In children and adolescents, chronic low-grade inflammation has been implicated in the pathogenesis of co- and multi-morbid conditions to mental health disorders. Diet quality is a potential mechanism of action that can exacerbate or ameliorate low-grade inflammation. A good quality diet, high in vegetable and fruit intake, wholegrains, fibre and healthy fats ameliorates low-grade inflammation, and therefore represents a promising therapeutic approach, as well as an important element for disease prevention in both children and adolescents.
- dietary intake
- dietary pattern
- macronutrients
- biomarkers
- inflammation
- CRP
- cytokine
- inter-leukin
- children
- adolescent
1. Introduction
Inflammation is a physiological response to cellular and tissue damage. It is designed to protect the host from bacteria, viruses and infections by eliminating pathogens, promoting cellular repair and restoring homeostatic conditions [1]. However, a prolonged inflammatory state through chronic low-grade inflammation has deleterious effects, including irreparable damage to tissues and organs, and increased risk of disease status [2].
Low-grade inflammation, reflected in the overproduction of acute phase proteins such as C-reactive protein (CRP), pro-inflammatory cytokines such as interleukin-6 (IL-6) and tumour necrosis factor-alpha (TFN-α) has been established as a risk factor for several neuropsychiatric disorders [3], including depression [4][5][6][7] and schizophrenia [8]. Moreover, low-grade inflammation in children and adolescents has been associated with the development of co- and multi-morbid conditions to mental health pathologies [9][10][11][12], including cardiovascular disease [13][14], metabolic syndrome [15], type-II diabetes [16] and obesity [17], therefore making inflammation an important therapeutic target to study, especially for individuals suffering from those conditions.
The potential factors that promote low-grade chronic inflammation are diverse. Stressors such as trauma through adverse childhood experiences, psychosocial stress, as well as modifiable lifestyle sources such as limited physical exercise or smoking are all capable of evoking a deleterious inflammatory response. Increasingly, attention has been given to diet quality as a potential mechanism of action that can exacerbate or ameliorate low-grade inflammation and subsequently influence mental health [18][19]. Certainly, healthy dietary patterns of high quality, such as adherence to a Mediterranean Diet [20], or eating foods such as vegetables and fruit [1], or macro/micronutrients, such as omega-3 poly-unsaturated fatty acids (PUFAs) [21] or vitamins C and E [22], respectively, have been shown to reduce systemic inflammation [23][24]. In observational and interventional studies, a higher quality diet, comprised of these nutrients, has been associated with a reduced risk of adverse mental health in both children [25] and adolescents [26][27]. In contrast, the prevailing Western dietary pattern, which is high in refined grains, red meat, refined sugar and saturated fat, elicits a pro-inflammatory response and increasing levels of circulating inflammatory biomarkers [21].
Moreover, it is well established that a healthy diet in childhood and adolescence is crucial for optimal growth and development and for disease prevention [28]. For example, higher vegetable intake in childhood has been associated with a lower risk of developing mental health pathologies later in life [29], such as depression. In addition, a healthy diet can contribute to the prevention of cardio-metabolic multi-morbidities, often seen in adult patients with neuropsychiatric conditions [30]. As such, modifying dietary intake as early as during childhood and adolescence represents a promising therapeutic strategy in order to maintain a regular immune response, and to reduce the risk of adverse mental health disorders and associated co- and multi-morbid conditions later in life.
Former literature reviews in children and adolescents have focused on various aspects of diet and various biomarkers that, however, are not specifically related to the immune system function and response [31][32][33]. Therefore, to the best of our knowledge this is the first systematic review bringing together the current evidence base from observational and interventional studies investigating associations between dietary intake, by means of dietary patterns, food groups, macronutrients or micronutrients, and biological markers of low-grade inflammation, including CRP, IL-6 and TNF-α among others, in both children and adolescents.
2. Discussion
This review provides the first evidence for the association between dietary intake (dietary patterns, food groups, macronutrients and micronutrients) and biological markers of inflammation in children and adolescents. The main results (figure 1) indicate that adequate adherence to healthful dietary patterns, such as the DASH diet, low glycaemic index diets and the Mediterranean diet are associated with decreased levels of biomarkers, including CRP, IL-6 and TNF-α. Among the individual constituents of these diets, vegetable and fruit intake and wholegrains, as well as healthy fats were associated with a favourable inflammatory response. In contrast, a Western dietary pattern, as well as its separate constituents including saturated fatty acid, elicited a pro-inflammatory response increasing levels of pro-inflammatory biomarkers, such as CRP, IL-6, TNF-α and sVCAM-1. Associations across the studies included in this review were attenuated by gender, as well as the presence of underlying pathologies, independent of dietary intake.
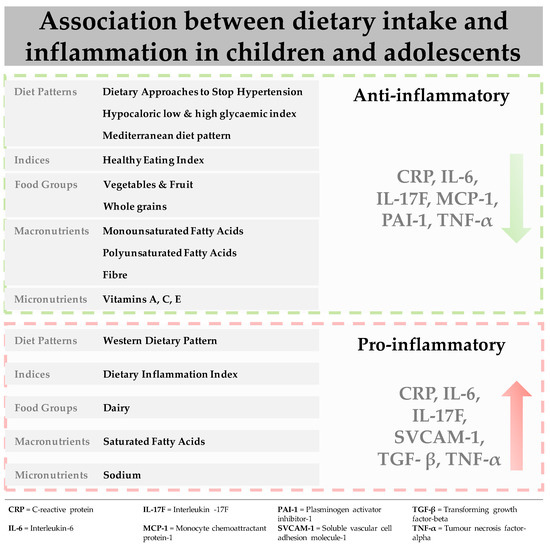
Traditionally diet–disease relationships have been examined by focusing on nutrients or food groups, which can be limiting. Foods are typically eaten in combination, and nutrients have both synergistic and antagonistic biochemical interactions [34]. More recently, dietary patterns that capture the whole diet, involving the combination of foods and nutrients have been examined [35]. The most examined dietary pattern in this review was the Mediterranean diet and in observational studies conducted in healthy populations adequate to high adherence resulted in decreased levels of pro-inflammatory biomarkers [36][37][38]. Similar results were found for studies examining low glycaemic index diets in obese populations [39][40][41] and one intervention study in females with metabolic syndrome which examined the DASH diet [42].
The mechanisms by which these healthful dietary patterns affect the inflammatory process are largely underexplored [18]. It has been hypothesised that the protective effect of these patterns may be derived from the anti-inflammatory properties of their constituents [43]. The Mediterranean diet is characterized by high intakes of vegetables, fruit, wholegrains, legumes, nuts, fish and low-fat dairy, and low intakes of red meat and adequate intakes of healthy fats [44]. As such, the diet is rich in antioxidants, folate, and flavonoids which are anti-inflammatory. The high dietary fibre content supports gut health and the growth of microbial species which potentially regulate the inhibition or production of pro-inflammatory chemokines and cytokines [45]. Omega-3 PUFAs, found in high concentration in oily fish such as salmon, have been shown to regulate the immune response by inhibiting the activation of pro-inflammatory pathways and reducing cytokine expression [3]. High-dose eicosapentaenoic acid has been shown to improve cognitive symptoms in Attention deficit hyperactivity disorder (ADHD) youth with low baseline levels [46][47], while research in animal models has demonstrated inflammation-induced reductions in neurogenesis can be prevented through omega-3 PUFAs intake [48]. Lastly, sodium intake has been implicated in the regulation of the immune response [49]. The DASH diet, which is similar to the Mediterranean diet, with a greater focus on minimal red meat and processed foods, also restricts sodium intake [50]. Similar to sodium, the studies included in this review also examined other dietary components with strong anti-inflammatory properties. For example, studies showed that high intakes of vegetables and fruit [51][52][53][54][55][56][57], and whole-grains [56][58] resulted in lower levels of inflammatory biomarkers, such as CRP, IL-6 and TNF-α. The same was for various micronutrients such as beta-carotene [55] and vitamins C and E [59] all of which are considered to have anti-inflammatory properties [60].
In contrast to healthful dietary patterns, studies that examined the Western dietary pattern, characterised by high amounts of refined grains, red meat, high fat dairy, ultra-processed food intake and trans fatty acids while being low in omega-3 PUFAs [61][62] showed positive associations with pro-inflammatory markers [63][64]. Similarly, in the studies that examined the DII, diets with high inflammatory potential, inducing a higher inflammatory response were positively associated with pro-inflammatory biomarkers in males and females [65][66][67][68]. In terms of separate components comprising these diets, saturated fatty acid intake is known to be a significant pro-inflammatory contributor that stimulates IL-6 secretion, while in contrast high intakes of healthier fats, such as omega-3 PUFAs found in oily fish can inhibit the inflammatory response [69]. Across the studies that examined saturated fatty acids in this review there was evidence for a positive association between saturated fatty acid and CRP, as well as various cytokines, IL-6, sVCAM and sICAM [51].
As demonstrated in this review, the relationship between diet and inflammation is attenuated by a number of different factors [1][70][71]. For example, gender differences were evident across the studies included in this review, however, they were not specific to one particular diet, food group, macro- or micronutrient. Nor where they specific to any inflammatory biomarker. We hypothesize the gender difference is potentially attributable to the influence of hormones. Sex hormones affect immune function, whereby estrogens stimulate auto-immunity and androgens exhibit protective properties [72][73][74][75]. Other evidence indicates genetic, epigenetic and environmental factors may also contribute to gender differences; however, no included studies explored these potential associations [75][76]. Furthermore, we acknowledge the heterogeneity in the cohorts of the studies included in this review, and as such some associations could have been confounded by the sample population. A number of studies examined cohorts with underlying pathologies, with overweight/obesity [77][39][40][41][78][65][79][80][81][82][83][84], and type-1 diabetes [85][86][87] being the most studied. In overweight and obese populations excess adipose tissue has been linked to an increase in sub-chronic levels of key pro-inflammatory cytokines, mainly CRP, IL-6 and TNF-α [88], and this may prevent or attenuate any potential therapeutic effect exerted by a healthful diet [89]. Taken together, physiologic mechanisms specific to disease state or population may inhibit any beneficial influence of a healthy dietary pattern, potentially explaining the lack of significant associations in the studies discussed in our review [87].
With regards to methodology, it is widely accepted that nutrition epidemiology studies are affected by reporting bias. Imprecision in the measurement of dietary intake is often observed, particularly in children and adolescents, which can cause the over- or under- estimation of the impact of exposure [90][91][92]. Moreover, the measurement tools themselves are often flawed, for example the KidMed questionnaire, used to evaluate the level of adherence to the Mediterranean diet, has a strong bias toward healthy foods and as such may not adequately capture hidden constituents such as sodium [93]. These methods also do not consider the biological effect of food (intake versus absorption) [94]. More recently, several studies have examined associations between biological markers of dietary intake and pro-inflammatory biomarkers. Using these methods, an inverse association between fatty acid composition in erythrocytes and pro-inflammatory biomarkers (IL-1β and IL-6) has been observed in children and adolescents [95], and higher omega-6/omega-3 PUFAs ratio has been associated with higher levels of inflammation [96] and subsequent adverse mental health effects [97]. These biological, rather than self-reported, dietary measures are a more accurate and reliable way of investigating dietary intake, which should be more often used in future research studies [98].
Lastly, there is currently no consensus regarding the inflammatory biomarkers best used to represent chronic low-grade inflammation in children and adolescents, and biomarker measurement error such as sampling, storage and laboratory errors also cannot be excluded [99]. The majority of the studies in this review used a single static measurement of inflammation, however, inflammatory markers owing to their role in homeostasis and immune response are by nature not static and when measured in the fasting state are recognised as being insensitive, and producing highly variable results [2]. Multiple, non-fasting state measures would provide for more accurate and meaningful outcomes.
This entry is adapted from the peer-reviewed paper 10.3390/nu13020356
References
- Calder, P.C.; Ahluwalia, N.; Brouns, F.; Buetler, T.; Clement, K.; Cunningham, K.; Esposito, K.; Jö Nsson, L.S.; Kolb, H.; Lansink, M.; et al. Dietary factors and low-grade inflammation in relation to overweight and obesity commissioned by the ILSI Europe Metabolic Syndrome and Diabetes Task Force. Br. J. Nutr. 2011, 106, S1–S78.
- Minihane, A.M.; Vinoy, S.; McArdle, H.J.; Kremer, B.H.A.; Sterkman, L.; Vafeiadou, K.; Benedetti, M.M.; Williams, C.M.; Calder, P.C.; Russell, W.R.; et al. Low-grade inflammation, diet composition and health: Current research evidence and its translation. Br. J. Nutr. 2015, 114, 999–1012.
- Giacobbe, J.; Benoiton, B.; Zunszain, P.; Pariante, C.M.; Borsini, A. The Anti-Inflammatory Role of Omega-3 Polyunsaturated Fatty Acids Metabolites in Pre-Clinical Models of Psychiatric, Neurodegenerative, and Neurological Disorders. Front. Psychiatry 2020, 11.
- Kiecolt-Glaser, J.K.; Derry, H.M.; Fagundes, C.P. Inflammation: Depression Fans the Flames and Feasts on the Heat. Am. J. Psychiatry 2015, 172, 1075–1091.
- Zunszain, P.A.; Hepgul, N.; Pariante, C.M. Inflammation and Depression. Behavioral Neurobiology of Depression and its Treatment. Springer: Berlin, Germany, 2012; pp. 135–151.
- Sawyer, K.M.; Zunszain, P.A.; Dazzan, P.; Pariante, C.M. Intergenerational transmission of depression: Clinical observations and molecular mechanisms. Mol. Psychiatry 2019, 24, 1157–1177.
- Cattaneo, A.; The Neuroimmunology of Mood Disorders and Alzheimer’s Disease (NIMA) Consortium; Ferrari, C.; Turner, L.; Mariani, N.; Enache, D.; Hastings, C.; Kose, M.; Lombardo, G.; McLaughlin, A.P.; et al. Whole-blood expression of inflammasome- and glucocorticoid-related mRNAs correctly separates treatment-resistant depressed patients from drug-free and responsive patients in the BIODEP study. Transl. Psychiatry 2020, 10, 1–14.
- Osimo, E.F.; Cardinal, R.N.; Jones, P.B.; Khandaker, G.M. Prevalence and correlates of low-grade systemic inflammation in adult psychiatric inpatients: An electronic health record-based study. Psychoneuroendocrinology 2018, 91, 226–234.
- Barbaresko, J.; Koch, M.; Schulze, M.B.; Nöthlings, U. Dietary pattern analysis and biomarkers of low-grade inflammation: A systematic literature review. Nutr. Rev. 2013, 71, 511–527.
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832.
- Stein, D.J.; Benjet, C.; Gureje, O.; Lund, C.; Scott, K.M.; Poznyak, V.; Van Ommeren, M. Integrating mental health with other non-communicable diseases. BMJ 2019, 364, l295.
- Bennett, J.M.; Reeves, G.; Billman, G.E.; Sturmberg, J.P. Inflammation–Nature’s way to efficiently respond to all types of challenges: Implications for understanding and managing “the epidemic” of chronic diseases. Front. Med. 2018, 5, 316.
- Singh, J.; Merrill, E.D.; Sandesara, P.B.; Schoeneberg, L.; Dai, H.; Raghuveer, G. Vitamin D, low-grade inflammation and cardio-vascular risk in young children: A pilot study. Pediatr. Cardiol. 2015, 36, 1338–1343.
- Amaral, G.A.; Alves, J.D.; Honorio-França, A.C.; Fagundes, D.L.; Araujo, G.G.; Lobato, N.S.; Lima, V.V.; Giachini, F.R. Interleukin 1-beta is Linked to Chronic Low-Grade Inflammation and Cardiovascular Risk Factors in Overweight Adolescents. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 887–894.
- Al-Hamad, D.; Raman, V. Metabolic syndrome in children and adolescents. Transl. Pediatr. 2017, 6, 397–407.
- Reinehr, T. Inflammatory markers in children and adolescents with type 2 diabetes mellitus. Clin. Chim. Acta 2019, 496, 100–107.
- Stroescu, R.F.; Mărginean, O.; Bizerea, T.; Gafencu, M.; Voicu, A.; Doroș, G. Adiponectin, leptin and high sensitivity C-reactive protein values in obese children—Important markers for metabolic syndrome? J. Pediatr. Endocrinol. Metab. 2019, 32, 27–31.
- Marx, W.; Lane, M.; Hockey, M.; Aslam, H.; Berk, M.; Walder, K.; Borsini, A.; Firth, J.; Pariante, C.M.; Berding, K.; et al. Diet and depression: Exploring the biological mechanisms of action. Mol. Psychiatry 2020, 2020, 1–17.
- Berk, M.; Williams, L.J.; Jacka, F.N.; O’Neil, A.; Pasco, J.A.; Moylan, S.; Allen, N.B.; Stuart, A.L.; Hayley, A.C.; Byrne, M.L.; et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013, 11, 200.
- Grosso, G.; Mistretta, A.; Frigiola, A.; Gruttadauria, S.; Biondi, A.; Basile, F.; Vitaglione, P.; D’Orazio, N.; Galvano, F. Mediterranean Diet and Cardiovascular Risk Factors: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2013, 54, 593–610.
- Silveira, B.K.S.; Oliveira, T.M.S.; Andrade, P.A.; Hermsdorff, H.H.M.; Rosa, C.d.O.B.; Franceschini, S.d.C.C. Dietary Pattern and Macro-nutrients Profile on the Variation of Inflammatory Biomarkers: Scientific Update. Cardiol. Res. Pract. 2018, 1–18.
- Sun, C.-H.; Li, Y.; Zhang, Y.; Zhou, X.-L.; Wang, F. The effect of vitamin–mineral supplementation on CRP and IL-6: A systemic review and meta-analysis of randomised controlled trials. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 576–583.
- Esposito, K.; Marfella, R.; Ciotola, M.; Di Palo, C.; Giugliano, F.; Giugliano, G.; D’Armiento, M.; D’Andrea, F.; Giugliano, D. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: A randomized trial. J. Am. Med Assoc. 2004, 292, 1440–1446.
- Giugliano, D.; Ceriello, A.; Esposito, K. The effects of diet on inflammation: Emphasis on the metabolic syndrome. J. Am. Coll. Cardiol. 2006, 48, 677–685.
- Kohlboeck, G.; Sausenthaler, S.; Standl, M.; Koletzko, S.; Bauer, C.P.; Von Berg, A.; Berdel, D.; Krämer, U.; Schaaf, B.; Lehmann, I.; et al. Food Intake, Diet Quality and Behavioral Problems in Children: Results from the GINI-plus/LISA-plus Studies. Ann. Nutr. Metab. 2012, 60, 247–256.
- Jacka, F.N.; Kremer, P.J.; Berk, M.; De Silva-Sanigorski, A.M.; Moodie, M.; Leslie, E.R.; Pasco, J.A.; Swinburn, B.A. A Prospective Study of Diet Quality and Mental Health in Adolescents. PLoS ONE 2011, 6, e24805.
- Jacka, F.N.; Kremer, P.; Leslie, E.; Berk, M.; Patton, G.; Toumbourou, J.W.; Williams, J.W. Associations Between Diet Quality and Depressed Mood in Adolescents: Results from the Australian Healthy Neighbourhoods Study. Aust. N. Z. J. Psychiatry 2010, 44, 435–442.
- Van der Velde, L.A.; Nguyen, A.N.; Schoufour, J.D.; Geelen, A.; Jaddoe, V.W.; Franco, O.H.; Voortman, T. Diet quality in childhood: The Generation R Study. Eur. J. Nutr. 2019, 58, 1259–1269.
- Lassale, C.; Batty, G.D.; Baghdadli, A.; Jacka, F.; Sánchez-Villegas, A.; Kivimäki, M.; Akbaraly, T. Healthy dietary indices and risk of depressive outcomes: A systematic review and meta-analysis of observational studies. Mol. Psychiatry 2019, 24, 965–986.
- Saugo, E.; Lasalvia, A.; Bonetto, C.; Cristofalo, D.; Poli, S.; Bissoli, S.; Bertani, M.; Lazzarotto, L.; Gardellin, F.; Ceccato, E.; et al. Dietary habits and physical activity in first-episode psychosis patients treated in community services. Effect on early anthropometric and cardio-metabolic alterations. Schizophr. Res. 2020, 216, 374–381.
- Hilger-Kolb, J.; Bosle, C.; Motoc, I.; Hoffmann, K. Associations between dietary factors and obesity-related biomarkers in healthy children and adolescents—A systematic review. Nutr. J. 2017, 16, 1–12.
- Suhett, L.G.; Hermsdorff, H.H.M.; Cota, B.C.; Ribeiro, S.A.V.; Shivappa, N.; Hebert, J.R.; Franceschini, S.; De Novaes, J.F. Dietary inflammatory potential, cardiometabolic risk and inflammation in children and adolescents: A systematic review. Crit. Rev. Food Sci. Nutr. 2021, 61, 407–416.
- Rocha, N.P.; Milagres, L.C.; Longo, G.Z.; Ribeiro, A.Q.; Novaes, J.F.d. Association between dietary pattern and cardiometabolic risk in children and adolescents: A systematic review. J. Pediatr. 2017, 93, 214–222.
- Zhang, R.; Chen, J.; Zheng, H.; Li, Y.; Huang, H.; Liang, Z.; Jiang, H.; Sun, J. Effects of medium chain triglycerides on body fat dis-tribution and adipocytokine levels in children with acute lymphoblastic leukemia under chemotherapy. Medicine 2019, 98, e16811.
- Moeller, S.M.; Reedy, J.; Millen, A.E.; Dixon, L.B.; Newby, P.; Tucker, K.L.; Krebs-Smith, S.M.; Guenther, P.M. Dietary Patterns: Challenges and Opportunities in Dietary Patterns Research. J. Am. Diet. Assoc. 2007, 107, 1233–1239.
- Agostinis-Sobrinho, C.; Ramírez-Vélez, R.; García-Hermoso, A.; Rosário, R.; Moreira, C.; Lopes, L.; Martinkenas, A.; Mota, J.; Santos, R. The combined association of adherence to Mediterranean diet, muscular and cardiorespiratory fitness on low-grade inflammation in adolescents: A pooled analysis. Eur. J. Nutr. 2018, 58, 2649–2656.
- De Carvalho, K.M.B.; Ronca, D.B.; Michels, N.; Huybrechts, I.; Cuenca-García, M.; Marcos, A.; Molnar, D.; Dallongeville, J.; Manios, Y.; Schaan, B.; et al. Does the Mediterranean Diet Protect against Stress-Induced Inflammatory Activation in European Adolescents? The HELENA Study. Nutrients 2018, 10, 1770.
- Sureda, A.; Bibiloni, M.D.M.; Julibert, A.; Bouzas, C.; Argelich, E.; Llompart, I.; Pons, A.; Tur, J.A. Adherence to the Mediterranean Diet and Inflammatory Markers. Nutrients 2018, 10, 62.
- Iannuzzi, A.; Licenziati, M.R.; Vacca, M.; De Marco, D.; Cinquegrana, G.; Laccetti, M.; Bresciani, A.; Covetti, G.; Iannuzzo, G.; Rubba, P.; et al. Comparison of two diets of varying glycemic index on carotid subclinical atherosclerosis in obese children. Hear. Vessel. 2009, 24, 419–424.
- Parillo, M.; Licenziati, M.R.; Vacca, M.; De Marco, D.; Iannuzzi, A. Metabolic changes after a hypocaloric, low-glycemic-index diet in obese children. J. Endocrinol. Investig. 2011, 35, 629–633.
- Rouhani, M.H.; Kelishadi, R.; Hashemipour, M.; Esmaillzadeh, A.; Surkan, P.J.; Keshavarz, A.; Azadbakht, L. The Impact of a Low Glycemic Index Diet on Inflammatory Markers and Serum Adiponectin Concentration in Adolescent Overweight and Obese Girls: A Randomized Clinical Trial. Horm. Metab. Res. 2016, 48, 251–256.
- Saneei, P.; Hashemipour, M.; Kelishadi, R.; Esmaillzadeh, A. The Dietary Approaches to Stop Hypertension (DASH) Diet Affects Inflammation in Childhood Metabolic Syndrome: A Randomized Cross-Over Clinical Trial. Ann. Nutr. Metab. 2014, 64, 20–27.
- Casas, R.; Sacanella, E.; Estruch, R. The immune protective effect of the Mediterranean diet against chronic low-grade inflam-matory diseases. Endocr. Metab. Immune Disord.-Drug Targets 2014, 14, 245–254.
- Dai, J.; Miller, A.H.; Bremner, J.D.; Goldberg, J.; Jones, L.; Shallenberger, L.; Buckham, R.; Murrah, N.V.; Veledar, E.; Wilson, P.W. Ad-herence to the Mediterranean diet is inversely associated with circulating interleukin-6 among middle-aged men: A twin study. Circulation 2008, 117, 169.
- Davis, R.; Day, A.S.; Barrett, J.; VanLint, A.; Andrews, J.M.; Costello, S.P.; Bryant, R.V. Habitual dietary fibre and prebiotic intake is inadequate in patients with inflammatory bowel disease: Findings from a multicentre cross-sectional study. J. Hum. Nutr. Diet. 2020.
- Chang, J.P.-C.; Su, K.-P.; Mondelli, V.; Satyanarayanan, S.K.; Yang, H.-T.; Chiang, Y.-J.; Chen, H.-T.; Pariante, C.M. High-dose eicosa-pentaenoic acid (EPA) improves attention and vigilance in children and adolescents with attention deficit hyperactivity disorder (ADHD) and low endogenous EPA levels. Transl. Psychiatry 2019, 9, 1–9.
- Chang, J.P.-C.; Su, K.-P.; Mondelli, V.; Pariante, C.M. Omega-3 polyunsaturated fatty acids in youths with attention deficit hyper-activity disorder: A systematic review and meta-analysis of clinical trials and biological studies. Neuropsychopharmacology 2018, 43, 534–545.
- Borsini, A.; Alboni, S.; Horowitz, M.A.; Tojo, L.M.; Cannazza, G.; Su, K.-P.; Pariante, C.M.; Zunszain, P.A. Rescue of IL-1β-induced reduction of human neurogenesis by omega-3 fatty acids and antidepressants. Brain Behav. Immun. 2017, 65, 230–238.
- Fogarty, A.; Lewis, S.A.; McKeever, T.M.; Britton, J.R. Is higher sodium intake associated with elevated systemic inflammation? A population-based study. Am. J. Clin. Nutr. 2009, 89, 1901–1904.
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R.; Simons-Morton, D.G.; et al. Effects on Blood Pressure of Reduced Dietary Sodium and the Dietary Approaches to Stop Hypertension (DASH) Diet. N. Engl. J. Med. 2001, 344, 3–10.
- Navarro, P.; De Dios, O.; Jois, A.; Gavela-Pérez, T.; Gorgojo, L.; Martín-Moreno, J.M.; Soriano-Guillén, L.; Garcés, C. Vegetable and Fruit Intakes Are Associated with hs-CRP Levels in Pre-Pubertal Girls. Nutrients 2017, 9, 224.
- Gonzalez-Gil, E.M.; Santabárbara, J.; Russo, P.; Ahrens, W.; Claessens, M.; Lissner, L.; Börnhorst, C.; Krogh, V.; Iacoviello, L.; Molnar, D.; et al. Food intake and inflammation in European children: The IDEFICS study. Eur. J. Nutr. 2015, 55, 2459–2468.
- Qureshi, M.M.; Singer, M.R.; Moore, L.L. A cross-sectional study of food group intake and C-reactive protein among children. Nutr. Metab. 2009, 6, 40.
- Hagin, S.; Lobato, D.J.; Sands, B.E.; Korzenik, J.R.; Merrick, M.; Shah, S.A.; Bancroft, B.; Bright, R.; Law, M.; Moniz, H.; et al. Dietary be-haviors in newly diagnosed youth with inflammatory bowel disease. Child Health Care. 2017, 46, 408–420.
- Holt, E.M.; Steffen, L.M.; Moran, A.; Basu, S.; Steinberger, J.; Ross, J.A.; Hong, C.-P.; Sinaiko, A.R. Fruit and Vegetable Consumption and Its Relation to Markers of Inflammation and Oxidative Stress in Adolescents. J. Am. Diet. Assoc. 2009, 109, 414–421.
- Han, Y.-Y.; Forno, E.; Brehm, J.M.; Acostaperez, E.; Álvarez, M.; Colón-Semidey, A.; Rivera-Soto, W.; Campos, H.; Litonjua, A.A.; Alcorn, J.F.; et al. Diet, interleukin-17, and childhood asthma in Puerto Ricans. Ann. Allergy Asthma Immunol. 2015, 115, 288–293.e1.
- Cabral, M.; Araújo, J.; Lopes, C.; Ramos, E. Food intake and high-sensitivity C-reactive protein levels in adolescents. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 1067–1074.
- Hur, I.Y.; Reicks, M. Relationship between Whole-Grain Intake, Chronic Disease Risk Indicators, and Weight Status among Adolescents in the National Health and Nutrition Examination Survey, 1999–2004. J. Acad. Nutr. Diet. 2012, 112, 46–55.
- Del Mar Bibiloni, M.; Maffeis, C.; Llompart, I.; Pons, A.; Tur, J.A. Dietary factors associated with subclinical inflammation among girls. Eur. J. Clin. Nutr. 2013, 67, 1264–1270.
- Schultz, H.; Ying, G.-S.; Dunaief, J.L.; Dunaief, D.M. Rising Plasma Beta-Carotene Is Associated With Diminishing C-Reactive Protein in Patients Consuming a Dark Green Leafy Vegetable–Rich, Low Inflammatory Foods Everyday (LIFE) Diet. Am. J. Lifestyle Med. 2019, 2019.
- Li, T.; Qiu, Y.; Yang, H.S.; Li, M.Y.; Zhuang, X.J.; Zhang, S.H.; Feng, R.; Chen, B.L.; He, Y.; Zeng, Z.R.; et al. Systematic review and meta-analysis: Association of a pre-illness Western dietary pattern with the risk of developing inflammatory bowel disease. J. Dig. Dis. 2020, 21, 362–371.
- Okręglicka, K. Health effects of changes in the structure of dietary macronutrients intake in western societies. Rocz. Państwowego Zakładu Hig. 2015, 66, 97–105.
- Oddy, W.H.; Allen, K.L.; Trapp, G.S.; Ambrosini, G.L.; Black, L.J.; Huang, R.-C.; Rzehak, P.; Runions, K.C.; Pan, F.; Beilin, L.J.; et al. Dietary patterns, body mass index and inflammation: Pathways to depression and mental health problems in adolescents. Brain Behav. Immun. 2018, 69, 428–439.
- Khayyatzadeh, S.S.; Bagherniya, M.; Fazeli, M.; Khorasanchi, Z.; Bidokhti, M.S.; Ahmadinejad, M.; Khoshmohabbat, S.; Arabpour, M.; Afkhamizadeh, M.; Ferns, G.A.; et al. A Western dietary pattern is associated with elevated level of high sensitive C-reactive protein among adolescent girls. Eur. J. Clin. Investig. 2018, 48, e12897.
- Lazarou, C.; Panagiotakos, D.; Chrysohoou, C.; Andronikou, C.; Matalas, A.-L. C-Reactive protein levels are associated with adiposity and a high inflammatory foods index in mountainous Cypriot children. Clin. Nutr. 2010, 29, 779–783.
- Almeida-De-Souza, J.; Santos, R.; Barros, R.; Abreu, S.; Moreira, C.; Lopes, L.; Mota, J.; Moreira, P. Dietary inflammatory index and inflammatory biomarkers in adolescents from LabMed physical activity study. Eur. J. Clin. Nutr. 2017, 72, 710–719.
- Seremet Kurklu, N.; Karatas Torun, N.; Ozen Kucukcetin, I.; Akyol, A. Is there a relationship between the dietary inflammatory index and metabolic syndrome among adolescents? J. Pediatr. Endocrinol. Metab. JPEM 2020, 33, 495–502.
- Shivappa, N.; Hebert, J.R.; Marcos, A.; Diaz, L.-E.; Gomez, S.; Nova, E.; Michels, N.; Arouca, A.; González-Gil, E.; Frederic, G.; et al. As-sociation between dietary inflammatory index and inflammatory markers in the HELENA study. Mol. Nutr. Food Res. 2017, 61.
- Zimmermann, M.B.; Aeberli, I. Dietary determinants of subclinical inflammation, dyslipidemia and components of the metabolic syndrome in overweight children: A review. Int. J. Obes. 2008, 32, S11–S18.
- Navarro, S.L.; Kantor, E.D.; Song, X.; Milne, G.L.; Lampe, J.W.; Kratz, M.; White, E. Factors Associated with Multiple Biomarkers of Systemic Inflammation. Cancer Epidemiol. Biomark. Prev. 2016, 25, 521–531.
- Cunningham-Rundles, S.; McNeeley, D.F.; Moon, A. Mechanisms of nutrient modulation of the immune response. J. Allergy Clin. Immunol. 2005, 115, 1119–1128.
- Zolin, S.J.; Vodovotz, Y.; Forsythe, R.M.; Rosengart, M.R.; Namas, R.; Brown, J.B.; Peitzman, A.P.; Billiar, T.R.; Sperry, J.L. The Early Evolving Sex Hormone Enviorment is Associated with Significant Outcome and Inflamatory Response Differences Post-injury. J. Trauma Acute Care Surg 2015, 78, 451.
- Da Silva, C.T.B.; de Abreu Costa, M.; Kapczinski, F.; de Aguiar, B.W.; Salum, G.A.; Manfro, G.G. Inflammation and internalizing dis-orders in adolescents. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2017, 77, 133–137.
- Whitacre, C.C. Sex differences in autoimmune disease. Nat. Immunol. 2001, 2, 777–780.
- Ortona, E.; Pierdominici, M.; Maselli, A.; Veroni, C.; Aloisi, F.; Shoenfeld, Y. Sex-based differences in autoimmune diseases. Ann. Ist. Super. Sanità 2016, 52, 205–212.
- Ağirbaşli, M.; Agaoglu, N.B.; Orak, N.; Caglioz, H.; Ocek, T.; Poci, N.; Salaj, A.; Maya, S. Sex hormones and metabolic syndrome in children and adolescents. Metabolism 2009, 58, 1256–1262.
- Damsgaard, C.T.; Papadaki, A.; Jensen, S.M.; Ritz, C.; Dalskov, S.-M.; Hlavaty, P.; Saris, W.; Martínez, J.A.; Handjieva-Darlenska, T.; Andersen, M.R.; et al. Higher Protein Diets Consumed Ad Libitum Improve Cardiovascular Risk Markers in Children of Overweight Parents from Eight European Countries. J. Nutr. 2013, 143, 810–817.
- Karampola, M.; Argiriou, A.; Hitoglou-Makedou, A. Study on dietary constituents, hs-CRP serum levels and investigation of correlation between them in excess weight adolescents. Hippokratia 2019, 23, 3.
- Çağiran Yilmaz, F.; Çağiran, D.; Özçelik, A.Ö. Adolescent Obesity and Its Association with Diet Quality and Cardiovascular Risk Factors. Ecol. Food Nutr. 2019, 58, 207–218.
- Aeberli, I.; Molinari, L.; Spinas, G.; Lehmann, R.; L’Allemand, D.; Zimmermann, M.B. Dietary intakes of fat and antioxidant vitamins are predictors of subclinical inflammation in overweight Swiss children. Am. J. Clin. Nutr. 2006, 84, 748–755.
- Hajihashemi, P.; Azadbakht, L.; Hashemipor, M.; Kelishadi, R.; Esmaillzadeh, A. Whole-grain intake favorably affects markers of systemic inflammation in obese children: A randomized controlled crossover clinical trial. Mol. Nutr. Food Res. 2014, 58, 1301–1308.
- Prihaningtyas, R.; Widjaja, N.; Irawan, R.; Hanindita, M.; Hidajat, B. Dietary Intakes and High Sensivity crp (hsCRP) in Adolescents with Obesity. Carpathian J. Food Sci. Technol. 2019, 11, 83–88.
- Machado, A.M.; De Paula, H.; Cardoso, L.D.; Costa, N.M.B. Effects of brown and golden flaxseed on the lipid profile, glycemia, inflammatory biomarkers, blood pressure and body composition in overweight adolescents. Nutrients 2015, 31, 90–96.
- Miller, S.J.; Batra, A.K.; Shearrer, G.E.; House, B.T.; Cook, L.T.; Pont, S.J.; Goran, M.I.; Davis, J.N. Dietary fibre linked to decreased in-flammation in overweight minority youth. Pediatr. Obes. 2016, 11, 33–39.
- Liese, A.D.; Ma, X.; Ma, X.; Mittleman, M.A.; Catherine, P.; Standiford, D.A.; Lawrence, J.M.; Pihoker, C.; Marcovina, S.M.; Mayer-Davis, E.J.; et al. Dietary quality and markers of inflammation: No association in youth with type 1 diabetes. J. Diabetes Its Complicat. 2018, 32, 179–184.
- Sanjeevi, N.; Lipsky, L.M.; Nansel, T.R. Cardiovascular Biomarkers in Association with Dietary Intake in a Longitudinal Study of Youth with Type 1 Diabetes. Nutrients 2018, 10, 1552.
- Jaacks, L.M.; Crandell, J.; Liese, A.D.; Lamichhane, A.P.; Bell, R.A.; Dabelea, D.; D’Agostino, R.B.; Dolan, L.M.; Marcovina, S.; Reynolds, K.; et al. No association of dietary fiber intake with inflammation or arterial stiffness in youth with type 1 diabetes. J. Diabetes Its Complicat. 2014, 28, 305–310.
- Reilly, S.M.; Saltiel, A.R. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 2017, 13, 633–643.
- Monteiro, R.; Azevedo, I. Chronic Inflammation in Obesity and the Metabolic Syndrome. Mediat. Inflamm. 2010, 2010, 1–10.
- Livingstone, M.B.E.; Robson, P.J. Measurement of dietary intake in children. Proc. Nutr. Soc. 2000, 59, 279–293.
- Foster, E.; Adamson, A.J. Challenges involved in measuring intake in early life: Focus on methods. Proc. Nutr. Soc. 2014, 73, 201–209.
- Magarey, A.; Watson, J.; Golley, R.K.; Burrows, T.; Sutherland, R.; Mcnaughton, S.A.; Denney-Wilson, E.; Campbell, K.; Collins, C. As-sessing dietary intake in children and adolescents: Considerations and recommendations for obesity research. Int. J. Pediatr. Obes. 2011, 6, 2–11.
- Cowan, S.F.; Leeming, E.R.; Sinclair, A.; Dordevic, A.L.; Truby, H.; Gibson, S.J. Effect of whole foods and dietary patterns on markers of subclinical inflammation in weight-stable overweight and obese adults: A systematic review. Nutr. Rev. 2019, 78, 19–38.
- Potischman, N. Biologic and Methodologic Issues for Nutritional Biomarkers. J. Nutr. 2003, 133, 875S–880S.
- Cunha, A.L.P.d.; Costa, A.C.C.d.; Vasconcelos, Z.; Carmo, M.d.G.T.D.O.; Chaves, C.R.M.d.M. Fatty acid profile in erythrocytes associated with serum cytokines in pediatric cystic fibrosis patients. Rev. Nutr. 2018, 31, 455–466.
- Klein-Platat, C.; Drai, J.; Oujaa, M.; Schlienger, J.-L.; Simon, C. Plasma fatty acid composition is associated with the metabolic syndrome and low-grade inflammation in overweight adolescents. Am. J. Clin. Nutr. 2005, 82, 1178–1184.
- Berger, M.E.; Smesny, S.; Kim, S.-W.; Davey, C.G.; Rice, S.; Sarnyai, Z.; Schlögelhofer, M.; Schäfer, M.R.; Berk, M.; McGorry, P.D.; et al. Omega-6 to omega-3 polyunsaturated fatty acid ratio and subsequent mood disorders in young people with at-risk mental states: A 7-year longitudinal study. Transl. Psychiatry 2017, 7, e1220.
- Dragsted, L.O.; Gao, Q.; Scalbert, A.; Vergères, G.; Kolehmainen, M.; Manach, C.; Brennan, L.; Afman, L.A.; Wishart, D.S.; Andres-Lacueva, C.; et al. Validation of biomarkers of food intake—Critical assessment of candidate biomarkers. Genes Nutr. 2018, 13, 1–14.
- Tworoger, S.S.; Hankinson, S.E. Use of biomarkers in epidemiologic studies: Minimizing the influence of measurement error in the study design and analysis. Cancer Causes Control. 2006, 17, 889–899.
