Polyphenols are secondary plant metabolites mainly known for their antioxidant properties. Their use as feed additives in the nutrition of farm animals is becoming increasingly popular as they are particularly exposed to oxidative stress which is reflected in a lipoperoxidation of the final product. For this reason, it is essential to preserve the quality and the safety of meat and milk products by attenuating oxidative deterioration. Moreover, polyphenols present the advantage of being more acceptable to the consumers than synthetic counterparts, as they are considered to be “non-toxic”.
- polyphenols
- antioxidants
- feed additives
- food-producing animals
- pig
- poultry
- ruminants
- animal product quality
1. Introduction
There is a growing interest in producing healthier animal products with a higher ratio of polyunsaturated (PUFA) to saturated fatty acids by modulation of animal’s diet. This nutritional strategy has been associated with an increase in lipoperoxidation. It is essential to preserve the quality and the safety of meat and dairy products by attenuating oxidative deterioration. Antioxidant molecules can be added to feed or introduced directly into the final product to control and reduce the beginning of the oxidative process. Recently, the interest of food processing industries in the use of natural antioxidants rather than synthetic counterparts was increased, for either low environmental impact or economic reasons [1]. Furthermore, natural antioxidants represent a good candidate in this respect because they are also well accepted by the consumers since they are considered safe. In the last decade, there has been a growing interest in supplementing animal feeds with plant antioxidant to boost the nutritional value of meat for consumers’ health benefits [2][3]. For these reasons the calls to use botanical-based feed additives due to their claimed antioxidant activity and beneficial effects on farm animal performance and animal product quality is increasing.
2. Classification and Structure of Polyphenols
The polyphenols comprise a large group of more than 8000 different compounds with the phenolic hydroxyl groups being the common structural feature. In nature, polyphenols are usually found conjugated to sugars and organic acids, and, according to the number of aromatic rings and their binding affinity for different compounds, can be divided into three classes, flavonoids, non-flavonoids, and tannins [4] (Figure 1). Flavonoids comprise the largest group of polyphenols with more than 4000 compounds identified and share as a common structure two benzene rings connected by three carbon atoms forming an oxygenated heterocycle [5][6]. Based on the type of heterocycle, the following flavonoid subclasses can be distinguished: flavonols, flavones, flavanols, flavanones, anthocyanins, and isoflavones [7]. The group of flavonoids is responsible for the red, blue, and yellow colouration of plants. They are found mainly in onions, leeks, soybeans, berries, and tea [5][8]. The group of non-flavonoid-polyphenols comprises phenolic acids (cinnamic acid, such as ferulic, caffeic, coumaric, and sinapic acid, and the less abundant hydroxyl benzoic acids, such as gallic and vanillic acid), lignans (e.g., secoisolariciresinol, pinoresinol, syringaresinoland), and stilbenes (e.g., resveratrol). Tannins, commonly referred to as tannic acid, are water-soluble polyphenols that are present in many plant foods. They have been reported to be responsible for decreases in feed intake, growth rate, feed efficiency, net metabolizable energy, and protein digestibility in livestock animals [9].

Figure 1. Main classes of polyphenols: flavonoids, non-flavonoids (phenolic acids, lignans, stilbenes) and tannins.
3. Bioavailability of Polyphenols in Food-Producing Animals
Bioavailability can be defined as the proportion of bioactive compounds that are successfully absorbed into the bloodstream for metabolic utilization [10]. Polyphenols are considered as bioactive compounds, that unlike macro- and micronutrients, are not essential for life, but have an effect on specific cells and tissues. Their availability is determined by the type of compound, its chemical and physical properties, and the type and presence of functional groups [8][11].
In the monogastric gastrointestinal tract, phenolic compounds metabolism starts from the upper intestinal epithelia and proceeds to the lower intestine, the liver, and then to the peripheral tissues, which include adipose tissue and the kidneys. The route of phenolic absorption can either be via the stomach and small intestine or possibly absorbed by the colon after chemical modification by the colonic microbiota. The microbiota present in the colon allows polyphenols to be absorbed into the bloodstream and subsequently to be excreted either in the urine or via the bile [12]. It has been shown that out of 100% total polyphenolic intake, only 5–10% is absorbed in the small intestine, while the 90–95% will be in the large intestine lumen together with other conjugates excreted by the bile [12][13]. Subsequently, they are exposed to the intestinal enzymes and gut microbiota, which breaks down the polyphenolic structures into smaller molecules to facilitate absorption. In the animal, polyphenols are transformed into glycoside, ester, and polymeric forms that need to be hydrolysed to facilitate absorption by gut microbiota and intestinal enzymes. Phenolic compounds with fewer complex structures are subjected to extensive biotransformation (oxidation, reduction, or hydrolysis) which converts them into water-soluble metabolites in the enterocytes before reaching the liver. The complex phenolic compounds not absorbed in the small intestine reach the colon, where the gut microbiota hydrolyses glycosides resulting in the formation of aglycones. This process decreases the complex structure of the phenolic hydroxyl group into low-molecular-weight phenolic metabolites that can be absorbed. Once absorbed, the molecules reach the liver via the hepatocytes, where they are again subjected to a biotransformation process (conjugation) which improves easy absorption (hydrophobicity) of the molecules and aids in rapid elimination. Finally, the metabolites enter the systemic circulation where they are distributed to the targeted organs or eliminated in the urine [14][15]. A schematic illustration of the described absorption and metabolism of plant polyphenols is summarized in Figure 2.
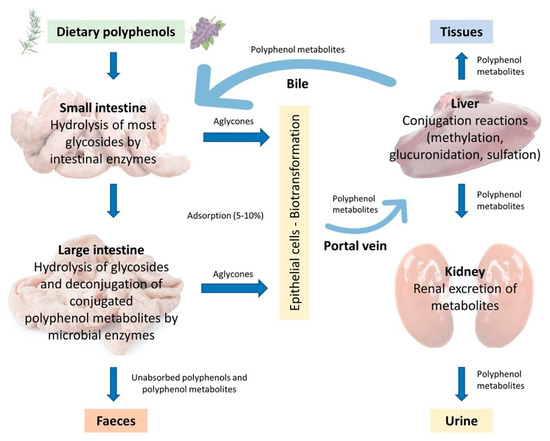
Figure 2. Adsorption and metabolism of plant polyphenols in monogastric farm animals (Adapted from Marín et al., 2014 [16]).
The content of polyphenols in body tissues is not directly related to their dietary levels [11][17][18]. For example, it has been shown that in pigs whose diets were supplemented with 50 mg/kg quercetin for 4 weeks, quercetin levels were higher in the kidneys (6.31 nmol/g) and colon (13.92 nmol/g) than in the liver (2.83 nmol/g) or plasma (0.67 μmol/L) [19]. On the contrary, when pig diets were supplemented with higher doses of quercetin (500 mg/kg) for 3 days, quercetin levels remained unchanged in bodily tissues (3.78 nmol/g in the liver, 1.84 nmol/g in the kidneys), but increased in plasma (1.1 μmol/L) [20]. In these works, elevated polyphenol concentrations were noted only in organs that participate in the metabolism of the polyphenolic compound. The levels of unabsorbed dietary phenolic compounds exert significant effects on the intestinal environment by suppressing or stimulating the growth of some of the components of intestinal microbiota. The dietary polyphenols present prebiotic properties and exert antimicrobial activities, they enhance the growth of specific beneficial bacteria strains (Bacillus spp., Lactobacillus spp.) in the intestinal tract while competitively excluding certain pathogenic bacteria and stabilizing gut microbiota; indirectly enhance the host’s immune system and overall health [21][22]. On intestinal morphology, they can exert a positive influence and improve nutrient absorption in monogastric animals [23].
Contrary to what is known about the monogastric, for which it has been clearly established that the small intestine is the main site of absorption for monomeric flavonoids, it is still unknown if flavonoids are absorbed across the rumen epithelium. However, several studies have shown an early peak in plasma flavonoid concentrations after intraruminal administration of quercetin [24] or consumption of a meal containing abundant isoflavones [25], thus suggesting that some flavonoids are adsorbed in the rumen, given that mean ruminal residence time of ingested feed is much greater than 1 h [26]. Consequently, flavonoids that come out of the rumen are probably absorbed in the small intestine due to the increase in the total plasma concentration of flavonol after intraduodenal infusion of quercetin [27]. In dairy cows, it has moreover been shown that the administration of feedstuffs rich in isoflavones (genistein, daidzein) such as soybean meal or red glover silage causes an increase in the concentrations of those isoflavones in blood and milk [28][29]. The supplementation of phenolic compounds in ruminants seems to be effective in preserving polyunsaturated fatty acids (PUFA), rumenic acid, and vaccenic acid from a complete biohydrogenation, with consequent enriching in health-promoting fatty acids in meat and milk, at the expense of saturated fatty acids. The effects of phenolic compounds on cellulolytic bacteria and protozoa probably are also associated with the reduction of both fibre degradability and, indirectly, with methane emission. However, a direct interaction between some specific phenolic compounds (such as hydrolysable tannins) and methanogen microbes was suggested [30].
4. Antioxidants and Their Importance in Food-Producing Animals
Τhe efficacy of animal production is based principally on balanced nutritional systems that meet the individual needs of farm animals. Animal growth can be influenced or even regulated by the supplementation of antioxidants in animal feed; thus, it is likely to be affected by the overall redox status of a productive animal [31].
It has been suggested that the high metabolic rate of growing tissues generates abnormally large amounts of free radicals. To avoid the formation of free radicals that usually lead to oxidative stress and other redox-related pathologies, they must be safely scavenged or removed via the administration of antioxidant compounds [32][33]. Oxidative stress is defined as "the imbalance between oxidants and antioxidants in favour of the oxidants, potentially leading to damage" [34]. A particularly destructive aspect of oxidative stress is the excessive production of reactive oxygen species (ROS), such as free radicals and peroxides, that cannot be effectively neutralized by the body [35][36]. In homeostasis, ROS are deactivated by endogenous antioxidants represented by enzymatic antioxidants (superoxide dismutase, catalase, and glutathione peroxidase) and non-enzymatic antioxidants (uric acid, glutathione, coenzyme Q; [36]). In recent years, evidence has emerged that oxidative stress plays a crucial role in the development and perpetuation of inflammation [37], and the potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals is fully described by Gessner and colleagues [7]. In pigs, grape seed and grape marc meal extracts or hop extract (10 g/kg diet), have shown to be able to lower the expression of several proinflammatory genes in various portions of the intestine (duodenum, ileum, colon; [38]). The abundance of some potentially pathogenic bacteria was lowered, thus suggesting that polyphenols exerted an antimicrobial effect on pathogenic bacteria in the intestine. The positive effect was reflected in an improvement of the gain-to-feed ratio in pigs, due to an inhibition of pro-inflammatory processes in the intestine and to antimicrobial effects. An improvement in growth performance may be related to the improvement of immune defence and suppression of excessive apoptosis among intestinal epithelial cells, as observed in piglets [39] and poultry [40]. Another action of antioxidants is represented by an improvement of food status and animal feed intake as some plant extracts enhance the flavour and palatability of feed, which improves feed intake and productive performance [41][42]. The increased stimulation of appetite results in higher feed consumption and weight gain. With regards to plant extracts rich in polyphenols, they have been shown to increase digestive secretion such as saliva and digestive enzymes, thus improving the adsorption and utilization of nutrients which increases, in turn, the growth of the animals [43]. Some phenolic compounds like genistein, daidzein, soybean isoflavone, and ferulic acid have been proposed to exert effects on animal metabolism, thus acting as growth promoters by modulating animal metabolism in favour of increasing muscle tissue [44][45] or increasing the bioavailability of nutrients [39]. Studies conducted in ruminants have mainly been focused on inducing changes in the microbial populations of the rumen and its subsequent effects on ruminal fermentation. However, dietary supplementation with ferulic acid to steers has been shown to exert effects similar to β-adrenergic agonists that are used in the final phase of intensive fattening of beef cattle [46].
Moreover, in intensive breeding systems, animals are frequently exposed to oxidation of fatty acids (lipoperoxidation), not only because of their frequent exposure to oxidative stress but also because of their diet [47]. On this basis, numerous studies over the past few years have examined the hypothesis that the supplementation of feed that is enriched with antioxidants to farm animals will provide an improvement of derived product quality [48][49][50][51][52]. As regards meat quality, parameters, such as colour, water-holding capacity, and the oxidative stability of lipids and proteins are usually referred to [52]. Lipid oxidation is a major quality deteriorative process in muscle foods resulting in a variety of breakdown products that produce off-odours and flavours, drip losses, discoloration, loss of nutrient value, decrease in shelf life, and accumulation of toxic compounds [53]. The interaction of alkyl and peroxyl radicals, which are formed during the oxidation of lipids, leads to the formation of non-radical products such as aldehydes [54], directly related to the deterioration of meat colour and flavour, protein stability, and functionality [53].
Another phenomenon closely associated with deteriorative processes that can affect meat and meat products is the oxidation of proteins, which play a fundamental role in meat quality concerning the sensory, nutritional and physico-chemical properties [53]. Protein oxidation occurs through a chain reaction of free radicals like oxidation of lipids in animal muscle. This process induces multiple physico-chemical changes and nutritional value in meat proteins including a decrease in the bioavailability of amino acid protein, change in amino acid composition, decrease in protein solubility due to protein polymerization [55]. Oxidative damage in tissues should be prevented at an early stage by controlling animal diets [12][55][56] and consequently, stabilizing the antioxidant potential of products of animal origin. There is a growing interest in the nutritional aspect of polyphenolic compounds in light of their antioxidant capacity and they may become an important alternative as a partial substitute for vitamin E in animal diets [12]. Polyphenols have a saving action on vitamin antioxidants such as ascorbic acid and tocopherols [57]. Polyphenols, minimizing the adverse consequences of lipid peroxidation by decreasing malondialdehyde concentrations and increasing tocopherol levels in tissues, improve the quality of animal products [4].
This entry is adapted from the peer-reviewed paper 10.3390/ani11020401
References
- Jiang, J.; Xiong, Y. Natural antioxidants as food and feed additives to promote health benefits and quality of meat products: A review. Meat Sci. 2016, 120, 107–117.
- Bou, R.; Codony, R.; Tres, A.; Decker, E.A.; Guardiola, F. Dietary strategies to improve nutritional value, oxidative stability, and sensory properties of poultry products. Crit. Rev. Food Sci. Nutr. 2009, 49, 800–822.
- Nieto, G.; Estrada, M.; Jordán, M.J.; Garrido, M.D.; Bañón, S. Effects in ewe diet of rosemary by-product on lipid oxidation and the eating quality of cooked lamb under retail display conditions. Food Chem. 2011, 124, 1423–1429.
- Lipiński, K.; Mazur, M.; Antoszkiewicz, Z.; Purwin, C. Polyphenols in monogastric nutrition—A review. Ann. Anim. Sci. 2017, 17, 41–58.
- Jasiński, M.; Mazurkiewicz, E.; Rodziewicz, P.; Figlerowicz, M. Flawonoidy–budowa, właściwości i funkcja ze szczególnym uwzględnieniem roślin motylkowatych. Biotechnologia 2009, 2, 81–94.
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Asp. Med. 2010, 31, 435–445.
- Gessner, D.K.; Ringseis, R.; Eder, K. Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals. J. Anim. Physiol. Anim. Nutr. 2016, 101, 605–628.
- Landete, J.M. Dietary intake of natural antioxidants: Vitamins and polyphenols. Crit. Rev. Food. Sci. Nutr. 2013, 53, 706–721.
- Piluzza, G.; Sulas, L.; Bullitta, S. Tannins in forage plants and their role in animal husbandry and environmental sustainability: A review. Grass Forage Sci. 2014, 69, 32–48.
- Palafox-Carlos, H.; Ayala-Zavala, J.F.; González-Aguilar, G.A. The role of dietary fiber in the bioaccessibility and bioavailability of fruit and vegetable antioxidants. J. Food Sci. 2011, 76, R6–R15.
- D’Archivio, M.; Filesi, C.; Di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, dietary sources and bioavailability. Ann. Ist. Super Sanita 2007, 43, 348–361.
- Brenes, A.; Viveros, A.; Chamorro, S.; Arija, I. Use of polyphenol-rich grape by-products in monogastric nutrition. A review. Anim. Feed. Sci. Tech. 2016, 211, 1–17.
- Chiva-Blanch, G.; Visioli, F. Polyphenols and health: Moving beyond antioxidants. J. Berry Res. 2012, 2, 63–71.
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422.
- Lavefve, L.; Howard, L.R.; Carbon, F. Berry polyphenols metabolism and impact on human gut microbiota and health. Food Funct. 2020, 11, 45–65.
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of dietary polyphenols and gut microbiota metabolism: Antimicrobial properties. BioMed Res. Int. 2015, 905215.
- Fotina, A.A.; Fisinin, V.I.; Surai, P.F. Recent developments in usage of natural antioxidants to improve chicken meat production and quality. Bulg. J. Agric. Sci. 2013, 19, 889–896.
- Surai, P.F. Polyphenol compounds in the chicken/animal diet: From the past to the future. J. Anim. Physiol. Anim. Nutr. 2014, 98, 19–31.
- Bieger, J.; Cermak, R.; Blank, R.; de Boer, V.C.; Hollman, P.C.; Kamphues, J.; Wolffram, S. Tissue distribution of quercetin in pigs after long-term dietary supplementation. J. Nutr. 2008, 138, 1417–1420.
- Boer de, V.C.; Dihal, A.A.; van der Woude, H.; Arts, I.C.; Wolffram, S.; Alink, G.M.; Hollman, P.C. Tissue distribution of quercetin in rats and pigs. J. Nutr. 2005, 135, 1718–1725.
- Hashemi, S.R.; Davoodi, H. Herbal plants and their derivatives as growth and health promoters in animal nutrition. Vet. Res. Commun. 2011, 35, 169–180.
- Paszkiewicz, M.; Budzynska, A.; Rozalska, B.; Sadowska, B. The immunomodulatory role of plant polyphenols. Post. Hig. Med. Dosw. 2012, 66, 637–646.
- Kamboh, A.A.; Arain, M.A.; Mughal, M.J.; Zaman, A.; Arain, Z.M.; Soomro, A.H. Flavonoids: Health promoting phytochemicals for animal production—A review. J. Anim. Health Prod. 2015, 3, 6–13.
- Berger, L.M.; Wein, S.; Blank, R.; Metges, C.C.; Wolffram, S. Bioavailability of the flavonol quercetin in cows after intraruminal application of quercetin aglycone and rutin. J. Dairy Sci. 2012, 95, 5047–5055.
- Lundh, T.J.H.; Pettersson, H.I.; Martinsson, K.A. Comparative levels of free and conjugated plant estrogens in blood plasma of sheep and cattle fed estrogenic silage. J. Agric. Food Chem. 1990, 38, 1530–1534.
- Warner, D.; Dijkstra, J.; Hendriks, W.H.; Pellikaan, W.F. Stable isotope-labelled feed nutrients to assess nutrient-specific feed passage kinetics in ruminants. J. Sci. Food Agric. 2014, 94, 819–824.
- Gohlke, A.; Ingelmann, C.J.; Nürnberg, G.; Starke, A.; Wolffram, S.; Metges, C.C. Bioavailability of quercetin from its aglycone and its glucorhamnoside rutin in lactating dairy cows after intraduodenal administration. J. Dairy Sci. 2013, 96, 2303–2313.
- Höjer, A.; Adler, S.; Purup, S.; Hansen-Møller, J.; Martinsson, K.; Steinshamn, H.; Gustavsson, A.M. Effects of feeding dairy cows different legume-grass silages on milk phytoestrogen concentration. J. Dairy Sci. 2012, 95, 4526–4540.
- Cools, S.; Van den Broeck, W.; Vanhaecke, L.; Heyerick, A.; Bossaert, P.; Hostens, M.; Opsomer, G. Feeding soybean meal increases the blood level of isoflavones and reduces the steroidogenic capacity in bovine corpora lutea, without affecting peripheral progesterone concentrations. Anim. Reprod. Sci. 2014, 144, 79–89.
- Vasta, V.; Daghio, M.; Cappucci, A.; Buccioni, A.; Serra, A.; Viti, C.; Mele, M. Invited review: Plant polyphenols and rumen microbiota responsible for fatty acid biohydrogenation, fiber digestion, and methane emission: Experimental evidence and methodological approaches. J. Dairy Sci. 2019, 102, 3781–3804.
- Rollo, C.D. Growth negatively impacts the life span of mammals. Evol. Dev. 2002, 4, 55–61.
- Oriani, G.; Corino, C.; Pastorelli, G.; Pantaleo, L.; Ritieni, A.; Salvatori, G. Oxidative status of plasma and muscle in rabbits supplemented with dietary vitamin E. J. Nutr. Biochem. 2001, 12, 138–143.
- Pastorelli, G.; Rossi, R.; Corino, C. Influence of Lippia citriodora verbascoside on growth performance, antioxidant status, and serum immunoglobulins content in piglets. Czech. J. Anim. Sci. 2012, 57, 312–322.
- Sies, H. What is oxidative stress? In Oxidative Stress and Vascular Disease; Keaney, J.F., Ed.; Developments in Cardiovascular Medicine; Springer: Boston, MA, USA, 2000; p. 224.
- Durand, D.; Damon, M.; Gobert, M. Oxidative stress in farm animals: General aspects. Cah. Nutr. Diet. 2013, 48, 218–224.
- Zhong, R.; Zhou, D. Oxidative stress and role of natural plant derived antioxidants in animal reproduction. J. Integr. Agric. 2013, 12, 1826–1838.
- Lauridsen, C. From oxidative stress to inflammation: Redox balance and immune system. Poult. Sci. 2019, 98, 4240–4246.
- Fiesel, A.; Gessner, D.K.; Most, E.; Eder, K. Effects of dietary polyphenol-rich plant products from grape or hop on pro-inflammatory gene expression in the intestine, nutrient digestibility and faecal microbiota of weaned pigs. BMC Vet. Res. 2014, 10, 196.
- Chen, J.; Li, Y.; Yu, B.; Chen, D.; Mao, X.; Zheng, P.; Luo, J.; He, J. Dietary chlorogenic acid improves growth performance of weaned pigs through maintaining antioxidant capacity and intestinal digestion and absorption function. J. Anim. Sci. 2018, 96, 1108–1118.
- Liu, W.C.; Yan, G.; Zhi-Hui, Z.; Rajesh, J.; Balamuralikrishnan, B. Algae-derived polysaccharides promote growth performance by improving antioxidant capacity and intestinal barrier function in broiler chickens. Front. Vet. Sci. 2020, 7, 990.
- Al-Kassie, G.A. Influence of two plant extracts derived from thyme and cinnamon on broiler performance. Pak. Vet. J. 2009, 29, 169–173.
- Jiang, Z.Y.; Jiang, S.Q.; Lin, Y.C.; Xi, P.B.; Yu, D.Q.; Wu, T.X. Effects of soybean isoflavone on growth performance, meat quality, and antioxidation in male broilers. Poult. Sci. 2007, 86, 1356–1362.
- Hashemi, S.R.; Davoodi, H. Phytogenics as new class of feed additive in poultry industry. J. Anim. Vet. Adv. 2010, 9, 2295–2304.
- Macías-Cruz, U.; Perard, S.; Vicente, R.; Álvarez, F.D.; Torrentera-Olivera, N.G.; González-Ríos, H.; Soto-Navarro, S.A.; Rojo, R.; Meza-Herrera, C.A.; Avendaño-Reyes, L. Effects of free ferulic acid on productive performance, blood metabolites, and carcass characteristics of feedlot finishing ewe lambs. J. Anim. Sci. 2014, 92, 5762–5768.
- Valenzuela-Grijalva, N.V.; Pinelli-Saavedra, A.; Muhlia-Almazan, A.; Domínguez-Díaz, D.; González-Ríos, H. Dietary inclusion effects of phytochemicals as growth promoters in animal production. J. Anim. Sci. Technol. 2017, 59, 8.
- González-Ríos, H.; Dávila-Ramírez, J.L.; Peña-Ramos, E.A.; Valenzuela-Melendres, M.; Zamorano-García, L.; Islava-Lagarda, T.Y.; Valenzuela-Grijalva, N.V. Dietary supplementation of ferulic acid to steers under commercial feedlot feeding conditions improves meat quality and shelf life. Anim. Feed. Sci. Tech. 2016, 222, 111–121.
- Gladine, C.; Rock, E.; Morand, C.; Cauchart, D.; Durand, D. Bioavailability and antioxidant capacity of plant extracts rich in polyphenols, given as a single acute dose, in sheep made highly susceptible to lipoperoxidation. Br. J. Nutr. 2007, 98, 691–701.
- Pastorelli, G.; Rossi, R.; Ratti, S.; Corino, C. Plant extracts in heavy pig feeding: Effects on quality of meat and Cremona salami. Anim. Prod. Sci. 2016, 56, 1199–1207.
- Salvatori, G.; Pantaleo, L.; Di Cesare, C.; Maiorano, G.; Filetti, F.; Oriani, G. Fatty acid composition and cholesterol content of muscles as related to genotype and vitamin E treatment in crossbred lambs. Meat Sci. 2004, 67, 45–55.
- Corino, C.; Oriani, G.; Pantaleo, L.; Pastorelli, G.; Salvatori, G. Influence of dietary vitamin E supplementation on “heavy” pig carcass characteristics, meat quality, and vitamin E status. J. Anim. Sci. 1999, 77, 1755–1761.
- Corino, C.; Pastorelli, G.; Pantaleo, L.; Oriani, G.; Salvatori, G. Improvement of color and lipid stability of rabbit meat by dietary supplementation with vitamin E. Meat Sci. 1999, 52, 285–289.
- Wood, J.D.; Richardson, R.I.; Nute, G.R.; Fisher, A.V.; Campo, M.M.; Kasapidou, E.; Sheard, P.R.; Enser, M. Effects of fatty acids on meat quality: A review. Meat Sci. 2004, 66, 21–32.
- Falowo, A.B.; Fayemi, P.O.; Muchenje, V. Natural antioxidants against lipid–protein oxidative deterioration in meat and meat products: A review. Food Res. Int. 2014, 64, 171–181.
- Pastorelli, G.; Magni, S.; Rossi, R.; Pagliarini, E.; Baldini, P.; Dirinck, P.; Van Opstaele, F.; Corino, C. Influence of dietary fat, on fatty acid composition and sensory properties of dry-cured Parma ham. Meat Sci. 2003, 65, 571.
- Gobert, M.; Gruffat, D.; Habeanu, M.; Parafita, E.; Bauchart, D.; Durand, D. Plant extracts combined with vitamin E in PUFA-rich diets of cull cows protect processed beef against lipid oxidation. Meat. Sci. 2010, 85, 676–683.
- Jung, S.; Choe, J.H.; Kim, B.; Yun, H.; Kruk, Z.A.; Jo, C. The effect of dietary mixture of gallic acid and linoleic acid on antioxidative potential and quality of breast meat from broilers. Meat Sci. 2010, 86, 520–526.
- Dai, F.; Chen, W.F.; Zhou, B. Antioxidant synergism of green tea polyphenols with α-tocopherol and l-ascorbic acid in SDS micelles. Biochimie 2008, 90, 1499–1505.
