four-component nanocomposite was found to be the most effective against Gram-negative bacteria (Figure 10). The improved activity of the hybrid was ascribed to the synergistic effect of the different components: direct damage of the cellular membrane by the Ag nanoparticles, ROS generation by the TiO2 and ZnO, contact between the GO sharp edges and the bacteria, and the accumulation of the nanoparticles in the cytoplasm.
Ag/ZnO/rGO nanocomposites with different weight ratios were synthesized via rapid microwave irradiation [99]. Depending on the composition, the hybrids were more effective against Gram positive or Gram negative bacteria, and the optimal performance was found at 7 wt% Ag and 15 wt% ZnO. Further, multifunctional nanocomposites of GO incorporating both Ag and Fe2O3 nanoparticles were prepared and characterized [100]. The MIC values against E. coli and S. aureus were 0.025 and 0.05 mg/mL, respectively These composites display both antibacterial and magnetic properties, and can be harvested with a magnet.
On the other hand, hydroxyapatite (HA) (Ca10(PO4)6(OH)2), has excellent biocompatibility, osteoconductivity, is non-toxic, and is widely used for biomedical applications [101]. rGO/HA/Ag multicomponent nanocomposites have been prepared using the hydrothermal method [102] and characterized via different spectroscopic techniques. They showed improved antibacterial activity against B. cereus, S. aureus, E. coli and K. pneumonia than the pure components.
Overall, the comparison of the data in Table 1 reveals that the optimum bactericide efficacy at the lowest concentration (0.1 μg/mL) is attained for the hybrid nanocomposite comprising Ag+ NPs, rGO and Fe3O4, with an antibacterial performance superior to those ever reported for photothermal materials, arising from the synergistic effect of the three components [93].
This entry is adapted from the peer-reviewed paper 10.3390/ijms21103563
