By virtue of its anti-thrombotic and anti-inflammatory properties, resveratrol would be expected to lower COVID-19-associated mortality, which is well known to be increased by thrombosis and inflammation.
- resveratrol
- COVID-19
- inflammation
1. Introduction
On 11 March 2020, the Corona Virus Disease 2019 (COVID-19) was declared a global pandemic by the World Health Organization (WHO) (https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 06 February 2021)). The causative agent was determined to be the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This virus, which belongs to the Coronaviridae family, subfamily Orthocoronavirinae, has a positive single-stranded RNA genome and a characteristic crown-like spikes proteins on the outer surface [1]. Symptoms of COVID-19 are variable but the most common are fever, cough, breathing difficulties, as well as loss of smell and taste. Overall, most people affected by COVID-19 have mild to moderate symptoms and recover without special treatments, but older people and those with comorbidities, such as cardiovascular disease, diabetes, and chronic respiratory disease, are more likely to develop adverse outcomes [2].
The lungs are the most affected organs, probably because SARS-CoV-2 preferentially uses the angiotensin-converting enzyme 2 (ACE2) receptor, which is highly expressed in lung type II alveolar cells [3]. Indeed, a common condition of COVID-19 patients, often associated with high mortality rate, is the acute respiratory distress syndrome (ARDS), an acute and diffuse inflammatory lung injury marked by increased pulmonary vascular permeability and loss of aerated lung tissue [4]. In addition, the excessive intra-alveolar fibrin deposition, driven by an imbalance between activation of coagulation and inhibition of fibrinolysis, is a condition linked to ARDS pathophysiology [5]. Therefore, as a consequence of SARS-CoV-2 attack of cells in the pulmonary and vascular systems, COVID-19-patients with poor prognosis show the concurrence of ARDS with pulmonary vascular thrombosis and increased concentration of D-dimer, a small protein fragment resulting from clot degradation [6,7].
Overall, COVID-19 is a systemic and complex disease with a wide spectrum of clinical manifestations. Many COVID-19 patients show a severe proinflammatory state associated with both distinctive coagulopathy and procoagulant endothelial phenotype. Indeed, several lines of evidence suggest that the interconnection between coagulopathy and endotheliopathy can explain the microvascular and macrovascular thrombotic events that participate in the multiorgan dysfunctions in severe COVID-19 cases. [8,9]. Indeed, endotheliopathy, responsible for both the microvascular thrombotic events and the microcirculatory impairment observed in many COVID-19 patients, is a direct consequence of the virus endothelial infection and an indirect damage caused by the disease-associated inflammatory status [8,9]. Increased levels of Von Willebrand factor (vWF) and factor VIII (FVIII) as well as platelet hyperactivation characterize the endothelial status during COVID-19 [9].
COVID-19 is also a systemic inflammatory vascular disease, evident by the increased concentrations of proinflammatory cytokines in severe cases. These cytokines include tumor necrosis factor-α (TNF-α), interleukin 1 (IL1) and interleukin 6 (IL6), all of which are important regulators of coagulation [10]. In this context, treatment of COVID-19 patients with low molecular weight heparin (LMWH) has proven beneficial in reducing the risk of mortality resulting from thrombotic events [11,12]. However, since the use of these therapeutic anticoagulants is associated with increased bleeding, specific doses should be tuned to the patient’s overall condition [11,12,13,14]. Therefore, it remains crucial to develop additional anti-inflammatory, anticoagulant, or antithrombotic strategies to prevent and treat COVID-19.
There is an increasing need for alternative therapeutic approaches that can prevent or reduce the risk factors associated with both pre-existing conditions and comorbidities responsible for worsening COVID-19 patients’ outcomes [15]. Employing natural compounds characterized by anti-inflammatory and antithrombotic properties may provide an attractive avenue towards this goal. Among the several phytonutrients of interest, resveratrol (3,5,4′-trihydroxy-trans-stilbene), a polyphenol found in various plants, especially grapes, berries, peanuts, cacao and soybeans, may represent a good candidate [16]. This phytochemical possesses a wide range of biological activities, including anti-inflammatory, anticancer, antiviral, antioxidant, cardioprotective and neuroprotective [17]. Despite skepticism concerning its bioavailability and potential adverse effects, a crescent number of in vivo models showed the beneficial effects of resveratrol in several disease conditions [18,19]. Specifically, accumulating evidence supports the anti-inflammatory, anticoagulant and antithrombotic role of resveratrol [20,21,22]. Because of its polyvalent action in preventing or attenuating coagulation disorder, inflammation and vascular damage, major hallmarks of COVID-19, we believe resveratrol may be a good addition in the management of this disease.
2. Hemostatic Modulatory Properties of Resveratrol
When a blood vessel is injured, three mechanisms occur in a rapid sequence to control bleeding at the injury site: (1) a brief and intense vessel wall contraction, (2) formation of the platelet plug by platelet adhesion and aggregation, and (3) coagulation, which reinforces the platelet plug with fibrin. Platelet activation and aggregation in the initial plug is also called primary hemostasis. In contrast, secondary hemostasis refers to the coagulation cascade of enzymatic reactions that ultimately lead to the conversion of fibrinogen in fibrin monomers by the action of the clotting enzyme thrombin [23]. Furthermore, secondary hemostasis is traditionally divided into two pathways: the contact activation pathway (or intrinsic pathway) and the tissue factor pathway (or extrinsic pathway). In this regard, resveratrol has been shown to exert a protective role in vascular hemostasis by acting in both primary and secondary hemostasis. Indeed, besides having anti-platelet aggregation properties, resveratrol has also been shown to modulate factors involved in the coagulation cascade [20,21,22,24] (Figure 1).
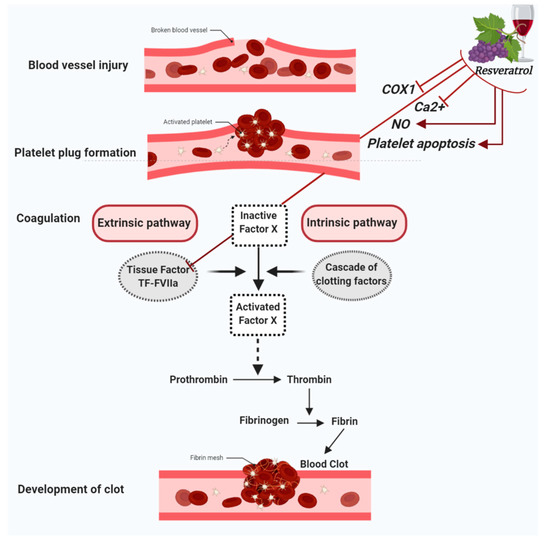
Figure 1. Figure summarizes the antiplatelet aggregation properties of resveratrol. Resveratrol inhibits cyclooxygenase-1 (COX-1), lowers nitric oxide (NO) concentration, decreases cytoplasmatic Ca2+ and blocks Ca2+ entry into platelets, which turn results in the suppression of platelet aggregation. Further antiplatelet aggregation properties of resveratrol are due to the activation of platelet apoptosis and the inhibition of Tissue factor (TF):FactorVIIa (FVIIa) complex (TF:FVIIa) formation.
This entry is adapted from the peer-reviewed paper 10.3390/molecules26040856
