Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Clinical Neurology
The insular cortex is an important information integration center. Numerous imaging studies have documented increased activity of the insular cortex in the presence of neuropathic pain; however, the specific role of this region remains controversial.
- neuropathic pain
- insular cortex
- analgesic response
1. Introduction
Neuropathic pain occurs when the somatosensory nervous system is damaged due to disease or trauma. Its main symptoms include hyperalgesia, allodynia, and spontaneous pain. Hyperalgesia and allodynia refer to increased pain perception in response to stimuli that usually do and do not cause pain, respectively [1]. Neuropathic pain is difficult to cure, and the long-term suffering that it causes can affect an individual’s quality of life and psychological state. For this reason, it is often comorbid with mood disorders, such as depression and anxiety [2]. Nonsteroidal anti-inflammatory drugs (NSAIDs) are effective for nociceptive pain, but have little effect on neuropathic pain [3].
Mu-opioid receptor (MOR) agonists, such as morphine, are the gold standard of analgesia for various types of pain, such as cancer and severe acute pain [4]. However, for neuropathic pain, the effect is not as obvious [5]. Some researchers believe that the delta-opioid receptor (DOR) may be an important target for the treatment of neuropathic pain [6,7]. Repeated administrations of SCN80, a DOR agonist, could improve some symptoms of neuropathic pain in rats with chronic constriction injury (CCI) [8]. The exploration of analgesic drugs indicates that there are many difficulties in the treatment of neuropathic pain. In many cases, antidepressants and anticonvulsants are used to relieve the symptoms of neuropathic pain [9].
Neurosurgical inventions are also options for the treatment of neuropathic pain [10], such as lesion of the dorsal root entry zone (DREZ) [11] and motor cortex neuromodulation [12]. Responses to neuropathic pain treatment differ among individuals, and sex-based differences in analgesia responses have been reported [13]. Exploration of the mechanisms underlying neuropathic pain from a broad range of perspectives may aid in the identification of better ways to relieve this condition.
As the insular cortex is relatively hidden, the embedding of a cannula or an electrode in the insular lobe for laboratory animal research is difficult. Thus, the insula is more mysterious to neuroscientists than other cortical regions. The rapid development of imaging technology has enabled researchers to explore the functions of the insula. Anatomic studies have confirmed that the insula is connected with many brain structures, such as the somatosensory cortex, limbic system, and frontal lobe [14]. Imaging studies suggest that the insular functions are diverse and include those related to interoception and emotion, reward and motivation, cognition, and decision-making [15].
Some researchers have suggested that the insula is an important information integration center [16] or an area of cross-modal integration [17]. The insular cortex has also been found to participate in the processing of empathy and awareness, and it may tell us “how we feel now” and even “who we are” [18]. In addition, the insula participates in the processing of pain, a complex multidimensional experience [19]. Studies have found that the insula is involved in the processing of autonomic responses to noxious stimuli [20] and in the affective–motivational component of pain [21]; however, increasing evidence indicates that the involvement of the insula in pain processing is more complex [22,23,24].
The role of the insula in neuropathic pain has been of interest for decades. The historical progress is shown in Figure 1. In 1956, a study reported a patient with neuropathic pain who had some small soft fused lesions in the insular cortex and parietal operculum [25,26]. Although this result was from an autopsy, it still suggests the relationship between the insular lobe and neuropathic pain. The development of imaging technology in the 1990s promoted progress in the research of the insular cortex. In 1995, Hsieh et al. found increased regional cerebral blood flow (rCBF) in the bilateral anterior insula and other brain regions in patients with painful mononeuropathy using positron emission tomography (PET) [27].
In 1999, Treede et al. proposed that the insular cortex may be involved in the affective–motivational dimensions of pain [19]. However, this may not be enough to explain the special role of the insula in neuropathic pain. The processing of synesthesia is an important feature of the insula. A 2006 study reported the synesthesia of neuropathic pain and odor and the associated insular activation using functional magnetic resonance imaging (fMRI) [28]. Since 2007, people have noticed the relationship between analgesia and the insula [29], as well as the molecules related to the insula and neuropathic pain [30]. In the past decade, neural network research related to the insular lobe has also provided a great deal of evidence revealing the pathogenesis of neuropathic pain [31]. In recent years, researchers have found that empathy may affect neuropathic pain and that the insular lobe is also involved [32].
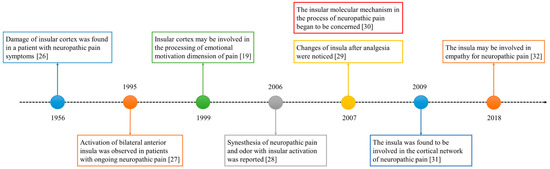
Figure 1. The development timeline of the insular lobe and neuropathic pain.
Although many studies have found that the insular cortex is essential for pain processing, it is still difficult to explain how it participates in pain. In this review, we introduce the structural and functional changes of the insular lobe in the neuropathic pain state and the related molecular mechanisms. There are other brain regions involved in pain, such as the thalamus, primary somatosensory cortex (SI), secondary somatosensory cortex (SII), anterior cingulate cortex (ACC), and frontal cortex [20,33]. Each brain region plays an important and unique role in the occurrence and development of neuropathic pain. However, to clearly describe the insular cortex, we ignore the changes in other brain areas during neuropathic pain.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22052648
This entry is offline, you can click here to edit this entry!
