Roxadustat is the first oral drug in the class of new erythropoiesis drugs and a potent HIF-PHD inhibitor, exerted to treat anemia in patients with CKD. In phase 1, 2 and 3 studies with CKD-affected patients, roxadustat was more effective to stimulate erythropoiesis for anemia correction than previously used drugs. Roxadustat can be orally given, unlike other erythropoiesis drugs with parenteral administration only, which grants roxadustat a considerable advantage.
- Roxadustat
- chronic kidney disease
- anemia
1. Introduction
Anemia is a disorder in which hemoglobin, hematocrit (Ht) and erythrocyte levels in the blood are reduced by >2 standard deviations vs. their normal values. Smaller erythrocyte numbers and reduced hemoglobin concentrations lead to decreased amounts of oxygen, transported to tissues and organs throughout the body, which translates directly into their compromised functioning [1].
The link between uremia and anemia was first described by Richard Bright almost 200 years ago [2]. Anemia is common in patients with chronic kidney disease (CKD). The incidence of anemia increases as the disease progresses. In patients with chronic kidney disease (CKD), anemia develops gradually, primarily due to inadequate renal synthesis of erythropoietin, disturbances in iron balance in the body (which may be in part due to increased hepcidin levels), blood loss, decreased red blood cell survival and inflammation [3][4].
It is thus obvious that the treatment of CKD-induced anemia is a clinically significant issue. The current treatment protocols use iron, vitamin B12, folic acid supplementation and only parenterally administered erythropoiesis stimulants.
Research is currently underway on new erythropoiesis drugs that could be orally given. When oxygen deficiency occurs, the oxygen homeostasis-regulating factors are activated in the cells. The main factors, responsible for the cellular responses to changes in oxygen deficiency, include hypoxia-inducible factors (HIFs), with two isoforms, HIF1α and HIF2α, prolyl hydroxylase (PHD) with three isoforms: PHD1, PHD2 and PHD3, aspartic hydroxylase (asparaginyl hydroxylase) and the factor inhibiting hypoxia-inducible factor 1α (HF) [4][5][6].
Hypoxia-induced factor (HIF) is a heterodimeric transcription factor responsible for stimulating the secretion of erythropoietin [EPO] and other oxygen-deficient genes. HIF prolyl hydroxylase (HIF-PHD) enzymes significantly affect the stability of the HIF-α subunit of the HIF transcription factor by promoting post-translational hydroxylation of HIF in an oxygen-dependent manner. This contributes to the balance between oxygen availability and HIF activity.
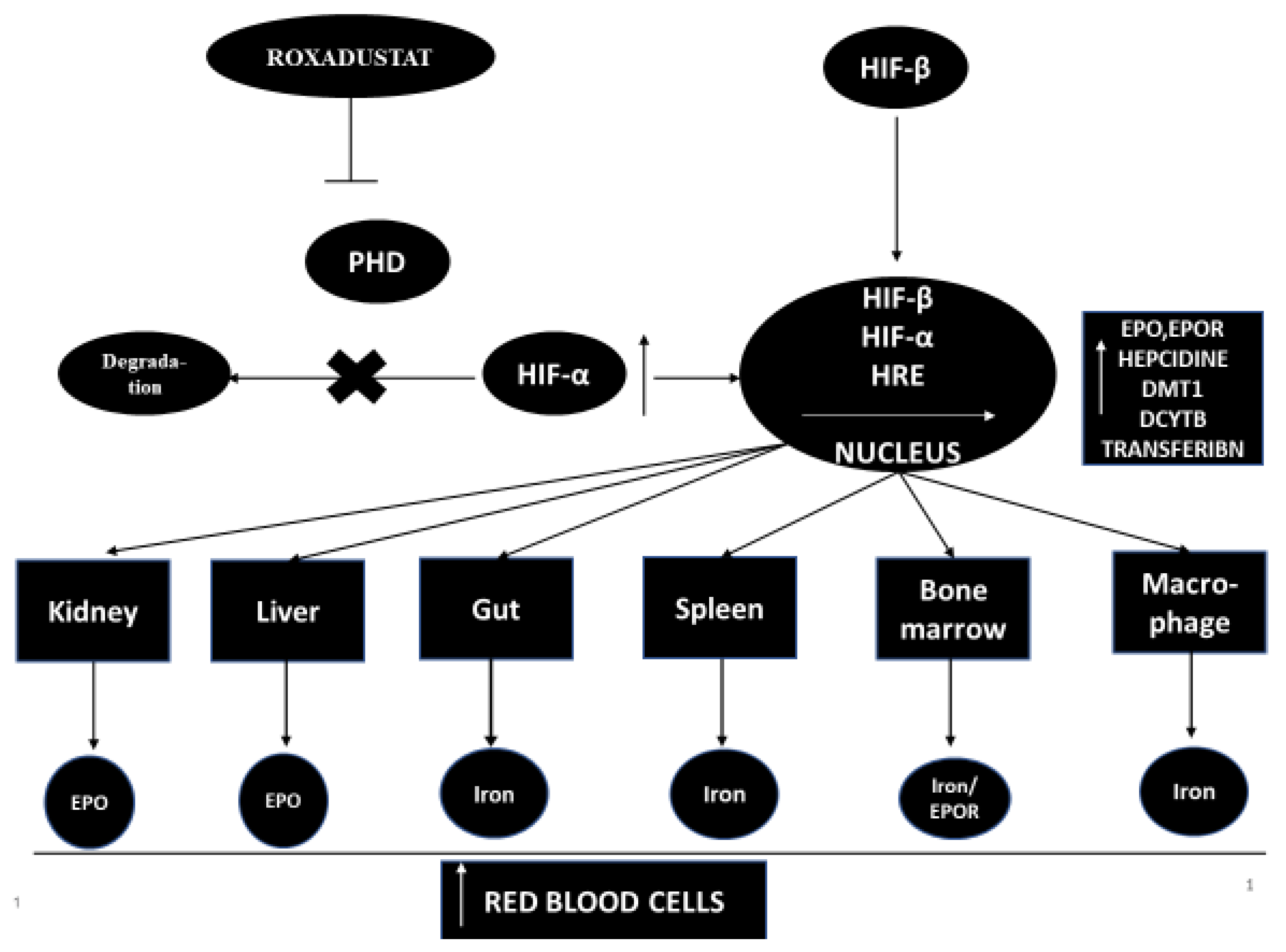
Hypoxia-inducible factor-propyl hydroxylase inhibitor (HIF-PHI)temporarily inhibits PHD catalysis and contributes to a transient increase in HIF expression, and effects the function of many genes, including kidney and liver EPO (or EPO/EPO receptor, proteins promoting iron absorption, iron transport and heme synthesis) [7][8]. However, the increase in plasma EPO concentration in patients with end-stage renal disease treated with the full therapeutic success of HIF-PHI is significantly lower than in patients treated with recombinant human erythropoietin [rhEPO] injections [9]. HIF-PHI has a beneficial effect on iron homeostasis as it reduces the level of hepcidins and ferritin and increases the total iron binding capacity (TIBC) in patients with end-stage renal disease [9]. HIF-PHI inhibitors are drugs that can become an alternative therapy to conventional erythropoiesis stimulating agents [ESA]. It has been shown that, in addition to stimulating erythropoiesis, HIF-PHI inhibitors have pleiotropic effects that affect cholesterol levels and blood pressure [9][10][11]. The mechanism of action of this medicine is shown in Figure 1.
Roxadustat (Ai RuiZhuo® in China) is the first oral drug in its class and a potent HIF-PHD inhibitor used to treat anemia in CKD patients not on dialysis and on dialysis, as well as in myelodysplastic syndromes.
Roxadustat has received approval in China to improve the severity of anemia in patients with CKD.
The FibroGen Company has submitted an application to the Food and Drug Administration [FDA] for permission to use roxadustat in patients with chronic renal failure accompanied by anemia [12]. The application is under consideration.
1.1. A Little Information about Roxadustat
Roxadustat was developed almost a decade ago [13]. Roxadustat (FG-4592) is a new oral drug that stimulates the synthesis of endogenous erythropoietin and also affects and regulates the body’s iron metabolism [14]. Roxadustat temporarily binds and significantly reduces the activity of HIF-PHD enzymes. This leads to a decrease in the degradation of HIF-α and to an increase in the transcriptional activity of HIF [11]. Increased HIF activity stimulates other genes that take an active part in erythropoiesis, such as the EPO gene, the EPO receptor gene, protein genes promoting iron absorption, iron transport and heme synthesis [11][14]. Roxadustat increases the level of hemoglobin in a dose-dependent manner and significantly reduces hepcidin levels [13]. Roxadustat significantly affects iron metabolism in patients with CKD, increasing serum transferrin levels and improving the intestinal absorption of iron in patients with CKD and anemia [9][13][15][16].
1.2. Pharmacokinetics of Roxadustat
Roxadustat clinical trials started in November 2005 [14]. The pharmacokinetics of roxadustat were studied in healthy Caucasian and non-Caucasian subjects. Those studies showed that, after the administration of roxadustat, the mean blood hemoglobin concentration was similar in Caucasians and other races. There was no accumulation of the drug after its repeated, three-times-a-week administration [17]. Neither food intake [18] nor moderate hepatic impairment affected the pharmacokinetics of the drug (NCT02805374) [12]. In another study (NCT 02252731), roxadustat had no significant effect on warfarin pharmacokinetics when both Lin et al. [19] drugs were simultaneously given.
2. Adverse Events and Potential Benefits of Roxadustat
The presented studies show that roxadustat, when administered in patients with terminal renal failure, significantly increases the level of hemoglobin, affects iron metabolism, increases iron absorption from the gastrointestinal tract and reduces the level of cholesterol, while not increasing blood pressure.
Phase III studies of roxadustat treatment were conducted in patients with CKD not treated or treated with renal replacement therapy (hemodialysis, peritoneal dialysis).
The studies, which compared the effects of roxadustat to those of placebo [20][21][22][23][24][25] demonstrated a significant increase in hemoglobin concentrations after roxadustat, both in conservatively treated and dialyzed patients.
The studies which juxtaposed the effects of roxadustat with those of erythropoietin [20][23][26][25][27] or darbapoietin [28], showed that, regarding changes in hemoglobin levels, the effects of roxadustat were not worse, while being usually better than the effects of erythropoietin
2.1. Adverse Events after Roxadustat
It has been shown that in Phase 2 trials involving CKD patients treated with dialysis who were treated with roxadustat (administered three times a week), the tolerance profile was similar to that of patients with CKD receiving dialysis and erythropoietin alfa [9]. Treatment-related serious adverse events (SAEs) were reported in 24% of patients treated with roxadustat and 17% of patients in the placebo group. The authors also analyzed the risk of a composite cardiovascular safety point (including death, myocardial infarction, stroke, hospitalization, heart failure, unstable angina requiring hospitalization, or thromboembolism) in patients after 19 weeks of treatment. The events described above occurred in 12% of patients treated with roxadustat compared with 17% of patients treated with erythropoietin alfa. There were three deaths in the group treated with roxadustat, none of which were related to the treatment [8].
Another phase 2 study (NCT01596855) [29], in which patients with CKD were studied, showed similar results. Treatment-related adverse events were reported in 43% (32/74) of roxadustatat-treated patients and 18% (4/22) of erythropoietin alfa-treated patients. The most common side effects (occurring in >5% of the subjects) were decreased appetite and muscle cramps (7% vs. 5% and 5% vs. 14% respectively, after treatment with roxadustat and erythropoietin alfa). In the Phase 2b trial in patients undergoing dialysis treatment (NCT01414075), roxadustat was a well-tolerated drug (24): back pain (5% vs. 4%), fatigue (5% vs. 0%) and hyperkalemia (5% vs. 0%).
In phase III trials, roxadustat was a well-tolerated drug in both dialyzed [18][30] and non-dialyzed [16][31] patients with CKD. In these studies, the most common treatment-related adverse events (AEs) after roxadustat were nasopharyngitis, back pain, diarrhea and vomiting [30][31]. We would like to emphasize here that hyperkalemia and infections of the upper respiratory tract occurred more often in the group of patients treated with roxadustat, and hypertension was more common in the group of patients treated with epoetin alfa [20].
In patients with CKD, the tolerance profile of roxadustat was similar to that of darberythropoietin [31]. Overall, the summary information on the safety of the treatment used, be it roxadustat or erythropoietin alfa, to the information on serious cardiovascular (CV) adverse events was similar. In patients treated with roxadustat, the most common adverse events were diarrhea, hypertension, pneumonia, headache and arteriovenous fistula thrombosis. Additional serious adverse events include sepsis and acute myocardial infarction.
Among the SAEs that occurred, in four patients treated with roxadustat, there were disturbances in vascular access to dialysis, hip fractures, non-cardiac pain in the chest and dyspnoea, and in one patient in the placebo group. Overall, treatment-related SAEs occurred in 13% (8/61) of roxadustat-treated patients and in 13% (4/30) of 471 placebo-treated subjects. We would like to emphasize that neither of them was found to be related to treatment [29]. A study of patients with CKD (NCT01244763) confirmed a good tolerance profile of roxadustat, and no treatment-related SAEs were reported [11].
2.2. Potential Benefits of Roxadustat, Assumed after Clinical Trials with CKD Patients, Both Non-Dialyzed and Dialyzed
The advantages of roxadustat include: (1) the increase of hemoglobin levels, (2)the maintenance of elevated Hb levels, (3) increased endogenous EPO expression within the physiological range, (4) positive effects on iron metabolism, e.g., by reducing the blood levels of hepcidin, (5) increased iron absorption in the gastrointestinal tract, (6) no inflammation-inducing effects in the body, (7) easy oral administration,(8) reversible and transient inhibition of HIF-PHD, (9) not increased blood pressure, (10) lowered cholesterol and(11) neither high levels of EPO nor side effects of iron supplementation.
2.3. Roxadustat Effects in Patients with Diabetes and Obesity
Following a thorough review of the results provided by the previously presented randomized trials in phase 2 and 3 [29][32][33][34][35][36], a question arises whether the effect of roxadustat (hemoglobin level and the number of red blood cells) is the same in patients with or without diabetes and with or without obesity. The authors of the above-mentioned studies did not take into account the separate effects of roxadustat on the level of hemoglobin and the number of erythrocytes in patients with or without diabetes and with or without obesity. After carefully reading the descriptions of particular study groups, it appears that the average study group may have included approximately 24–42% of patients suffering from diabetes. Many of the examined patients also suffered from obesity.
Analyzing the most recent data, it appears that roxadustat may have a beneficial pleiotropic effect, regardless of its primary effects, in patients with diabetes and obesity.
In obese people, blood flow through the organs is reduced and oxygen consumption is increased. These phenomena lead to insufficient tissue oxygenation. Insufficient tissue oxygenation leads to secretion of HIFs. Increased secretion of HIF-1α leads to the development of insulin resistance and other metabolic disorders. Increased concentration of HIF-1α is accompanied by intensification of inflammatory processes and fibrosis in obese subjects. Contrary to what we wrote, HIF-2α plays a protective role against the development of diabetes (of which HIF-1α is involved in the pathogenesis). Well-conducted pharmacological modulation of HIF activity may contribute to the effective fight against obesity and diabetes.
In order to find answers to the questions about the effectiveness of roxadustat in diabetic and obese patients, we need time and further studies.
This entry is adapted from the peer-reviewed paper 10.3390/ijerph18041612
References
- Podolak-Dawidziak, M. Anemia, Internal Medicine Szczeklik; Practical Medicine Krakow: Krakow, Poland, 2018; p. 1719.
- Bright, R. Cases and Observations Illustrative of Renal Disease, Accompanied with the Secretion of Albuminous Urine. Med. Chir. Rev. 1836, 49, 23–35.
- Becker, K.; Saad, M. A new approach to the management of anemia in CKD patients: A review on roxadustat. Adv. Ther. 2017, 34, 848–853.
- Locatelli, F.; Fishbane, S.; Block, G.A.; Macdougall, I.C. Targeting hypoxia-inducible factors for the treatment of anemia in CKD patients. Am. J. Nephrol. 2017, 5, 187–199.
- Semenza, G.L. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu. Rev. Pathol. 2014, 9, 47–71.
- Haase, V.H. HIF-prolyl hydroxylases as therapeutic targets in erythropoiesis and iron metabolism. Hemodial. Int. 2017, 21 (Suppl. 1), S110–S124.
- Bernhardt, W.M.; Wiesener, M.S.; Scigalla, P.; Chou, J.; Schmieder, R.; Gumzler, V.; Eckardt, K. Inhibition of prolyl hydroxylases increases erythropoietin production in ESRD. J. Am. Soc. Nephrol. 2010, 21, 2151–2156.
- Provenzano, R.; Besarab, A.; Wright, S.; Dua, S.; Zeig, S.; Nguyen, P.; Poole, L.; Saikali, K.G.; Saha, G.; Hemmerich, S.; et al. Roxadustat (FG-4592) versus epoetin alfa for anemia in patients receiving maintenance hemodialysis: A phase 2, randomise, 6- to 19-week, open-Label, active-comparator, dose-ranging, safety and exploratory efficacy study. Am. J. Kidney Dis. 2016, 67, 912–924.
- Provenzano, R.; Besarab, A.; Sun, C.H.; Diamond, S.A.; Durham, J.H.; Cangiano, J.L.; Aiello, J.R.; Novak, J.E.; Lee, T.; Leong, R.; et al. Oral hypoxia-inducible factor prolyl hydroxylase inhibitor roxadustat (FG-4592) for the treatment of anemia in patients with CKD. Clin. J. Am. Soc. Nephrol. 2016, 11, 982–991.
- Flamme, I.; Oehme, F.; Ellinghaus, P.; Jeske, M.; Keldenich, J.; Thuss, U. Mimicking hypoxia to treat anemia: HIF-stabilizer BAY 85-3934 (Molidustat) stimulates erythropoietin production without hypertensive effects. PLoS ONE 2014, 9, e111838.
- Besarab, A.; Provenzano, R.; Hertel, J.; Zabaneh, R.; Klaus, S.; Lee, T.; Leong, R.; Hemmerich, S.; Peony Yu, K.-H.; Neff, T. Randomise placebo controlled dose-ranging and pharmacodynamics study of roxadustat (FG-4592) to treat anemia in nondialysis-dependent CKD (NDD-CKD) patients. Nephrol. Dial. Transplant. 2015, 30, 1665–1673.
- FibroGen. FibroGen Announces Positive Topline Results from Three Global Phase 3 Trials of Roxadustat for Treatment of Anemia in Patients with CKD: Primary Efficacy Endpoints Met in All Three Studies, Non-Dialysis, Incident Dialysis, and Stable Dialysis Studies [Media Release]. 20 December 2018. Available online: (accessed on 20 December 2018).
- Groenendaal-van de Meent, D.; Adel, M.D.; Noukens, J.; Rijnders, S.; Krebs-Brown, A.; Mateva, L.; Alexiev, A.; Schaddelee, M. Effect of moderate hepatic impairment on the pharmacokinetics and pharmacodynamics of roxadustat, an oral hypoxia-inducible factor prolyl hydroxylase inhibitor. Clin. Drug Investig. 2016, 36, 743–751.
- Haase, V.H. Hypoxic regulation of erythropoiesis and iron metabolism. Am. J. Physiol. Renal. Physiol. 2010, 299, F1–F13.
- AstraZeneca. Phase III OLYMPUS and ROCKIES Trials for Roxadustat Met Their Primary Endpoints in CKD Patients with Anaemia [Media Release]. 2018. Available online: (accessed on 20 December 2018).
- Chen, N.; Hao, C.; Peng, X.; Lin, H.; Yin, A.; Hao, L.; Tao, Y.; Liang, X.; Liu, Z.; Xing, C.; et al. Roxadustat for anemia in patients with kidney disease not receiving dialysis. N. Engl. J. Med. 2019, 381, 1001–1010.
- Astellas Pharma. Astellas Announces Positive Topline Results for Global Phase 3 Trial of Roxadustat in CKD (CKD) Patients with Anemia Not on Dialysis [Media Release]. 20 September 2018. Available online: (accessed on 20 September 2018).
- Chen, N.; Hao, C.; Liu, B.; Lin, H.; Wang, C.; Xing, C.; Liang, X.; Jiang, G.; Liu, Z.; Li, X.; et al. Roxadustat Treatment for Anemia in Patients Undergoing Long-Term Dialysis. N. Engl. J. Med. 2019, 381, 1011–1020.
- Groenendaal-van de Meent, D.; den Adel, M.; Rijnders, S.; Krebs- Brown, A.; Kerbusch, V.; Golor, G.; Schaddelee, M. The hypoxia-inducible factor prolyl-hydroxylase inhibitor roxadustat (FG-4592) and warfarin in healthy volunteers: A pharmacokinetic and pharmacodynamic drug-drug interaction study. Clin. Ther. 2016, 38, 918–928.
- Dhillon, S. Roxadustat: First Global Approval. Drugs 2019, 79, 563–572.
- Besarab, A.; Chernyavskaya, E.; Motylev, I.; Shutov, E.; Kumbar, L.; Gurevich, K.; Tak Mao Chan, M.; Leong, R.; Poole, L.; Zhong, M.; et al. Roxadustat (FG4592): Correction of anemia in incident dialysis patients. J. Am. Soc. Nephrol. 2016, 27, 1225–1233.
- Rabinowitz, M.H. Inhibition of hypoxia-inducible factor prolyl hydroxylase domain oxygen sensors: Tricking the body into mounting orchestrated survival and repair responses. J. Med. Chem. 2013, 56, 9369–9402.
- Li, Z.; Tu, Y.; Liu, B. Treatment of renal anemia with roxadustat: Advantages and achievement. Kidney Dis. 2020, 6, 65–73.
- Del Balzo, U.; Signore, P.E.; Walkinshaw, G.; Seeley, T.; Brenner, M.; Wang, Q.; Guo, G.; Arend, M.; Flippin, L.; Chow, A.; et al. Nonclinical Characterization of the HIF-Prolyl Hydroxylase Inhibitor Roxadustat, a Novel Treatment for Anemia of Chronic Kidney Disease Fast Forward. J. Pharmacol. Exp. Ther. 2020.
- Provenzano, R.; Tumlin, J.; Zabaneh, R.; Chou, J.; Hemmerich, S.; Neff, T.B.; Yu, K.P. Oral Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitor Roxadustat (FG-4592) for Treatment of Anemia in Chronic Kidney Disease: A Placebo-Controlled Study of Pharmacokinetic and Pharmacodynamic Profiles in Hemodialysis Patients. J. Clin. Pharmacol. 2020.
- Noonan, M.; Clinkenbeard, E.; Ni, P.; Swallow, E.; Tippen, S.; Agoro, R.; Allen, M.; White, K. Erythropoietin and a hypoxia-inducible factor prolyl hydroxylase inhibitor (HIF-PHDi) lowers FGF23 in a model of chronic kidney disease (CKD). Physiol. Rep. 2020, 8, e14434.
- Pirri, D.; Fragiadaki, M.; Evans, P. Diabetic atherosclerosis: Is there a role for the hypoxia-inducible factors? Biosci. Rep. 2020, 40.
- Akizawa, T.; Yamagushi, Y.; Otsuka, T. A Phase 3, Multicenter, Randomized, Two-Arm, Open-Label Study of Intermittent Oral Dosing of Roxadustat for the Treatment of Anemia in Japanese Erythropoiesis-Stimulating Agent-Naïve Chronic Kidney Disease Patients Not on Dialysis. Nephron 2020, 144, 372–382.
- Chen, N.; Qian, J.; Chen, J.; Yu, X.; Mei, C.; Hao, C.; Jiang, G.; Lin, H.; Zhang, X.; Zuo, L.; et al. Phase 2 studies of oral hypoxiainducible factor prolyl hydroxylase inhibitor FG-4592 for treatment of anemia in China. Nephrol. Dial. Transplant. 2017, 32, 1373–1386.
- Akizawa, T.; Otsuka, T.; Reusch, M.; Ueno, M. Intermittent Oral Dosing of Roxadustat in Peritoneal Dialysis Chronic Kidney Disease Patients with Anemia: A Randomized, Phase 3, Multicenter, Open-Label Study. Ther. Apheresis Dial. 2020, 24, 115–125.
- Akizawa, T.; Iwasaki, M.; Yamaguchi, Y.; Majikawa, Y.; Reusch, M. Phase 3, randomise, double-blind, active-comparator (darberythropoietin alfa) conversion study of oral roxadustat in CKD patients with anemia on hemodialysis in Japan. J. Am. Soc. Nephrol. 2020, 31, 1628–1639.
- Riopel, M.; Moon, J.; Bandyopadhyay, G.; You, S.; Lam, K.; Liu, X.; Kisseleva, T.; Brenner, D.; Lee, S. Inhibition of prolyl hydroxylases increases hepatic insulin and decreases glucagon sensitivity by an HIF-2a-dependent mechanism. Mol. Metab. 2020, 41, 101039.
- Rahtu-Korpela, L.; Karsikas, S.; Horkko, S.; Sequeiros, R.; Lammentausa, E.; Makela, K.; Herzig, K.; Walkinshaw, G.; Kivirikko, K.; Myllyharju, J.; et al. HIF Prolyl 4-Hydroxylase-2 Inhibition Improves Glucose and Lipid Metabolism and Protects Against Obesity and Metabolic Dysfunction. Diabetes 2014, 63, 3324–3333.
- Dallas, A.; Trotsyuk, A.; Ilves, H.; Bonham, C.; Rodrigues, M.; Engel, K.; Barrera, J.; Kosaric, N.; Stern-Buchbnder, Z.; White, A.; et al. Acceleration of Diabetic Wound Healing with PHD2- and miR-210-Targeting Oligonucleotides. Tissue Eng. 2019, 25, 44–54.
- Laitakari, A.; Tapio, J.; Makela, K.; Herzig, K.; Dengler, F.; Gylling, H.; Walkinshaw, G.; Myllyharju, J.; Dimova, E.; Serpi, R.; et al. HIF-P4H-2 inhibition enhances intestinal fructose metabolism and induces thermogenesis protecting against NAFLD. J. Mol. Med. 2020, 98, 719–731.
- Saito, H.; Tanaka, T.; Sugahara, M.; Tanaka, S.; Fukui, K.; Wakashima, T.; Nangaku, M. Inhibition of prolyl hydroxylase domain (PHD) by JTZ-951 reduces obesity-related diseases in the liver, white adipose tissue, and kidney in mice with a high-fat diet. Lab. Investig. 2019, 99, 1217–1232.