Porphyria refers to a group of fascinating diseases from a metabolic and nutritional standpoint as it provides an example of how metabolic manipulation can be used for therapeutic purposes. It is characterized by defects in heme synthesis, particularly in the erythrocytes and liver. Specific enzymes involved in heme biosynthesis directly depend on adequate levels of vitamins and minerals in the tissues. Moreover, micronutrients that are required for producing succinyl CoA and other intermediates in the Krebs (TCA) cycle are indirectly necessary for heme metabolism. This review summarizes articles that describe the nutritional status, supplements intake and dietary practices of patients affected by porphyria, paying special attention to the therapeutic use of nutrients that may help or hinder this group of diseases.
- porphyria, vitamins, glucose metabolism
Diet
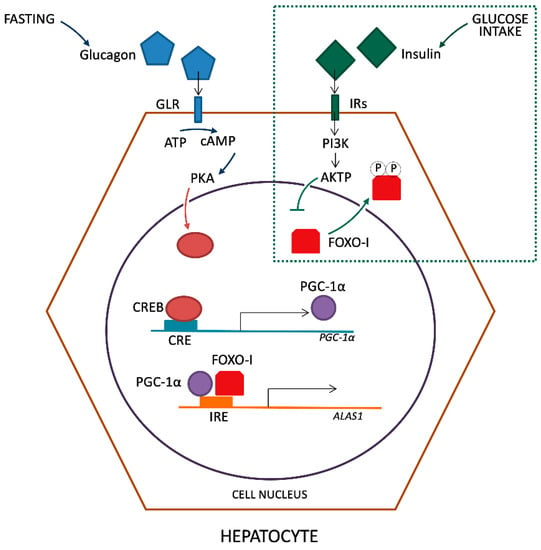
Glucose
Iron
Vitamins
Vitamin B6
| Vitamins | Fat-Soluble | Water-Soluble | Deficiency | Main Foods | Nutraceuticals Characteristics |
|---|---|---|---|---|---|
| Vitamin B6 | x | Rare | Meats, fishery products, offal, cereals, legumes, oilseeds. |
|
|
| Beta-carotene (provit-A) | x | Ultra-rare (more likely to exceed) | In most fruit, cereals, oils, green leafy vegetables. |
|
|
| Vitamin E | x | Uncommon | Nuts, plant seeds, plant oils. |
|
|
| Vitamin C | x | Uncommon | Fresh fruits and vegetables (decreases with cooking and during the seasons). |
|
|
| Vitamin D | x | Common | Oily fish, liver oil, egg yolks, shiitake mushrooms, liver or organ meats |
|
β-. Carotene
Vitamin E
Vitamin C
Vitamin D
- Egger, N.G.; Lee, C.; Anderson, K.E. Disorders of Heme Biosynthesis. In Inborn Metabolic Diseases; Fernandes, J., Saudubray, J.M., Van den Berghe, G., Walter, J.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Welland, F.H.; Hellman, E.S.; Gaddis, E.M.; Collins, G.; Hunter, G.W., Jr.; Tschudy, D.P. factors affecting the excretion of porphyrin precursors by patients with acute intermittent porphyria. I. The effect of diet. Metabolism 1964, 13, 232–250. [Google Scholar] [CrossRef]
- Storjord, E.; Dahl, J.A.; Landsem, A.; Ludviksen, J.K.; Karlsen, M.B.; Karlsen, B.O.; Brekke, O.L. Lifestyle factors including diet and biochemical biomarkers in acute intermittent porphyria: Results from a case-control study in northern Norway. Mol. Genet. Metab. 2019, 128, 254–270. [Google Scholar] [CrossRef]
- Slimani, N.; Ferrari, P.; Ocke, M.; Welch, A.; Boeing, H.; Liere, M.; Pala, V.; Amiano, P.; Lagiou, A.; Mattisson, I.; et al. Standardization of the 24-h diet recall calibration method used in the european prospective investigation into cancer and nutrition (EPIC): General concepts and preliminary results. Eur. J. Clin. Nutr. 2000, 54, 900–917. [Google Scholar] [CrossRef]
- Romaguera, D.; Puigros, M.A.; Palacin, C.; Pons, A.; Tur, J.A. Nutritional assessment of patients affected by porphyria variegata. Ann. Nutr. Metab. 2006, 50, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Diz, L.; Murcia, M.A.; Gris, J.L.; Pons, A.; Monteagudo, C.; Martinez-Tome, M.; Jimenez-Monreal, A.M. Assessing nutritional status of acute intermittent porphyria patients. Eur. J. Clin. Investig. 2012, 42, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Miranda, C.; De La Calle, M.; Larumbe, S.; Gomez-Izquierdo, T.; Porres, A.; Gomez-Gerique, J.; de Enriquez, S.R. Lipoprotein abnormalities in patients with asymptomatic acute porphyria. Clin. Chim. Acta 2000, 294, 37–43. [Google Scholar] [CrossRef]
- Muller, M.J.; Bosy-Westphal, A.; Kutzner, D.; Heller, M. Metabolically active components of fat-free mass and resting energy expenditure in humans: Recent lessons from imaging technologies. Obes Rev. 2002, 3, 113–122. [Google Scholar] [CrossRef]
- Marsden, J.T.; Rees, D.C. Urinary excretion of porphyrins, porphobilinogen and delta-aminolaevulinic acid following an attack of acute intermittent porphyria. J. Clin. Pathol. 2014, 67, 60–65. [Google Scholar] [CrossRef]
- Robert, T.L.; Varella, L.; Meguid, M.M. Nutrition management of acute intermittent porphyria. Nutrition 1994, 10, 551–555. [Google Scholar]
- Pischik, E.; Kauppinen, R. An update of clinical management of acute intermittent porphyria. Appl. Clin. Genet. 2015, 8, 201–214. [Google Scholar] [CrossRef]
- Anderson, K.E.; Bloomer, J.R.; Bonkovsky, H.L.; Kushner, J.P.; Pierach, C.A.; Pimstone, N.R.; Desnick, R.J. Recommendations for the diagnosis and treatment of the acute porphyrias. Ann. Intern. Med. 2005, 142, 439–450. [Google Scholar] [CrossRef]
- Giger, U.; Meyer, U.A. Induction of delta-aminolevulinate synthase and cytochrome P-450 hemoproteins in hepatocyte culture. Effect of glucose and hormones. J. Biol. Chem. 1981, 256, 11182–11190. [Google Scholar]
- Tschudy, D.P.; Welland, F.H.; Collins, A.; Hunter, G., Jr. The effect of carbohydrate feeding on the induction of delta-aminolevulinic acid synthetase. Metabolism 1964, 13, 396–406. [Google Scholar] [CrossRef]
- Handschin, C.; Lin, J.; Rhee, J.; Peyer, A.K.; Chin, S.; Wu, P.H.; Meyer, U.A.; Spiegelman, B.M. Nutritional regulation of hepatic heme biosynthesis and porphyria through PGC-1alpha. Cell 2005, 122, 505–515. [Google Scholar] [CrossRef]
- Herzig, S.; Long, F.; Jhala, U.S.; Hedrick, S.; Quinn, R.; Bauer, A.; Rudolph, D.; Schutz, G.; Yoon, C.; Puigserver, P.; et al. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature 2001, 413, 179–183. [Google Scholar] [CrossRef]
- Varone, C.L.; Giono, L.E.; Ochoa, A.; Zakin, M.M.; Canepa, E.T. Transcriptional regulation of 5-aminolevulinate synthase by phenobarbital and cAMP-dependent protein kinase. Arch. Biochem. Biophys. 1999, 372, 261–270. [Google Scholar] [CrossRef]
- Scassa, M.E.; Guberman, A.S.; Varone, C.L.; Canepa, E.T. Phosphatidylinositol 3-kinase and Ras/mitogen-activated protein kinase signaling pathways are required for the regulation of 5-aminolevulinate synthase gene expression by insulin. Exp. Cell Res. 2001, 271, 201–213. [Google Scholar] [CrossRef]
- Brunet, A.; Bonni, A.; Zigmond, M.J.; Lin, M.Z.; Juo, P.; Hu, L.S.; Anderson, M.J.; Arden, K.C.; Blenis, J.; Greenberg, M.E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 1999, 96, 857–868. [Google Scholar] [CrossRef]
- Nakae, J.; Kitamura, T.; Silver, D.L.; Accili, D. The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. J. Clin. Investig. 2001, 108, 1359–1367. [Google Scholar] [CrossRef]
- Li, D. PGC-1alpha: Looking behind the sweet treat for porphyria. Cell 2005, 122, 487–489. [Google Scholar] [CrossRef]
- Collantes, M.; Serrano-Mendioroz, I.; Benito, M.; Molinet-Dronda, F.; Delgado, M.; Vinaixa, M.; Sampedro, A.; de Enriquez, S.R.; Prieto, E.; Pozo, M.A.; et al. Glucose metabolism during fasting is altered in experimental porphobilinogen deaminase deficiency. Hum. Mol. Genet. 2016, 25, 1318–1327. [Google Scholar] [CrossRef]
- Matkovic, L.B.; D’Andrea, F.; Fornes, D.; de San Martin Viale, L.C.; Mazzetti, M.B. How porphyrinogenic drugs modeling acute porphyria impair the hormonal status that regulates glucose metabolism. Their relevance in the onset of this disease. Toxicology 2011, 290, 22–30. [Google Scholar] [CrossRef]
- Lithner, F. Beneficial effect of diabetes on acute intermittent porphyria. Diabetes Care 2002, 25, 797–798. [Google Scholar] [CrossRef]
- Storjord, E.; Dahl, J.A.; Landsem, A.; Fure, H.; Ludviksen, J.K.; Goldbeck-Wood, S.; Karlsen, B.O.; Berg, K.S.; Mollnes, T.E.; Nielsen, W.; et al. Systemic inflammation in acute intermittent porphyria: A case-control study. Clin. Exp. Immunol. 2017, 187, 466–479. [Google Scholar] [CrossRef]
- Delaby, C.; To-Figueras, J.; Deybach, J.C.; Casamitjana, R.; Puy, H.; Herrero, C. Role of two nutritional hepatic markers (insulin-like growth factor 1 and transthyretin) in the clinical assessment and follow-up of acute intermittent porphyria patients. J. Intern. Med. 2009, 266, 277–285. [Google Scholar] [CrossRef]
- Bernstein, L.H.; Ingenbleek, Y. Transthyretin: Its response to malnutrition and stress injury. Clinical usefulness and economic implications. Clin. Chem. Lab. Med. 2002, 40, 1344–1348. [Google Scholar] [CrossRef]
- Johnson, A.M. Low levels of plasma proteins: Malnutrition or inflammation? Clin. Chem. Lab. Med. 1999, 37, 91–96. [Google Scholar] [CrossRef]
- Clark, M.A.; Hentzen, B.T.; Plank, L.D.; Hill, G.I. Sequential changes in insulin-like growth factor 1, plasma proteins, and total body protein in severe sepsis and multiple injury. JPEN J. Parenter. Enter. Nutr. 1996, 20, 363–370. [Google Scholar] [CrossRef]
- Biolo, G.; Toigo, G.; Ciocchi, B.; Situlin, R.; Iscra, F.; Gullo, A.; Guarnieri, G. Metabolic response to injury and sepsis: Changes in protein metabolism. Nutrition 1997, 13, 52S–57S. [Google Scholar] [CrossRef]
- Dowman, J.K.; Gunson, B.K.; Bramhall, S.; Badminton, M.N.; Newsome, P.N. Liver transplantation from donors with acute intermittent porphyria. Ann. Intern. Med. 2011, 154, 571–572. [Google Scholar] [CrossRef]
- Soonawalla, Z.F.; Orug, T.; Badminton, M.N.; Elder, G.H.; Rhodes, J.M.; Bramhall, S.R.; Elias, E. Liver transplantation as a cure for acute intermittent porphyria. Lancet 2004, 363, 705–706. [Google Scholar] [CrossRef]
- Stojeba, N.; Meyer, C.; Jeanpierre, C.; Perrot, F.; Hirth, C.; Pottecher, T.; Deybach, J.C. Recovery from a variegate porphyria by a liver transplantation. Liver Transpl. 2004, 10, 935–938. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products NaA. Scientific Opinion on Dietary Reference Values for iron1. EFSA J. 2015, 13, 4254–4369. [Google Scholar] [CrossRef]
- Barman-Aksoezen, J.; Girelli, D.; Aurizi, C.; Schneider-Yin, X.; Campostrini, N.; Barbieri, L.; Minder, E.I.; Biolcati, G. Disturbed iron metabolism in erythropoietic protoporphyria and association of GDF15 and gender with disease severity. J. Inherit. Metab. Dis. 2017, 40, 433–441. [Google Scholar] [CrossRef]
- Sampietro, M.; Fiorelli, G.; Fargion, S. Iron overload in porphyria cutanea tarda. Haematologica 1999, 84, 248–253. [Google Scholar]
- Dereure, O.; Jumez, N.; Bessis, D.; Gallix, B.; Guillot, B. Measurement of liver iron content by magnetic resonance imaging in 20 patients with overt porphyria cutanea tarda before phlebotomy therapy: A prospective study. Acta Derm. Venereol. 2008, 88, 341–345. [Google Scholar]
- Di Pierro, E.; Brancaleoni, V.; Granata, F. Advances in understanding the pathogenesis of congenital erythropoietic porphyria. Br. J. Haematol. 2016, 173, 365–379. [Google Scholar] [CrossRef]
- Phillips, J.D.; Bergonia, H.A.; Reilly, C.A.; Franklin, M.R.; Kushner, J.P. A porphomethene inhibitor of uroporphyrinogen decarboxylase causes porphyria cutanea tarda. Proc. Natl. Acad. Sci. USA 2007, 104, 5079–5084. [Google Scholar] [CrossRef]
- Dabrowska, E.; Jablonska-Kaszewska, I.; Falkiewicz, B. Effect of high fiber vegetable-fruit diet on the activity of liver damage and serum iron level in porphyria cutanea tarda (PCT). Med. Sci. Monit. 2001, 7 (Suppl. S1), 282–286. [Google Scholar]
- Singal, A.K. Porphyria cutanea tarda: Recent update. Mol. Genet. Metab. 2019, 128, 271–281. [Google Scholar] [CrossRef]
- Mirmiran, A.; Poli, A.; Ged, C.; Schmitt, C.; Lefebvre, T.; Manceau, H.; Daher, R.; Moulouel, B.; Peoc’h, K.; Simonin, S.; et al. Phlebotomy as an efficient long-term treatment of congenital erythropoietic porphyria. Haematologica 2020. [Google Scholar] [CrossRef]
- Egan, D.N.; Yang, Z.; Phillips, J.; Abkowitz, J.L. Inducing iron deficiency improves erythropoiesis and photosensitivity in congenital erythropoietic porphyria. Blood 2015, 126, 257–261. [Google Scholar] [CrossRef]
- Landefeld, C.; Kentouche, K.; Gruhn, B.; Stauch, T.; Rossler, S.; Schuppan, D.; Whatley, S.D.; Beck, J.F.; Stolzel, U. X-linked protoporphyria: Iron supplementation improves protoporphyrin overload, liver damage and anaemia. Br. J. Haematol. 2016, 173, 482–484. [Google Scholar] [CrossRef]
- Balwani, M. Erythropoietic Protoporphyria and X-Linked Protoporphyria: Pathophysiology, genetics, clinical manifestations, and management. Mol. Genet. Metab. 2019, 128, 298–303. [Google Scholar] [CrossRef]
- Minder, E.I.; Barman-Aksozen, J. Iron and erythropoietic porphyrias. Blood 2015, 126, 130–132. [Google Scholar] [CrossRef]
- Barman-Aksozen, J.; Halloy, F.; Iyer, P.S.; Schumperli, D.; Minder, A.E.; Hall, J.; Minder, E.I.; Schneider-Yin, X. Delta-aminolevulinic acid synthase 2 expression in combination with iron as modifiers of disease severity in erythropoietic protoporphyria. Mol. Genet. Metab. 2019, 128, 304–308. [Google Scholar] [CrossRef]
- Gordeuk, V.R.; Brittenham, G.M.; Hawkins, C.W.; Mukhtar, H.; Bickers, D.R. Iron therapy for hepatic dysfunction in erythropoietic protoporphyria. Ann. Intern. Med. 1986, 105, 27–31. [Google Scholar] [CrossRef]
- McClements, B.M.; Bingham, A.; Callender, M.E.; Trimble, E.R. Erythropoietic protoporphyria and iron therapy. Br. J. Dermatol. 1990, 122, 423–424. [Google Scholar] [CrossRef]
- Milligan, A.; Graham-Brown, R.A.; Sarkany, I.; Baker, H. Erythropoietic protoporphyria exacerbated by oral iron therapy. Br. J. Dermatol. 1988, 119, 63–66. [Google Scholar] [CrossRef]
- Holme, S.A.; Thomas, C.L.; Whatley, S.D.; Bentley, D.P.; Anstey, A.V.; Badminton, M.N. Symptomatic response of erythropoietic protoporphyria to iron supplementation. J. Am. Acad. Dermatol. 2007, 56, 1070–1072. [Google Scholar] [CrossRef]
- Hemminger, A.; Wills, B.K. Vitamin B6 Toxicity; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Linkswiler, H. Biochemical and physiological changes in vitamin B6 deficiency. Am. J. Clin. Nutr. 1967, 20, 547–561. [Google Scholar] [CrossRef]
- Hamfelt, A.; Wetterberg, L. Pyridoxal phosphate in acute intermittent porphyria. Ann. N. Y. Acad. Sci. 1969, 166, 361–364. [Google Scholar] [CrossRef]
- Elder, T.D.; Mengel, C.E. Effect of pyridoxine deficiency on porphyrin precursor excretion in acute intermittent porphyria. Am. J. Med. 1966, 41, 369–374. [Google Scholar] [CrossRef]
- Mydlik, M.; Derzsiova, K. Vitamin B6 and oxalic acid in clinical nephrology. J. Ren. Nutr. 2010, 20, S95–S102. [Google Scholar] [CrossRef] [PubMed]
- Scheer, J.B.; Mackey, A.D.; Gregory, J.F., III. Activities of hepatic cytosolic and mitochondrial forms of serine hydroxymethyltransferase and hepatic glycine concentration are affected by vitamin B-6 intake in rats. J. Nutr. 2005, 135, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.S.; Richardson, K.E. Isolation and characterization of an L-alanine: Glyoxylate aminotransferase from human liver. J. Biol. Chem. 1967, 242, 3614–3619. [Google Scholar] [PubMed]
- To-Figueras, J.; Lopez, R.M.; Deulofeu, R.; Herrero, C. Preliminary report: Hyperhomocysteinemia in patients with acute intermittent porphyria. Metabolism 2010, 59, 1809–1810. [Google Scholar] [CrossRef] [PubMed]
- Chabner, B.A.; Stein, J.A.; Tschudy, D.P. Effect on dietary pyridoxine deficiency on experimental porphyria. Metabolism 1970, 19, 189–191. [Google Scholar] [CrossRef]
- Balic, A.; Mokos, M. Do We Utilize Our Knowledge of the Skin Protective Effects of Carotenoids Enough? Antioxidants 2019, 8, 259. [Google Scholar] [CrossRef]
- Mathews-Roth, M.M.; Pathak, M.A.; Fitzpatrick, T.B.; Harber, L.H.; Kass, E.H. Beta carotene therapy for erythropoietic protoporphyria and other photosensitivity diseases. Arch. Dermatol. 1977, 113, 1229–1232. [Google Scholar] [CrossRef]
- Mathews-Roth, M.M. Beta-carotene therapy for erythropoietic protoporphyria and other photosensitivity diseases. Biochimie 1986, 68, 875–884. [Google Scholar] [CrossRef]
- Alemzadeh, R.; Feehan, T. Variable effects of beta-carotene therapy in a child with erythropoietic protoporphyria. Eur. J. Pediatr. 2004, 163, 547–549. [Google Scholar] [CrossRef]
- Minder, E.I.; Schneider-Yin, X.; Steurer, J.; Bachmann, L.M. A systematic review of treatment options for dermal photosensitivity in erythropoietic protoporphyria. Cell. Mol. Biol. (Noisy -le-grand) 2009, 55, 84–97. [Google Scholar]
- Balwani, M.; Desnick, R. X-Linked Protoporphyria; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Balwani, M.; Bloomer, J.; Desnick, R. Erythropoietic Protoporphyria, Autosomal Recessive; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Tintle, S.; Alikhan, A.; Horner, M.E.; Hand, J.L.; Davis, D.M. Cutaneous porphyrias part II: Treatment strategies. Int. J. Dermatol. 2014, 53, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Bafteh, P.R.; Siegesmund, M.; Hanneken, S.; Neumann, N.J. Protective effects of beta-carotene and melanin against protoporphyrine IX-induced phototoxicity in the photo hen’s egg test. Photodermatol. Photoimmunol. Photomed. 2012, 28, 12–16. [Google Scholar] [CrossRef]
- Rocchi, E.; Stella, A.M.; Cassanelli, M.; Borghi, A.; Nardella, N.; Seium, Y.; Casalgrandi, G. Liposoluble vitamins and naturally occurring carotenoids in porphyria cutanea tarda. Eur. J. Clin. Investig. 1995, 25, 510–514. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the re-evaluation of Mixed Carotenes (E 160a (i)) and beta-Carotene (E 160a (ii)) as a food additive. EFSA J. 2012, 10, 2593–2660. [Google Scholar]
- Beetch, M.; Harandi-Zadeh, S.; Shen, K.; Lubecka, K.; Kitts, D.D.; O’Hagan, H.M.; Stefanska, B. Dietary antioxidants remodel DNA methylation patterns in chronic disease. Br. J. Pharmacol. 2020, 177, 1382–1408. [Google Scholar] [CrossRef]
- Jeanes, Y.M.; Hall, W.L.; Ellard, S.; Lee, E.; Lodge, J.K. The absorption of vitamin E is influenced by the amount of fat in a meal and the food matrix. Br. J. Nutr. 2004, 92, 575–579. [Google Scholar] [CrossRef]
- Monteiro, H.P.; Abdalla, D.S.; Faljoni-Alario, A.; Bechara, E.J. Generation of active oxygen species during coupled autoxidation of oxyhemoglobin and delta-aminolevulinic acid. Biochim. Biophys. Acta 1986, 881, 100–106. [Google Scholar] [CrossRef]
- Thunell, S.; Andersson, C.; Carlmark, B.; Floderus, Y.; Gronqvist, S.O.; Harper, P.; Henrichson, A.; Lindh, U. Markers for vulnerability in acute porphyria. A hypothesis paper. Eur. J. Clin. Chem. Clin. Biochem. 1995, 33, 179–194. [Google Scholar] [CrossRef]
- Rocchi, E.; Casalgrandi, G.; Masini, A.; Giovannini, F.; Ceccarelli, D.; Ferrali, M.; Marchini, S.; Ventura, E. Circulating pro- and antioxidant factors in iron and porphyrin metabolism disorders. Ital. J. Gastroenterol. Hepatol. 1999, 31, 861–867. [Google Scholar]
- Ferrer, M.D.; Tauler, P.; Sureda, A.; Palacin, C.; Tur, J.A.; Pons, A. Variegate porphyria induces plasma and neutrophil oxidative stress: Effects of dietary supplementation with vitamins E and C. Br. J. Nutr. 2010, 103, 69–76. [Google Scholar] [CrossRef]
- Rocchi, E.; Ventura, P.; Ronzoni, A.; Rosa, M.C.; Gozzi, C.; Marri, L.; Casalgrandi, G.; Cappellini, M.D. Pro-oxidant and antioxidant factors in acute intermittent porphyria: Family studies. J. Inherit. Metab. Dis. 2004, 27, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Pinelli, A.; Trivulzio, S.; Tomasoni, L.; Bertolini, B.; Pinelli, G. High-dose vitamin E lowers urine porphyrin levels in patients affected by porphyria cutanea tarda. Pharmacol. Res. 2002, 45, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Szekely, E.; Vereckei, A.; Almasi, A.; Rapavi, E.; Tasnadi, G.; Varnai, K.; Pallai, Z.; Lugasi, A.; Blazovics, A. Effects of vitamin E administration on the hemorheological status and redox homeostasis of patients with porphyria cutanea tarda treated with phlebotomy. Clin. Hemorheol. Microcirc. 2007, 36, 13–23. [Google Scholar]
- Thunell, S.; Andersson, D.; Harper, P.; Henrichson, A.; Floderus, Y.; Lindh, U. Effects of administration of antioxidants in acute intermittent porphyria. Eur. J. Clin. Chem. Clin. Biochem. 1997, 35, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Adjarov, D.; Ribarova, F.; Koytcheva, N.; Shishkov, S.; Ivanova, A.; Antonov, K. Impaired antioxidant status in porphyria cutanea tarda. Acta Medica Bulg. 2001, 28, 135–143. [Google Scholar]
- Johnson, J.A.; Fusaro, R.M. Possible use of vitamins C and-or E in erythropoietic protoporphyria. JAMA 1973, 224, 901–902. [Google Scholar] [CrossRef]
- Fryer, M.J. Evidence for the photoprotective effects of vitamin E. Photochem. Photobiol. 1993, 58, 304–312. [Google Scholar] [CrossRef]
- Komatsu, H.; Ishii, K.; Imamura, K.; Maruyama, K.; Yonei, Y.; Masuda, H.; Tsuchihashi, T.; Sajima, Y. A case of erythropoietic protoporphyria with liver cirrhosis suggesting a therapeutic value of supplementation with alpha-tocopherol. Hepatol. Res. 2000, 18, 298–309. [Google Scholar] [CrossRef]
- Phillips, K.M.; Tarrago-Trani, M.T.; McGinty, R.C.; Rasor, A.S.; Haytowitz, D.B.; Pehrsson, P.R. Seasonal variability of the vitamin C content of fresh fruits and vegetables in a local retail market. J. Sci. Food Agric. 2018, 98, 4191–4204. [Google Scholar] [CrossRef]
- Traber, M.G.; Stevens, J.F. Vitamins C and E: Beneficial effects from a mechanistic perspective. Free Radic. Biol. Med. 2011, 51, 1000–1013. [Google Scholar] [CrossRef]
- Boffa, M.J.; Ead, R.D.; Reed, P.; Weinkove, C. A double-blind, placebo-controlled, crossover trial of oral vitamin C in erythropoietic protoporphyria. Photodermatol. Photoimmunol. Photomed. 1996, 12, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Granata, F.; Duca, L.; Graziadei, G.; Brancaleoni, V.; Missineo, P.; De Luca, G.; Fustinoni, S.; Di Pierro, E. Inflammatory involvement into phototoxic reaction in erythropoietic protoporphyria (EPP) patients. Immunol. Res. 2019, 67, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jiang, H.; Li, W.; Qiang, M.; Dong, T.; Li, H. Role of Vitamin C in Skin Diseases. Front. Physiol. 2018, 9, 819. [Google Scholar] [CrossRef] [PubMed]
- Percy, V.A.; Naidoo, D.; Joubert, S.M.; Pegoraro, R.J. Ascorbate status of patients with porphyria cutanea tarda symptomatica and its effect on porphyrin metabolism. S. Afr. J. Med. Sci. 1975, 40, 185–196. [Google Scholar]
- Sinclair, P.R.; Gorman, N.; Shedlofsky, S.I.; Honsinger, C.P.; Sinclair, J.F.; Karagas, M.R.; Anderson, K.E. Ascorbic acid deficiency in porphyria cutanea tarda. J. Lab. Clin. Med. 1997, 130, 197–201. [Google Scholar] [CrossRef]
- Fedeles, F.; Murphy, M.; Rothe, M.J.; Grant-Kels, J.M. Nutrition and bullous skin diseases. Clin. Dermatol. 2010, 28, 627–643. [Google Scholar] [CrossRef]
- Duarte, T.L.; Lunec, J. Review: When is an antioxidant not an antioxidant? A review of novel actions and reactions of vitamin C. Free Radic. Res. 2005, 39, 671–686. [Google Scholar] [CrossRef]
- Kechichian, E.; Ezzedine, K. Vitamin D and the Skin: An Update for Dermatologists. Am. J. Clin. Dermatol. 2018, 19, 223–235. [Google Scholar] [CrossRef]
- Chang, S.W.; Lee, H.C. Vitamin D and health—The missing vitamin in humans. Pediatr. Neonatol. 2019, 60, 237–244. [Google Scholar] [CrossRef]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 Dietary Reference Intakes for Calcium and Vitamin D: What dietetics practitioners need to know. J. Am. Diet. Assoc. 2011, 111, 524–527. [Google Scholar] [CrossRef]
- Major, J.M.; Graubard, B.I.; Dodd, K.W.; Iwan, A.; Alexander, B.H.; Linet, M.S.; Freedman, D.M. Variability and reproducibility of circulating vitamin D in a nationwide U.S. population. J. Clin. Endocrinol. Metab. 2013, 98, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. The vitamin D deficiency pandemic and consequences for nonskeletal health: Mechanisms of action. Mol. Asp. Med. 2008, 29, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Touvier, M.; Deschasaux, M.; Montourcy, M.; Sutton, A.; Charnaux, N.; Kesse-Guyot, E.; Assmann, K.E.; Fezeu, L.; Latino-Martel, P.; Druesne-Pecollo, N.; et al. Determinants of vitamin D status in Caucasian adults: Influence of sun exposure, dietary intake, sociodemographic, lifestyle, anthropometric, and genetic factors. J. Investig. Dermatol. 2015, 135, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Holme, S.A.; Anstey, A.V.; Badminton, M.N.; Elder, G.H. Serum 25-hydroxyvitamin D in erythropoietic protoporphyria. Br. J. Dermatol. 2008, 159, 211–213. [Google Scholar] [CrossRef] [PubMed]
- Spelt, J.M.; de Rooij, F.W.; Wilson, J.H.; Zandbergen, A.A. Vitamin D deficiency in patients with erythropoietic protoporphyria. J. Inherit. Metab. Dis. 2010, 33 (Suppl. S3), S1–S4. [Google Scholar] [CrossRef]
- Rhodes, L.E.; Webb, A.R.; Berry, J.L.; Felton, S.J.; Marjanovic, E.J.; Wilkinson, J.D.; Vail, A.; Kift, R. Sunlight exposure behaviour and vitamin D status in photosensitive patients: Longitudinal comparative study with healthy individuals at U.K. latitude. Br. J. Dermatol. 2014, 171, 1478–1486. [Google Scholar] [CrossRef]
- Allo, G.; del Carmen Garrido-Astray, M.; Mendez, M.; De Salamanca, R.E.; Martinez, G.; Hawkins, F. Bone mineral density and vitamin D levels in erythropoietic protoporphyria. Endocrine 2013, 44, 803–807. [Google Scholar] [CrossRef]
- Biewenga, M.; Matawlie, R.H.S.; Friesema, E.C.H.; Koole-Lesuis, H.; Langeveld, M.; Wilson, J.H.P.; Langendonk, J.G. Osteoporosis in patients with erythropoietic protoporphyria. Br. J. Dermatol. 2017, 177, 1693–1698. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
This entry is adapted from the peer-reviewed paper 10.3390/ijms21103462
