The measurement assays have been developed to fulfill these requirements. It was considered that the optimal laboratory assays were dependent on the cases and the tests were classified as two types as follows: qualitative (presence or absence) and quantitative (ng/mL).
Qualitative Assays for DOACs
DOACs can interfere with clot-based coagulation assay because these drugs inhibit thrombin or factor Xa. Many researchers reported that the effect on clotting assays were largely dependent on principles like PT and APTT, which are the global assays for the screening of coagulation cascade. Endogenous coagulation activity changes and coagulation inhibitors can also affect the measurement results of PT and APTT, and these global assays lack the specificity for the measurements of DOACs anticoagulation. However, these tests have been performed as routine tests in many clinical laboratories, which indicates that the results are obtained in short turn-around-times. Timely evaluation is important because the half-lives of DOACs are short and it is critical in several scenarios, such as life-threatening bleeding or acute stroke management [
72].
PT is prolonged by dabigatran and rivaroxaban in a concentration-dependent manner, and PT has higher sensitivity to rivaroxaban than dabigatran [
73,
74]. Specifically, the investigation for rivaroxaban has been performed by many researchers, it was reported that two-fold clotting times was 66–258 ng/mL among PT reagents in the spiked samples and it indicated that the variability among the reagents was wide. Although the relation was linear in the spiked samples, the correlation between PT and the concentration measured by mass spectrometry in clinical samples was weak (R
2 = 0.3178) and the discrepancy samples were also recognized in the correlation [
74,
75]. The sensitivity to apixaban was lower than that of rivaroxaban. Although the sensitivity is largely dependent on PT reagents, PT may be normal with apixaban concentrations up to 200 ng/mL [
76,
77,
78]. The sensitivity to edoxaban is equivalent or less than rivaroxaban, but PT is more sensitive than that of APTT, the prolongation of the PT is also concentration- and reagent-dependent [
79,
80]. Although INR and ISI are widely used from the view of standardization, this system had been established for warfarin monitoring based on VKA sensitivity. This system is not suitable for DOACs measurements and data interpretation [
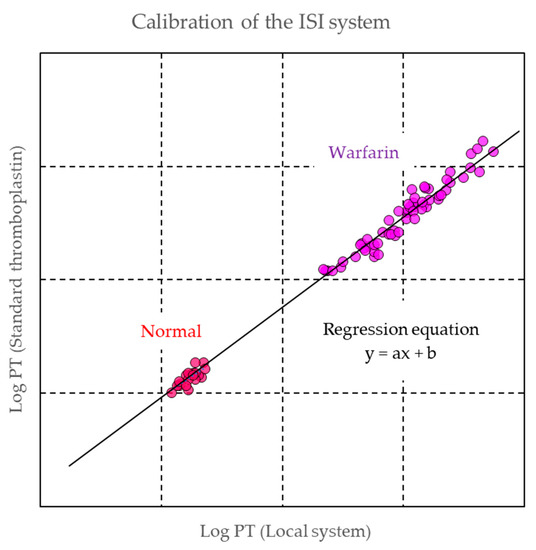
81].
It was also reported that APTT had sensitivity for dabigatran, rivaroxaban, apixaban, and edoxaban in concentration-dependent prolongation of clotting times. Particularly, the sensitivity to dabigatran was higher than those of other drugs [
74,
82,
83] and the prolongation of APTT increased in a non-linear manner with increasing concentrations of dabigatran in clinical samples [
84]. As described above, APTT is a commonly used parameter for the measurement of the degrees of anticoagulation in patients receiving anticoagulant medications. It has been used for the monitoring of treatment with UFH and has been discussed more recently in the clinical evaluation of direct thrombin inhibitors, such as hirudin [
85,
86,
87]. Although commercial APTT reagents differ in their sensitivity, it was estimated that APTT had higher sensitivity to thrombin inhibitor than PT and the principle might be useful for the detection of dabigatran.
Overall, APTT is more sensitive with dabigatran and PT with rivaroxaban, whilst apixaban has very little influence on both PT and APTT. The specificity was low and the sensitivity to drugs is not so high. It indicates that the ability of these tests to quantify DOAC concentrations is poor and reagent-dependent. In addition, PT and APTT are not recommended at all for monitoring DOACs.
Thrombin time (TT) is highly sensitive to dabigatran because the reagent includes thrombin in lower concentration than fibrinogen reagent, converts fibrinogen into fibrin, and the clotting time is detected. Dabigatran concentrations in trough levels lower than 30 ng/mL lead to significant prolongation of the TT; a normal TT indicates that little or no dabigatran is present, but a prolonged TT does not necessarily equate to a high dabigatran level [
88,
89]. Thus, TT is only useful in the very low dabigatran concentration range to exclude its presence in plasma samples. The reagent is not affected by direct factor Xa inhibitor.
In addition to these classical coagulation tests, other assays had been developed to measure DOACs in clotting principles, and one of them is diluted prothrombin time (dPT). In routine PT tests, the quantity of thromboplastin is much higher than in physiological conditions. dPT experiments were performed to increase the sensitivity of the test because the conditions of this test might be closer to the actual physiological conditions. In these dPT experiments, PT reagents were diluted with 25 mM CaCl
2 solution to obtain normal clotting times of 30–40 s. The dilution ratios were different in each reagent, but these were 1/8–1/750 (one part of reagent/x parts of CaCl
2 solution) [
73,
90]. Although the results obtained with several dPT reagents still varied, depending on the reagents used, it was confirmed that the clotting times of dPT were prolonged in rivaroxaban samples and dPT assay demonstrated weak correlation with rivaroxaban concentrations. The sensitivity to rivaroxaban in dPT was increased as the dilution ratios became higher [
75,
90]. Ieko et al. developed a novel dPT assay with a new formula to normalize the effect of thrombin generation. They used 500-fold diluted PT reagent for the measurements, the results were expressed as the ratio of thrombin generation in their formula, and they investigated the correlation between the formula and the drug concentration in the samples of rivaroxaban, apixaban, and edoxaban. The results showed that the formula was positively correlated with the concentrations of all drugs, and it was also recognized that the formula showed a significant decrease in cases with thrombosis and increase in those with hemorrhage [
91]. Recently, we also developed a modified dPT (mdPT) assay and reported that the reagent had good correlation against the drug concentration of rivaroxaban, apixaban, and edoxaban, and the sensitivity of mdPT was higher than that of commercial PT reagents in these clinical samples. In addition, the discrepancy analysis in the correlation between mdPT and the drug concentrations were performed, and the results showed that the discrepancy might be related to the lower coagulation activity of factors II, V, VII, and X. Thus, it was considered that mdPT reagent reflected not only DOAC inhibition but also coagulation factor activity and this assay may predict bleeding risk in patients treated with DOAC at a lower level of coagulation activity [
92,
93]. Letertre et al. developed an interesting assay based on dPT. They hypothesized that the levels of anticoagulant drugs could be measured with use of the dPT and diluted factor II and X deficient plasma-PT (dFiix-PT), which is only affected by reduced levels of active factor II and X. In the dFiix-PT assays, FII- and FX-depleted plasma was mixed with the test plasma to correct for any factor deficiency other than FII or FX, and the test plasma was mixed with diluted thromboplastin, CaCl
2 solution, and factor II and X depleted plasma; and the clotting time was detected. This assay showed good correlation against drug concentration in dabigatran, rivaroxaban, and apixaban. They also showed that the dFiix-PT with drug-specific calibration curves estimated the drug concentration in plasma samples from patients taking these agents and suggested that the assay may be suitable for screening for the presence of anticoagulant drug in plasma and the test might be suitable for emergency testing of anticoagulant concentrations or effects [
94]. Overall, while these global assays are not specific for DOAC, they are useful for screening for anticoagulant drugs and finding the effects of drug concentration with coagulation factor activity. Although further studies are required from the perspective of clinical insight, it is considered that these assays are helpful to understand the status of patients taking anticoagulants.
Quantitative Assay for DOAC Measurement
Liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS) is considered the gold standard method for the quantitative assay for DOAC measurement because it has high degree of specificity, sensitivity, selectivity, and reproducibility, and this method is often used in clinical development to evaluate DOAC pharmacokinetics [
95,
96,
97]. The lower limits of detection (LoD) and quantitation (LoQ) in LC-MS/MS for DOAC detection has been reported as 0.025–3 ng/mL and the reportable range of quantitation has also been reported as 5–500 ng/mL [
98,
99,
100,
101,
102,
103]. Although LC-MS/MS is considered the most accurate method for DOAC measurements, it is not widely used because of the limitation of availability and complexity associated with this testing. In addition, LC-MS/MS has a high intra- and inter-assay coefficient of variation and it needs to be calibrated with the purified drug, which contributes to series-to-series variations. Thus, the drug-calibrated clot-based or chromogenic methods have been developed and adapted to automated coagulation analyzers.
One of the clot-based kits for dabigatran measurement is Hemoclot thrombin inhibitor (Hyphen BioMed, Neuville-sur-Oise, France). For detection, the diluted tested plasma is mixed with normal pooled plasma. Then, clotting is initiated by addition of human thrombin and the clotting time is measured in coagulation analyzers. The clotting time is directly related to the concentration of dabigatran, and the concentration is calculated from the calibration curve obtained from the dabigatran calibrator. It was shown that the clotting method and LC-MS/MS analysis correlated well, and the agreement was good [
98]. Stangier et al. also reported that this kit enabled the accurate assessment of dabigatran concentrations within the therapeutic concentration range without inter-laboratory variability [
104]. The correlation between the clotting method and qualitative tests as APTT and thrombin time has also been investigated. The APTT demonstrated a modest correlation with the dabigatran concentration measured by the clotting method but the correlation became less reliable at higher dabigatran levels and it was recognized that the sensitivity was different among APTT reagents. The TT was sensitive to the presence of dabigatran and showed >300 s in more than 60 ng/mL concentration. It was confirmed that the TT was too sensitive to quantify dabigatran levels [
105]. The other study also demonstrated that APTT and TT could not identify the drug presence in too low or too high concentrations, respectively [
106]. For dabigatran concentration detection, it is considered that the clotting method is a more suitable assay. In addition, there was an accurate correlation reported for ecarin clotting time or ecarin chromogenic assay and dabigatran concentrations [
74]. Although the upper limit of the measurements is dependent on the ecarin unit and the composition, it was reported that these assays could detect dabigatran up to 940 ng/mL.
Chromogenic anti-Xa assay has been used in clinical laboratories for several decades as a method to assess heparin concentration. This assay is appropriate for measuring direct factor Xa inhibitor drug concentration. The chromogenic anti-Xa assay principle is a kinetics method based on the inhibition of factor Xa at a constant and limiting concentration by the tested factor Xa inhibitor. The samples are mixed with an excess amount of factor Xa, and factor Xa inhibitor binds to factor Xa. The remaining factor Xa is then measured by its amidolytic activity on a factor Xa specific chromogenic substrate, and the residual factor Xa cleaves the substrate. The amount of cleaved substrate is inversely proportional to the concentration of factor Xa inhibitor in the tested samples. The concentration is calculated from the calibration curve obtained from rivaroxaban, apixaban, and edoxaban calibrator, respectively. It has been proved that this kit has the correlation against LC-MS/MS, and it was also shown that the inter variability was small [
100,
107,
108]. Douxfils et al. reported that the correlation between PT and LC-MS/MS was not linear and the sensitivity to drugs differed among reagents [
100], it could be difficult to perform the standardization to estimate drug concentration in PT reagents. Biophen DiXaI is useful to quantify drug concentration because the kit has high sensitivity to drugs and adapted to automated coagulation analyzers. DOACs have short half-lives, their concentration is time-dependent, and the concentration of peak time is quite different from that of trough time. The information when patients take the drugs is useful for the interpretation of the drug concentration data.