The structures of these gold derivatives (i.e. gold nanoparticles, gold (I) and (III) complexes and carbene-based gold complexes) were synthesized to evaluate the influence of increased activity and/or selectivity on their pharmacological effects.
- gold derivatives
- cancer treatment
- breast cancer
- cytotoxicity
- antitumor activity
1.Introduction
Approximately 9 million people around the world fall ill and die from cancer diseases every year, many of who do not receive adequate treatment due to the high cost. It is esteemed that the incidence of cancer will double by 2035 [1]. In particular, breast cancer is the most frequent type diagnosed, about 25% of all cancers [2,3]. The anticancer therapy currently in use consists of three main approaches: surgical removal of the tumor mass, chemotherapy and radiotherapy. Unfortunately, several types of metastatic tumors have been found to be chemoresistant [4]. The most common form of breast cancer, which makes up 60% of all diagnoses, is hormone receptor (HR) positive and human epidermal growth factor receptor 2 (HER2) negative [5]. Several studies have shown that HR positive patients have a better response to hormone therapy. However, the lack of HER2 proteins causes a disposal of drugs specially designed to target these proteins as therapeutic options. The retinoblastoma (Rb) protein, a tumor suppressor, regulates RNA transcription during the G1 cell growth phase to stop the proliferation of malignant cells [6]. Cyclin D1 proteins bind to cyclin-dependent kinase (CDK) enzymes 4 and 6 thereby inhibiting the regulatory function of the Rb protein. The National Comprehensive Cancer Network (NCCN) recognized CDK 4/6 inhibitors are palbociclib, abemaciclib and ribociclib in combination with aromatase inhibitors. Combination therapy provides treatment for HR positive/HER2 negative advanced or metastatic breast cancer by lowering estrogen levels to inhibit cell growth and cyclin-dependent kinase to block malignant cell division and proliferation [7,8]. Abemaciclib and ribociclib showed a relative reduction in the risk of death of 25–30% [9]. By combining an aromatase inhibitor with palbociclib, progression free survival (PFS) is increased by 10 months, compared with hormone monotherapy [10]. Likewise, adding palbociclib to fulvestrant resulted in a double increase in PFS compared to those taking fulvestrant alone [11]. There is currently limited evidence to support the use of CDK 4/6 inhibitors as monotherapy. Increased data on the safety and tolerability of CDK 4/6 inhibitors in patients may help clinicians in the selection of initial therapy for patients with HR positive/HER2 negative breast cancer [12]. Platinum-based anticancer drugs such as cisplatin, carboplatin and oxaliplatin have been widely employed in the treatment of several types of cancer, such as lung, colorectal, ovarian and breast cancers [13]. However, the efficacy and, therefore, the use of such platinum-based drugs have decreased due to the high risk of serious toxicity, such as neurotoxicity [14,15]. Major advances have recently been made in drug delivery systems, due to miniaturization technologies that have improved the performance of existing drugs to provide new, more effective therapies [16]. The use of biomolecules such as peptides, nucleic acids and others reduced the amount of drug required by improving its targeted action [17]. The reduction in size at the nanometer level (<100 nm) has led to dramatic improvements in the way drugs are delivered [18]. All particles <100 nm in size could be formed via nanocrystals, drug–polymer complexation or using nanoscale shells that could trap drugs [19]. The fine size of nanoparticles or metal complexes allows for a loading of small molecules, peptides, proteins and nucleic acids that escape immunological detection unlike larger particles, which are easily excluded from the body. Recently, innovative research has developed towards metal-based anticancer drugs, such as gold derivatives, with the aim of improving the effectiveness, expanding the activity field and, above all, reducing the general toxicity [20,21]. The pharmacological activity of gold compounds has been tested since ancient times; these compounds have been used in a series of treatments, including that for the rheumatoid arthritis and as an antibacterial and antitumor. Different studies have shown that gold derivatives act differently from platinum anticancer drugs, since their primary target is the proteasome; in this regards future approaches will bring to the development gold complexes selective for specific cancer cells and tumor targets in order to increase their effectiveness and better control of undesired side effects [22,23]. The purpose of this review is to summarize the state of the art of the antitumor activity of gold compounds, complexes and nanoconjugates, providing a brief overview of their use against breast cancer. In particular, the following aspects will be treated:- Drug delivery systems;
- Gold nanosystems;
- Gold complexes.
2.Drug Delivery Systems
Conventional cancer treatments, such as chemotherapy and radiotherapy, act in biological systems in a non-specific way, affecting both malignant and healthy cells. This affects the optimal therapeutic and implementation of gold derivatives.Targeted delivery through gold derivatives can take place through two types: passive and active effect, reducing unwanted effects and the development of drug resistance [25]. Passive targeting allows the accumulation of a drug or drug transport system within a specific site due to the variation of physicochemical or pharmacological factors. This type of method exploits the size of the nanoparticles and the properties of the tumor vascular system, effectively improving the bioavailability and efficacy of the drug. The vascularity of the tumor is very different from normal tissue, in fact the blood vessels of the tumor tissues, unlike those in normal tissues, have spaces between the adjacent endothelial cells up to 600–800 nm. These pathophysiological features of tumor vessels induce the Enhanced permeability and retention EPR (Enhanced Permeability and Retention) effect, which allows macromolecules, including nanoparticles, to extravasate through these extravascular spaces and accumulate within tumor tissues [26]. The accumulation of tumor drugs is ten times greater when the drug is administered from a nanoparticle rather than as a free drug. Another contributor to passive targeting is the unique microenvironment that surrounds cancer cells, which is different from that of normal cells. Fast-growing hyperproliferative cancer cells use glycolysis for extra energy, resulting in an acidic environment. The pH-sensitive liposomes are designed to be stable at a physiological pH but degraded to release the active drug into target tissues where the pH is lower, such as in the acidic environment of cancer cells [27]. Active targeting involves the attachment of a fraction, such as a monoclonal antibody or a ligand, to deliver a drug to pathological sites or to cross biological barriers based on molecular recognition processes [28–30]. When designing the synthesis of nanoparticles, it is necessary to consider some factors: for example, the antigen or receptor should be expressed exclusively and homogeneously on tumor cells and not expressed on healthy ones. The internalization of conjugates occurs through receptor-mediated endocytosis. Indeed, when a conjugate binds to its receptor on the cell surface, the plasma membrane envelops the receptor and ligand complex to form an endosome, this is transferred to target organelles.When the pH value inside the endosome becomes acidic and lysozymes are activated, the drug is released from the conjugate and enters the cytoplasm. The receptor released by the conjugate returns to the cell membrane to begin a second transport cycle by binding with new conjugates. Ligands targeting cell surface receptors can be natural substances that have the advantages of a molar mass and lower immunogenicity than antibodies. Molecular targeted therapy is a potential solution to overcome these challenges, it can be achieved through smart design (Figure 1). Both methods allow increasing the concentration of the anticancer drug directly inside the tumor cell, causing the decrease of toxicity for healthy cells [30]. Gold derivatives (gold compounds, complexes and nanoparticles) can be conjugated to a wide range of biologically active organic molecules, designed to cross the blood–brain barrier, interact with specific receptors entering the cell through an alternative path. In particular, passive targeting of gold nanoparticles is based on the effect of enhanced permeability and retention (EPR) and tumor angiogenesis, while active targeting is based on direct binding from the ligand to receptors expressed by tumor cells [26]. Antitumor agents can be released as a function of pH or temperature [31,32].
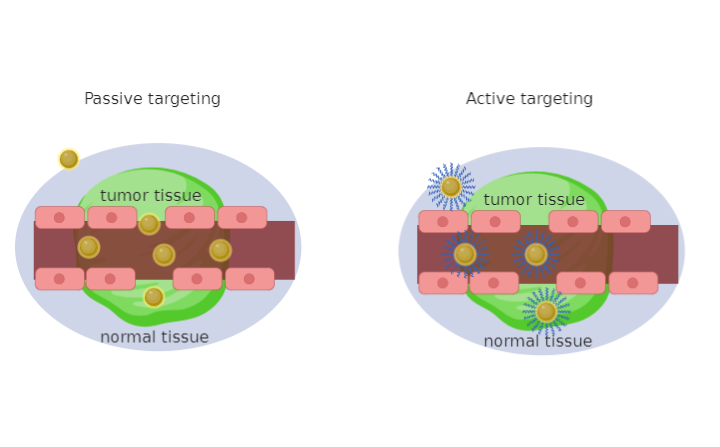
Figure 1. Schematic illustration of targeted strategies for cancer therapy by functional Gold Nanoparticles (Au-NPs, NPs=NanoParticles). Malignant tissue is distributed unevenly concerning healthy cells, hence the gold nanoparticles that cross the empty spaces due to the increase in permeability and the retention effect of passive drug release. This figure also illustrates active targeting with direct binding of gold nanoparticles to receptors with specific ligands.
3. Gold Nanosystems
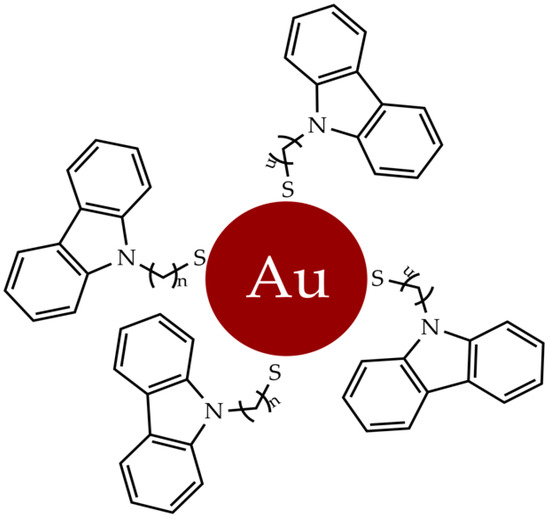
4. Gold Complexes
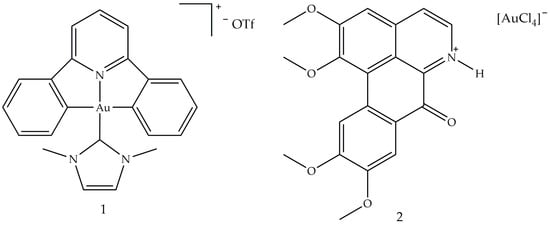
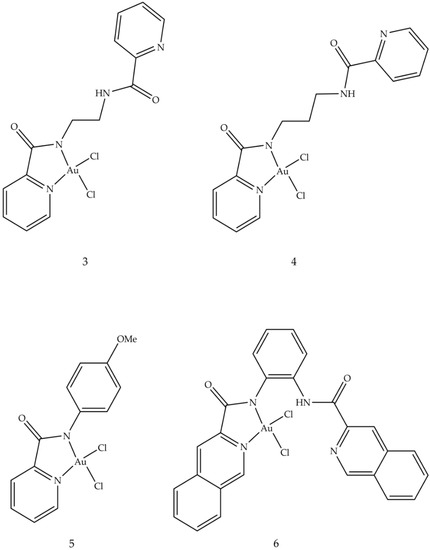
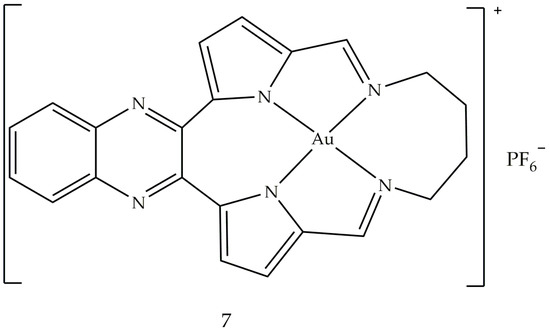
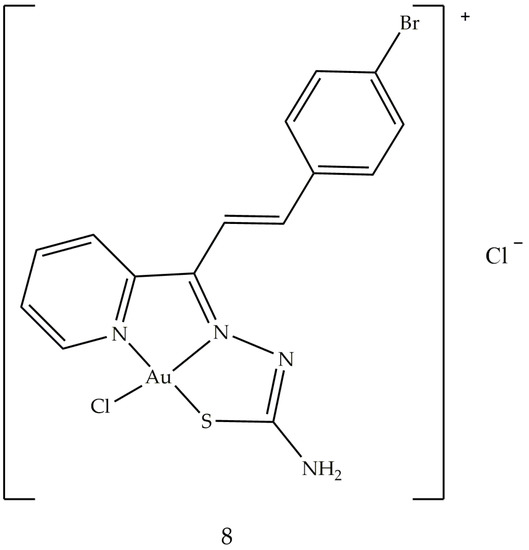
5. Gold Based Carbene Complexes
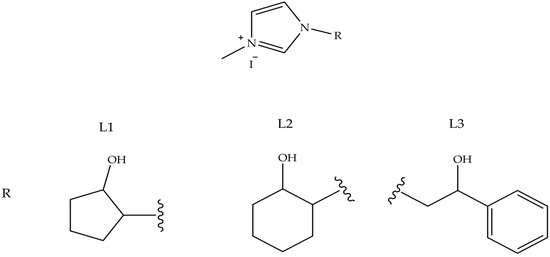
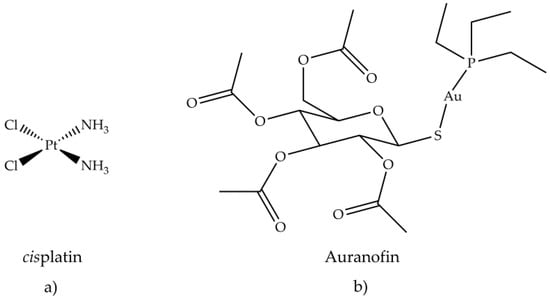
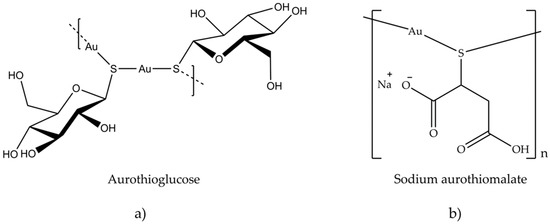
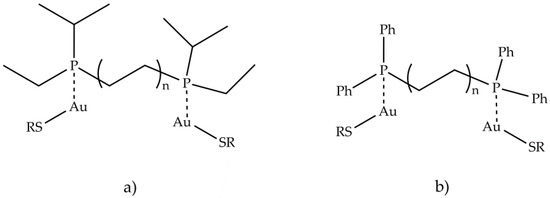
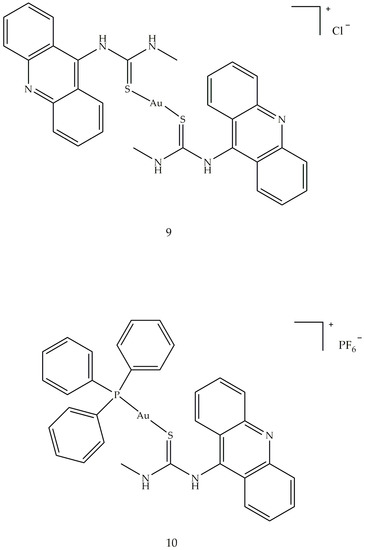
Antiarthritic Gold Compounds in Oncology
6. Conclusions and Prospects
This entry is adapted from the peer-reviewed paper 10.3390/app11052089
