Quiescent cancer cells (QCCs) are cancer cells that are reversibly suspended in G0 phase with the ability to re-enter the cell cycle and initiate tumor growth, and, ultimately, cancer recurrence and metastasis.
- quiescence
- dormancy
- cancer
- model
- detection
1. Introduction
Despite recent advances in cancer treatment, patients still succumb to recurrence and metastasis, which are the main causes of cancer-related mortality [1,2]. Quiescent cancer cells (QCCs) transiently exit the cell cycle to reside in G0 phase [3,4] and, depending on the triggers that promote re-entry into the cell cycle, their behavior can affect both the clinical course of the disease and treatment effectiveness. The G0-G1 reversibility of QCCs distinguish them from other nonproliferating cells, such as senescent cells that are irreversibly arrested [5]. Cells enter quiescence state to survive deficiency of nutrition and growth factor, while continuous stimuli, including activated oncogene, impaired mitochondrial function, and DNA damaging agents induce the affected cells to senescence, leading to subsequent cell death [5,6]. Quiescent cells on the contrary, can re-enter the cell cycle and re-proliferate, thus evading cell death [6].
The presence of QCCs is common, and evident in various tumor types [1,4,7,8], suggesting that they are a fundamental property of cancer independent of histology. As the tumor develops in patients, cancer cells grow uncontrollably as they lose the “contact inhibition” property. The feature of “contact inhibition” in normal cells restrict the growth of a cell upon contact [9]. Cancer cells grow continuously even outreached the blood vessels and they become nutrient- and oxygen-deprived as they reside further from blood vessels. Entering into quiescence state allow these cancer cells to survive the oppressive environment [10]. The experimental QCCs models, such as nutrient deprivation, hypoxia-induced, and contact inhibition model, recapitulate the nutrient-, oxygen-deprived, and contact inhibition aspect of QCCs, respectively.
QCCs are similar to quiescent CSCs, in terms that they are both cancer cells in G0 phase. However, QCCs are distinct than cancer stem cells (CSCs) as CSCs can exist in any phase in the cell cycle, not necessarily in G0 phase. While quiescent CSCs are located at endosteal bone surface, QCCs are evident in tumor masses and everywhere as circulating tumor cells (CTCs) or disseminating tumor cells (DTCs) are frequently in quiescence. Moreover, self-renewal, “stem-ness” markers and specific transcription factors, which are expressed by the CSCs, but are absent in QCCs [1]. In addition to being in G0 phase and nonproliferating, other properties of QCCs are having less RNA content [11,12] and expressing Ki-67 negativity [13]. These characteristics of QCCs are the basis of the markers used to detect QCCs, for example Ki-67 that are applied in preclinical studies [14,15,16].
QCCs are nonproliferating, thus marking them as resistant to most conventional cancer treatments that act preferentially on proliferating cells [4]. Surviving QCCs can re-enter the cell cycle when conditions are suitable [3], and reproliferation gives rise to cancer progression and recurrence [17]. In addition, it is well-known that dissemination can occur early in the malignant process, but the basis for dormancy at secondary sites is cellular quiescence [18]. When QCCs survive in the local niche and are reactivated, clinically detectable metastases become apparent [1,7].
Therapeutic strategies targeting QCCs include blocking QCCs from re-entering the cell cycle, encouraging timing of therapies dependent on cell proliferation to match cell cycle re-entry points, or eradication of QCCs while in the G0 state. A rational understanding of these approaches, therefore, requires relevant models recapitulating QCCs behavior, as well as superior methods to evaluate QCCs activity. This review emphasizes the existing and emerging models of studying and measuring QCCs and discusses their respective features and applications.
2. Models Mimicking Quiesdcent Cancer Cells
Experimental models can be established through the use of either a homogenous or a heterogeneous QCC environment (Figure 1). Homogenously altered conditions of cell culture, including growing cancer cells until contact inhibition, depriving cell cultures of serum, nutrients or oxygen, induce the cultured cells to attain quiescence, thus enriching the culture with QCC. On the other hand, coculturing different cell populations (malignant and non-malignant) to mimic the tissue microenvironment results in models heterogeneously composed of proliferating and quiescent cells.
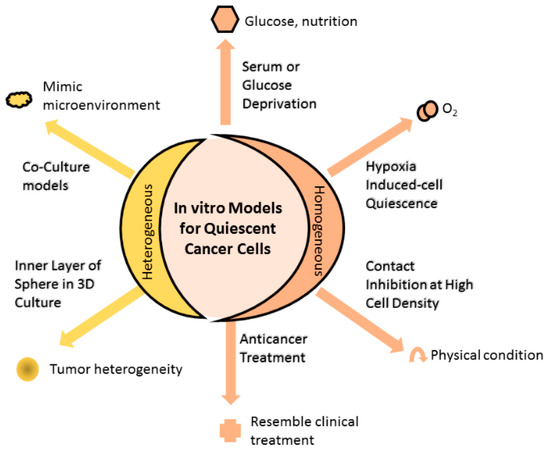
Figure 1. In vitro models for quiescent cancer cells (QCCs). Changing the physical condition of cell culture by allowing cultured cells to grow at high cell density until reaching contact inhibition or depriving essential substances (such as serum, glucose, or oxygen) of the culture, or administering anticancer treatment into cell culture that homogenously induces cells into quiescence. Alternatively, cell culture can heterogeneously comprise quiescent and proliferating cells using the 3D culture method. The inner and outer layers of 3D spheres are mainly composed of quiescent and proliferating cells, respectively, resembling the tumor heterogeneity observed in the clinical setting. Other means to achieve heterogeneous cell composition are coculturing different cell populations that mimic the microenvironment in which actual tumor cells grow, while anticancer treatment induces a portion of cells to quiescence, as found in the clinical setting.
This entry is adapted from the peer-reviewed paper 10.3390/cells10030562
