Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Pharmacology & Pharmacy
Methotrexate (MTX), a structural analogue of folic acid, that inhibits cell division (mainly in the S phase of the cell cycle) is commonly used for the treatment of many cancers as well for severe and resistant forms of autoimmune pathologies and inflammatory disorders. This paragraph of clinical overview presents state of knowledge with regards to different pathways of MTX active transport system, mechanisms of action and its applications as immunosuppressive drug and anticancer agent.
- methotrexate
- immunosuppressive drug
- anticancer drug
- mechanism
- disease modifying anti-rheumatic drug
MTX is an anti-metabolite (anti-vitamin) of folic acid (FA, vitamin B9), which acts as anticancer agent and immunosuppressant [[1],[2]]. MTX indirectly inhibits cell division through the blockage of folate-related enzymes, mainly DHFR, that catalyses the conversion of dihydrofolate to tetrahydrofolate (THF). THF serves as a significant coenzyme in several transmethylation reactions in pyrimidine and purine nucleotide synthesis pathways, essential in synthesis, repair or replication of DNA strands [[3],[4]]. Actually, the methyl-THF acts as proximal methyl donor in numerous methylation reactions of DNAs, RNAs, proteins, phospholipids and amino acids syntheses. Inhibition of intracellular THF production by MTX results in disruption of cell proliferation and its metabolic imbalance.
MTX crosses the biological barriers very poorly, being highly ionized and generally hydrophilic. Bioavailability and biodistribution of the drug are determined by an active transport system [[5][6]]. Intestinal tissue adsorption of MTX occurs by the proton-coupled folate transporters (PCFTs), which are a solute carrier transporter, while a cellular drug penetration is followed mainly by the reduced folate carrier 1 (RFC1), an ATP-binding cassette transporter. To a small extent, MTX also uses receptor-mediated endocytosis via folate receptors (FRs), the glycosyl-phosphatidyl-inositol (GPI)-anchored membrane proteins that may internalize bound folates and folate conjugates [[7][8]]. Intracellularly, MTX is metabolized by folylpolyglutamyl synthase (FPGS) to a polyglutamate derivatives (MTXGlu), that show significantly increased cell residence time and bioactivity in comparison to initial MTX form (Figure 1) [[9][10][11]]. This is a key pharmacokinetic step that determines the attributed effect of this drug, defining MTX as a representative type 1 prodrug, that undergoes bioactivation inside the cell [[12]]. Polyglutamated MTX is a superior anti-folate agent than MTX, capable of highly potent DHFR inhibition. Moreover, it also induces inhibition of other enzymes like thymidylate synthase (TYMS) [[13]], 5-aminoimidazole-4-carboxamide ribonucleotide transformylase (AICART) [[14][15]] and amido-phosphoribosyltransferase [[16][17]] participating in de novo biosynthesis of purine and pyrimidine nucleotides. Consequently, it is MTXGlu that deprives a cell of precursors for the synthesis of DNA and RNA necessary for cell proliferation (Figure 1), leading to DNA synthesis disturbances and subsequent cell apoptosis [[18]]. It should not be surprising that MTX activity is most visible in actively dividing cells, mainly in the S phase of the cell cycle, and in fact, that highly proliferating cancer cells are the most susceptible to the cytotoxic effect of this drug, indicating that antagonism of folate is related with the anti-tumour activity of MTX.
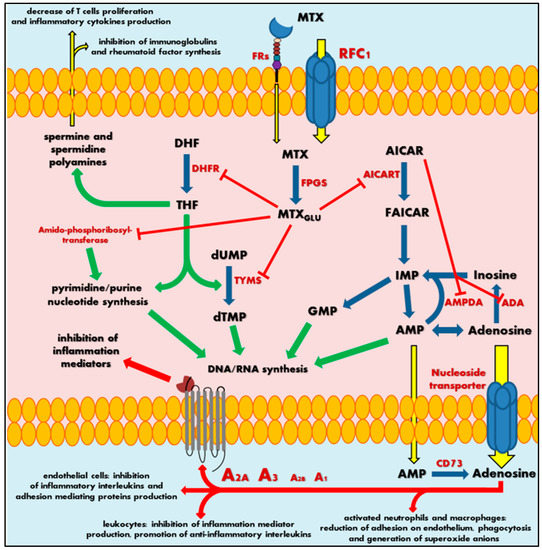
Figure 1. Scheme of mechanism of methotrexate action. MTX—methotrexate; MTXGlu—polyglutamated methotrexate; FRs—folate receptors; RFC1—reduced folate carrier 1; FPGS—folylpolyglutamyl synthase; DHF—dihydrofolate; DHFR—dihydrofolate reductase; THF—tetrahydrofolate; dUMP—deoxyuridine monophosphate; TYMS—thymidylate synthase; dTMP—deoxythymidine monophosphate; DNA—deoxyribonucleic acid; RNA—ribonucleic acid; AICAR—5-aminoimidazole-4-carboxamide ribonucleotide; AICART—5-aminoimidazole-4- carboxamide ribonucleotide transformylase; FAICAR—5-formamidoimidazole-4-carboxamide ribonucleotide; IMP—inosine monophosphate; GMP—guanosine monophosphate; AMPDA—adenosine monophosphate deaminase; ADA—adenosine deaminase; AMP—adenosine monophosphate; CD73—ecto-5′-nucleotidase; A1, A2A, A2B or A3—adenosine receptors. Green arrows represent stimulation, red arrows and sticks represent inhibition, blue arrows represent biochemical conversion, yellow arrows represent migration.
At the same time, the continuous action of MTX polyglutamates in cellular biochemistry results in intracellular accumulation of 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) by AICART inhibition. AICAR has an ability to diminish activity of adenosine deaminase (ADA) and adenosine monophosphate (AMP) deaminase (AMPDA) as well (Figure 1) [[14]]. An MTX-mediated excess of AICAR promotes the AMP and adenosine increase and subsequent release of these adenine derivatives outside the cell. Extracellularly, AMP follows easily dephosphorylation to adenosine via ecto-5′-nucleotidase, also known as the CD73 enzyme [[19]]. Adenosine, a significant signalling agent that modulates diverse physiological functions, acts as major mediator of anti-inflammatory action associated with MTX. In the local extracellular space, adenosine might interact with its specific receptors (A1, A2A, A2B or A3) present on the surface of the origin tissue, but also on immune system cells (Figure 1). Adenosine receptors belong to the seven-transmembrane receptor family, mediating signals into the cell by coupled specific G proteins depending on the receptor subtype. In RA patients, the occurrence of adenosine-specific receptors on immune and synovial cells is elevated, especially A2A and A3 that highly mediate adenosine regulation of immune response and inflammation [[20][21][22][23]]. The adenosine action on A2A and A2B stimulates intracellular production of cyclic adenosine monophosphate (cAMP), a significant cellular second messenger, while stimulation of A3 induces phospholipases C and D or inhibits adenylate cyclase producing cAMP. As a result, adenosine in activated neutrophils and macrophages reduces their ability of adhesion on endothelium and phagocytosis as well as generation of superoxide anions and further reactive oxygen species [[24][25]]. Moreover, leukocytes decrease the production of tumour necrosis factor α (TNF-α) and interleukin (IL)-12—the mediators of inflammation—and promotes anti-inflammatory IL-4 and IL-10. Additionally, it is observed that an inhibition of IL-1 action and suppression of TNF-α excretion in T cells and macrophages inhibit the prostaglandins and leucotrienes syntheses [[25][26][27][28][29][30][31]]. At the same time, adenosine disrupts the cytokine-mediated inflammation in endothelial cells by decrease in production of IL-6 and IL-8 and hindering leucocytes’ adhesion [[32]].
Thus, an adenosine-mediated effect seems to be a key mechanism in MTX anti-inflammatory action. Nevertheless, the MTXGlu also has some other impact on immune chemotaxis and reduction of the occurrence of inflammation mediators. Sustained decrease of THF-mediated methylation downregulates an accumulation of spermine and spermidine polyamines in the extracellular fluids and lymphocytes of patients with RA [[33][34]]. These polyamines are essential cellular growth factors among others in lymphocytes. MTX action inhibits T cell proliferation and synthesis of immunoglobulins or rheumatoid factor in RA patients [[35][36]], followed by a local reduction in lymphocytes interferon (IFN)-γ and IL-2 production, decrease of inflammation, convergent to adenosine receptor-mediated effects.
This entry is adapted from the peer-reviewed paper 10.3390/ijms21103483
References
- Li Huschtscha; Wa Bartier; Ce Ross; Mhn Tattersall; Characteristics of cancer cell death after exposure to cytotoxic drugs in vitro. British Journal of Cancer 1996, 73, 54-60, 10.1038/bjc.1996.10.
- Edwin S. L. Chan; Bruce N. Cronstein,; Mechanism of Action of Methotrexate. Bulletin of the Hospital for Joint Diseases 2013, 71 (Suppl. S1), S5–S8, .
- Laurent Genestier; Romain Paillot; Laurence Quemeneur; Kamel Izeradjene; J P Revillard; Mechanisms of action of methotrexate. Immunopharmacology 2000, 47, 247-257, 10.1016/s0162-3109(00)00189-2.
- Henghe Tian; Bruce N Cronstein; Understanding the mechanisms of action of methotrexate: implications for the treatment of rheumatoid arthritis.. Bulletin of the NYU hospital for joint diseases 2007, 65, 168-173, .
- Katsuhisa Inoue; Hiroaki Yuasa; Molecular Basis for Pharmacokinetics and Pharmacodynamics of Methotrexate in Rheumatoid Arthritis Therapy. Drug Metabolism and Pharmacokinetics 2014, 29, 12-19, 10.2133/dmpk.dmpk-13-rv-119.
- Larry H Matherly; I.David Goldman; Membrane Transport of Folates. Vitamins & Hormones 2003, 66, 403-456, 10.1016/s0083-6729(03)01012-4.
- Shefali Sabharanjak; Satyajit Mayor; Folate receptor endocytosis and trafficking. Advanced Drug Delivery Reviews 2004, 56, 1099-1109, 10.1016/j.addr.2004.01.010.
- Eugénia Nogueira; Marisa P. Sárria; Nuno Gonçalo Azoia; José Antunes; Ana Loureiro; Diana Guimarães; Jennifer Noro; Alexandra Rollett; Georg M. Guebitz; Artur Cavaco-Paulo; et al. Internalization of Methotrexate Conjugates by Folate Receptor-α. Biochemistry 2018, 57, 6780-6786, 10.1021/acs.biochem.8b00607.
- C.M. Baugh; C.L. Krumdieck; M.G. Nair; Polygammaglutamyl metabolites of methotrexate. Biochemical and Biophysical Research Communications 1973, 52, 27-34, 10.1016/0006-291x(73)90949-2.
- Thierry Dervieux; Daniel Fürst; Diana Orentas Lein; Robert Capps; Katie Smith; Michael Walsh; Joel M Kremer; Polyglutamation of methotrexate with common polymorphisms in reduced folate carrier, aminoimidazole carboxamide ribonucleotide transformylase, and thymidylate synthase are associated with methotrexate effects in rheumatoid arthritis. Arthritis & Rheumatism 2004, 50, 2766-2774, 10.1002/art.20460.
- B A Chabner; C J Allegra; G A Curt; N J Clendeninn; J Baram; S Koizumi; J C Drake; J Jolivet; Polyglutamation of methotrexate. Is methotrexate a prodrug?. Journal of Clinical Investigation 1985, 76, 907-912, 10.1172/jci112088.
- Kuei-Meng Wu; A New Classification of Prodrugs: Regulatory Perspectives. Pharmaceuticals 2009, 2, 77-81, 10.3390/ph2030077.
- C J Allegra; B A Chabner; J C Drake; R Lutz; D Rodbard; J Jolivet; Enhanced inhibition of thymidylate synthase by methotrexate polyglutamates.. Journal of Biological Chemistry 1985, 260, 9720–9726, .
- J E Baggott; W H Vaughn; B B Hudson; Inhibition of 5-aminoimidazole-4-carboxamide ribotide transformylase, adenosine deaminase and 5′-adenylate deaminase by polyglutamates of methotrexate and oxidized folates and by 5-aminoimidazole-4-carboxamide riboside and ribotide. Biochemical Journal 1986, 236, 193-200, 10.1042/bj2360193.
- C. J. Allegra; J. C. Drake; J. Jolivet; B. A. Chabner; Inhibition of phosphoribosylaminoimidazolecarboxamide transformylase by methotrexate and dihydrofolic acid polyglutamates.. Proceedings of the National Academy of Sciences 1985, 82, 4881-4885, 10.1073/pnas.82.15.4881.
- M E Sant; S D Lyons; L Phillips; R. I. Christopherson; Antifolates induce inhibition of amido phosphoribosyltransferase in leukemia cells. Journal of Biological Chemistry 1992, 267, 11038–11045, .
- Lynette D. Fairbanks; Katarzyna Ruckemann; Ying Qiu; Catherine M. Hawrylowicz; David F. Richards; Ramasamyiyer Swaminathan; Bernhard Kirschbaum; H. Anne Simmonds; Methotrexate inhibits the first committed step of purine biosynthesis in mitogen-stimulated human T-lymphocytes: a metabolic basis for efficacy in rheumatoid arthritis?. Biochemical Journal 1999, 342, 143-152, 10.1042/0264-6021:3420143.
- L Genestier; R Paillot; Sylvie Fournel; C Ferraro; P Miossec; J P Revillard; Immunosuppressive properties of methotrexate: apoptosis and clonal deletion of activated peripheral T cells.. Journal of Clinical Investigation 1998, 102, 322-328, 10.1172/jci2676.
- L Morabito; M. Carmen Montesinos; D M Schreibman; L Balter; L F Thompson; R Resta; G Carlin; M A Huie; Bruce N. Cronstein; Methotrexate and sulfasalazine promote adenosine release by a mechanism that requires ecto-5'-nucleotidase-mediated conversion of adenine nucleotides.. Journal of Clinical Investigation 1998, 101, 295-300, 10.1172/jci1554.
- M. Carmen Montesinos; Avani Desai; Dave Delano; Jiang-Fan Chen; J. Stephen Fink; Marlene A. Jacobson; Bruce N. Cronstein; Adenosine A2A or A3 receptors are required for inhibition of inflammation by methotrexate and its analog MX-68. Arthritis & Rheumatism 2003, 48, 240-247, 10.1002/art.10712.
- K Varani; F Vincenzi; A Tosi; M Targa; Ff Masieri; Alessia Ongaro; M De Mattei; L Massari; Pa Borea; Expression and functional role of adenosine receptors in regulating inflammatory responses in human synoviocytes. British Journal of Pharmacology 2010, 160, 101-115, 10.1111/j.1476-5381.2010.00667.x.
- Lisa Stamp; Jody Hazlett; R L Roberts; Christopher M.A. Frampton; John Highton; Paul Hessian; Adenosine receptor expression in rheumatoid synovium: a basis for methotrexate action. Arthritis Research & Therapy 2012, 14, R138-R138, 10.1186/ar3871.
- Fabrizio Vincenzi; Melissa Padovan; Martina Targa; Carmen Corciulo; Sarah Giacuzzo; Stefania Merighi; Stefania Gessi; Marcello Govoni; Pier Andrea Borea; Katia Varani; et al. A2A Adenosine Receptors Are Differentially Modulated by Pharmacological Treatments in Rheumatoid Arthritis Patients and Their Stimulation Ameliorates Adjuvant-Induced Arthritis in Rats. PLOS ONE 2013, 8, e54195, 10.1371/journal.pone.0054195.
- Bruce N. Cronstein; M. A. Eberle; H. E. Gruber; R. I. Levin; Methotrexate inhibits neutrophil function by stimulating adenosine release from connective tissue cells.. Proceedings of the National Academy of Sciences 1991, 88, 2441-2445, 10.1073/pnas.88.6.2441.
- B N Cronstein; R I Levin; J Belanoff; Gerald Weissmann; R Hirschhorn; Adenosine: an endogenous inhibitor of neutrophil-mediated injury to endothelial cells. Journal of Clinical Investigation 1986, 78, 760-770, 10.1172/jci112638.
- J L Leroux; M Damon; C Chavis; A Crastes De Paulet; F Blotman; Effects of methotrexate on leukotriene and derivated lipoxygenase synthesis in polynuclear neutrophils in rheumatoid polyarthritis. Revue du rhumatisme et des maladies osteo-articulaires 1992, 59, 587–591, .
- M Brody; I Böhm; R Bauer; Mechanism of action of methotrexate: experimental evidence that methotrexate blocks the binding of interleukin 1 beta to the interleukin 1 receptor on target cells.. European journal of clinical chemistry and clinical biochemistry : journal of the Forum of European Clinical Chemistry Societies 1993, 31, 667–674, 10.1515/cclm.1993.31.10.667.
- M G Bouma; R K Stad; F A Van Den Wildenberg; W A Buurman; Differential regulatory effects of adenosine on cytokine release by activated human monocytes.. The Journal of Immunology 1994, 153, 4159–4168, .
- Christoph Becker; Karina Barbulescu; Kai Hildner; Markus F. Neurath; Karl-Hermann Meyer Buschenfelde; Activation and Methotrexate-Mediated Suppression of the TNFalpha Promoter in T Cells and Macrophages. Annals of the New York Academy of Sciences 1998, 859, 311-314, 10.1111/j.1749-6632.1998.tb11153.x.
- Arnaud Constantin; Nathalie Lambert; Bader Yassine-Diab; Michel Abbal; Alain Cantagrel; Patrick Loubet‐Lescoulié; Bernard Mazières; Claude De Préval; Antiinflammatory and immunoregulatory action of methotrexate in the treatment of rheumatoid arthritis: Evidence of increased interleukin-4 and interleukin-10 gene expression demonstrated in vitro by competitive reverse transcriptase-polymerase chain reaction. Arthritis & Rheumatism 1998, 41, 48-57, 10.1002/1529-0131(199801)41:1<48::aid-art7>3.0.co;2-k.
- G Haskó; Adenosine: an endogenous regulator of innate immunity. Trends in Immunology 2004, 25, 33-39, 10.1016/j.it.2003.11.003.
- M. G. Bouma; F. A. Van Den Wildenberg; W. A. Buurman; Adenosine inhibits cytokine release and expression of adhesion molecules by activated human endothelial cells. American Journal of Physiology-Cell Physiology 1996, 270, C522-C529, 10.1152/ajpcell.1996.270.2.c522.
- Gideon Nesher; Terry L. Moore; The in vitro effects of methotrexate on peripheral blood mononuclear cells: Modulation by methyl donors and spermidine. Arthritis & Rheumatism 1990, 33, 954-959, 10.1002/art.1780330706.
- K Yukioka; S Wakitani; M Yukioka; Y Furumitsu; K Shichikawa; T Ochi; H Goto; I Matsui-Yuasa; S Otani; Y Nishizawa; et al. Polyamine levels in synovial tissues and synovial fluids of patients with rheumatoid arthritis.. The Journal of Rheumatology 1992, 19, 689–692, .
- G Nesher; T G Osborn; T L Moore; In vitro effects of methotrexate on polyamine levels in lymphocytes from rheumatoid arthritis patients.. Clinical and experimental rheumatology 1996, 14, 395-399, .
- G Nesher; T G Osborn; T L Moore; Effect of treatment with methotrexate, hydroxychloroquine, and prednisone on lymphocyte polyamine levels in rheumatoid arthritis: correlation with the clinical response and rheumatoid factor synthesis. Clinical and experimental rheumatology 1997, 15, 343–347, .
This entry is offline, you can click here to edit this entry!
