Tissue engineering has promoted structures that can simulate the extracellular matrix and are capable of guiding natural bone repair using signaling molecules to promote osteoinduction and angiogenesis essential in the formation of new bone tissues.
- tissue engineering
- drug delivery
- biomaterials
- polymer composites
- bone regeneration
- growth factor
- bone morphogenetic protein
- bioscaffold
1. Introduction
1. Introduction
Nonhealing chronic bone tissue defects represent a major problem in healthcare. Despite numerous reports [1][2], there is still a growing need to identify new high-impact compounds for bone tissue regeneration applications. A current approach for bone tissue engineering is based on scaffolds that release growth factors (GFs) required for bone regeneration. A bone scaffold is a 3D matrix that allows for and stimulates the attachment and proliferation of osteoinductive cells on its surface. An ideal scaffold should be biocompatible and should degrade with time to allow new bone deposition; it also should have suitable mechanical properties for load-bearing with proper architecture in terms of porosity and pore sizes for cellular infiltration and angiogenesis, and the ability to control the delivery of bioactive molecules and drugs [3][4][5][6].
Different factors that promote tissue growth have been found at the skeletal damage site and have a physiologic role in healing bone fractures. Osteoinductive GFs such as platelet-derived growth factors (PDGFs), bone morphogenic proteins (BMPs), insulin-like growth factors (IGFs), transforming growth factors (TGFs-ß), and vascular endothelial growth factors (VEGFs) have presented great application potentials in bone healing and osteogenesis for regulating cell behavior, including recruitment, migration, adhesion, proliferation, and differentiation [7][8][9].
Biomechanical stability and biological activity that furnishes an appropriate background for new bone formation are the basis for triumphant GF therapy in bone tissue engineering [9]. Thus, understanding GF biological features, action mechanisms, and delivery strategies are vital for scientists and surgeons.
Several in vivo and clinical studies showed that incorporating GFs into polymer carriers/scaffolds such as gelatin, chitosan, alginate, chitosan, collagen, and hyaluronic acid improved bone healing [2][10][11][12][13]. Among the different carrier materials, absorbable collagen sponges can be used as carriers not only for recombinant human bone morphogenetic protein 2 (rhBMP-2) but also for BMP-9 [14] and BMP-7 [15]. However, this protocol is still limited due to the effective delivery of GFs to tissue, such as release sustainability, stability, inflammation, and ectopic bone formation [16].
A very short duration of action and systemic toxicity by over-release have prevented GFs from being developed into effective regenerative treatments [17]. To circumvent the side effects (i.e., edema), it is foremost important to attain a controllable and sustained release of GFs [18]. Alternatives such as tissue transplantation procedures exist (allograft) but frequently have poor regenerating results, and a better option is needed. Although there is vast applicability for bone bioscaffolds, grafting extracellular matrix (ECM)-derived functional groups to the scaffold is an up-and-coming potential approach for biomaterial design [18]. Successful trials had in common the presence of a control vehicle, which categorically suggests that an effective therapeutic effect is achievable through spatiotemporal management over the targeted area and factor bioactivity [19][20][21].
Emerging and trailblazing materials that modulate the biological presentation of GFs are promising analeptic agents to aid in treating diseases [18][22]. This review considers various biomaterial polymer carriers and GF systemic delivery systems investigated to help the regeneration and repair of bone tissue. In the next sections, general approaches to the strategic use of these factors are discussed in detail and some specific applications for these factors in regenerative medicine are covered. Currently designed approaches and investigated essential topics related to polymer-based carriers for particular technical objectives are also addressed.
1.1. Growth Factors Roles in Bone Tissue Engineering
Studies have shown the projected perspectives of tissue engineering. However, triumphant translations into the clinical application are still restricted owing to the shortfall of delivery systems with optimal signaling. Thus, engineers and scientists are promptly developing biomimetic drug delivery systems that can take advantage of reproducing signaling molecules released by the native ECM during healing or regeneration processes. Designed drug delivery systems aim to provide control over the localization, time, and kinetics of the release pattern of signaling molecules such as GFs according to the drug chemical properties and specific biological mechanisms [23].
Biological signal molecules have a crucial function in modulating cellular activities and tissue regeneration. Bioactive compounds such as GFs are proteins that regulate many aspects of cellular function, including survival, proliferation, migration, and differentiation [24], and have an essential contribution to ECM synthesis [25]. Due to the essential role of GFs in controlling cellular functions and their ability to directly promote and engineer tissue regeneration, a wide range of GFs has been studied and tested for therapeutic applications [26], including bone regeneration [27]. Fibroblast GFs (FGFs), VEGFs, IGFs, TGFs-β, PDGFs, and BMPs are the main groups of GFs associated with bone regeneration [28]. Proteins such as recombinant human BMP-2, BMP-4, BMP-6, BMP-7, and BMP-9 that are currently used in clinical trials are expected to stimulate local bone regeneration by signaling the differentiation of mesenchymal stem cells (MSCs) into osteoblasts [29][30]. Currently, special focus has been given to BMP-2 and 7, as they were approved by the FDA (Food and Drug Administration) for bone-regeneration applications [31]. For instance, BMPs have been shown to elicit new bone formation both at the bone defect site and at heterotopic sites in a large number of species. The process of bone regeneration encompasses the initial inflammatory phase, soft callus formation, mineralization, and bone remodeling [32]. The different phases of bone regeneration engage multiple GFs in specific spatiotemporal patterns (Figure 1).
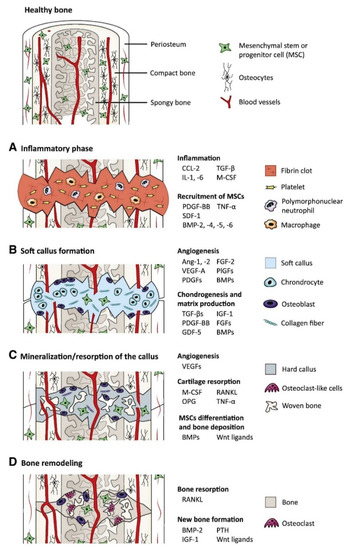
Figure 1. The main growth factors that are relevant to the bone-regeneration process: the bone-regeneration process is addressed in four overlapped, different phases of inflammation (phase A), soft callus formation (phase B), mineralization and resorption of the soft callus (phase C), and bone remodeling (phase D) (BMP: bone morphogenetic protein, FGF: fibroblast growth factor, GDF-5: growth/differentiation factor 5, IGF-1: insulin-like growth factor 1, PTH: parathyroid hormone, M-CSF: macrophage colony-stimulating factor, OPG: osteoprotegerin, PDGF: platelet-derived growth factor, PlGF: placental growth factor, RANKL: receptor activator of nuclear factor κB ligand, SDF-1: stromal cell-derived factor 1, TGF-β: transforming growth factor β, TNF-α: tumor necrosis factor α, and VEGF: vascular endothelial growth factor) [18].
In the bone-repair process, angiogenesis precedes the onset of osteogenesis. A combination of angiogenic (VEGF), cell recruiting (platelet-derived growth factor (PDGF)), and osteogenic (BMPs) GFs has been designed and demonstrated a synergistic effect that is more beneficial to bone repair than any GF delivered alone [33]. This synergism was also demonstrated through the immobilization of FGF-2 and BMP-2 in administered ratios on the surfaces of gelatin nanofibers to promote bone regeneration [34]. BMPs stimulate the osteogenic and chondrogenic differentiation of mesenchymal cells and play a significant role in structural development throughout the body, having a wide range of functions, including embryogenesis and regulation of cells widely expressed in several tissues [35]. BMPs also display sites for N- and O-glycosylation, allowing for an increase in BMP stability and half-life in the body and determination of the specificity of receptor coupling [36][37]. The integration of stem cells with BMP-2 to promote healthy bone regeneration has demonstrated great new bone formation, fast healing, and callus remodeling [2]. The therapeutic effect of collagen particles combined with BMP-2 with the collagen-binding domain has been shown to reconstruct vertebral laminar defects [38]. That being said, BMP-GFs have an osteoinductive potential for orthopedic clinical practice for the treatment of bone tissue regeneration.
At the surgical site, a specific delivery system should use GFs to exert and maintain biological activity in a controlled fashion and to avoid any systemic diffusion. Therefore, a delivery system is imperative to stabilize GFs and to provide long-term sustained release for in vivo efficacy. Understanding the biomolecular processes during the healing of injured organs is essential for developing GF-based therapeutics for tissue regeneration. An aspect of the natural healing process is the continuous delivery of GFs throughout recovery, avoiding a high variability of GF concentration at the target tissue and rapid clearance [39].
A successful delivery system can deliver GFs to areas besides the target spot through surgery. This system can maintain enough bioactive factors during the time needed to promote osteogenesis and low fundamental doses to prevent side effects due to supraphysiological GF doses [40]. Delivering osteogenic and angiogenesis-promoting GFs [41][42] together can be a feasible alternative to reestablishing vascularized bone tissue, which is a defying task in bone tissue engineering. Delivering distinct GFs simultaneously, overall, enhances the innate bone-healing process [43]. Local alendronate administration to control β-tricalcium phosphate (β-TCP) resorption and the induction of bone formation by rhBMP-2 were attempted [44]. However, the administration of rhBMP-2 promoted a burst release and reduced osteoclastic resorption of β-TCP induced by rhBMP-2, resulting in decreased bone formation. Supraphysiological delivery of bone tissue GFs resulted in the development of heterotopic bone and other side effects [45]. Octacalcium phosphate/collagen (OCP/Col) can also be used as a carrier system to reduce the rhBMP-2 effective dose. Bien et al. [46] implanted OCP/Col discs impregnated with rhBMP-2 (about 0.25 μg) in mice calvarial bone defects that resulted in no bone formation. Therefore, it is paramount to deliver an effective amount of drug to the defect site. To overcome the mentioned drawbacks, GF carrier systems may play a key role in determining GF bioactivity. Drug injection affecting the whole system or grafting of a polymeric scaffold modified with a bone-targeting moiety delivers a nonintrusive approach for site-specific or targeted therapy [47]. By changing the type of receptor and cell to which the GF binds, the same GF can convey different instructions (Figure 2). Moreover, the same receptor can translate different messages depending on the intracellular transduction pathways, which can differ from one cell type to another.
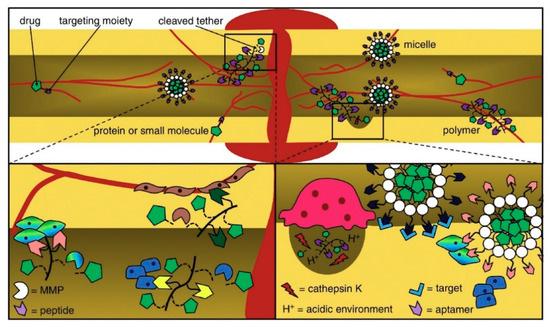
Figure 2. Peptides and aptamers are targeting moieties used to deliver drugs to bones through carriers that transit or infiltrate the blood stream and come out after targeting. The delivered drugs are metabolized owing to a pH media variation or via matrix metalloproteinases (MMP) and enzymes [48].
1.2. Scaffold Properties for Bone Tissue Engineering
Evidenced by the wide range of inflammatory, osteogenic, and angiogenic factors involved in all bone tissue regeneration processes, these processes can be directly related to biomolecular and cellular processes [47]. GFs’ therapeutic roles can be effectively attained by reaching the damaged tissue site without losing their bioactivity and remaining in the specific site over the healing process [49]. Thus, it is foremost important to develop release technologies to administer the release of signaling molecules in space and time. A proper GF material should be able to manage GF delivery system kinetics to realize tissue formation by efficiently loading the factor and by stimulating protein presentation to the surface of cells (Figure 3). GF release profiles involve prolonged, multifactorial, or sequential releases depending on the type of molecule being delivered and the biological demands [50]. An effective carrier for GFs not only should allow site-specific delivery but also should strengthen the infiltration of cells. Moreover, GFs should accurately load the bioactive factors to allow strong carrier/protein associations [51]. Ultimately, the fabrication process should be straightforward and viable and should maintain the bioactive status of the integrated protein. Overall, scaffold-based GF delivery aims to orchestrate cell response by connecting the transmission of signals from the cells to the kinetics of bone damage healing. Tissue engineering scaffolds not only should prevent ectopic bone formation by facilitating fast infiltration of host cells from margins to the center of the scaffold but also should present low immunogenic and antigenic responses [52]. When GFs are loaded into a scaffold, the incorporation levels and the kinetics that encompass sustained therapeutic doses should be achieved [53][54]. Moreover, the scaffold should degrade into harmless products at a rate that provides the host tissue with a successfully developed mechanical stability [55]. Considering that bones are composed of miscellaneous components such as hydroxyapatite (HA) mineral, organic components (type I collagen, lipids, and non-collagenous proteins), and water [56][57], this combination of materials likely allows the biological activity of scaffolds and their bio-architecture to be accomplished [54]. The bioactivity of tissue engineering scaffolds can also be improved by integrating compounds that correlate organs and cells at the cellular organizational level [58] and, therefore, lead to osteoconduction (bone cell ingrowth), osseointegration (steady attachment to the tissue defect), osteoinduction (stimulation of immature cells into osteogenic ones), and vascularization [59]. Due to the versatile roles of natural bone in the body, bone tissue engineering scaffolds should present several different characteristics to effectively function as a bone scaffold [60]. The main structural characteristics (such as high porosity, high mechanical properties, and tunable architecture), common compositions (polymers, ceramics, and composites), biological requirements (including nontoxicity, biocompatibility, low immunogenic response, and bioactivity), as well as conventional and advanced manufacturing methods (including freeze-drying, electrospinning, and solvent casting) for bone tissue engineering scaffolds are listed in Figure 3.

Figure 3. The main biological and structural properties, common compositions, and manufacturing technologies of bone tissue engineering scaffolds [61].
Such structures provide initial biomechanical support to the implanted tissue until cells can develop a proper ECM to support the regeneration process. It is expected that the scaffold is gradually degraded and metabolized during the formation, deposition, and organization of the ECM, allowing for the tissue to be reestablished with the same or improved function. Thus, such scaffolds are engineered to be biocompatible, biodegradable, and porous to assure vascularization, to show mechanical reinforcement, and to allow functional and bioactive responses [62]. Bone grafts should be biocompatible, bioresorbable, osteoconductive, osteoinductive, structurally similar to bone, easy to use, and cost-effective. The biomaterial properties and features determine the cascade of events that take place at the site of bone healing [63]. The biomaterial should be dissolved or absorbed by the body to be considered bioresorbable. Biomaterials directed for tissue regeneration should degrade continuously in vivo besides filling the defect [64]. As discussed, polymeric, ceramic, and composite scaffolds have been widely considered for bone tissue engineering scaffolds. Although the incorporation of metal nanoparticles in polymeric scaffolds is known to effectively improve scaffold mechanical properties [65][66], the application of metal scaffolds for GF delivery is limited due to the low biodegradability, high rigidity, limited integration to the host tissue, and infection possibility of metal scaffolds [61]. Moreover, compared to polymeric scaffolds, porous metallic scaffolds mostly can only be manufactured through complex procedures, such as electron beam melting [67], layer-by-layer powder fabrication using computer-aided design strategies [68], and extrusion [69], which further limits their architecture design and application in GF delivery [61]. To avoid compromising the function and structure of new bone, the degradation rate of bone biomaterials should match the growth rate of the new structure [70]. Osteoconductive materials allow vascularization of the tissue and further regeneration in addition to building its architecture, chemical structure, and surface charge. Osteoinduction is related to the mobility and propagation of embryonic stem cells as well as cell differentiation [63]. Briefly, scaffolds should present reduced immunogenic and antigenic responses whilst making host cell infiltration easier. Loading efficiency and release kinetics that account for controlled delivery of a therapeutic dosage of GFs are necessary; additionally, scaffolds should degrade into non-harmful substances in a way that the tissue can regenerate its mechanical properties [71][72].
2. Polymer Scaffolds for GF Delivery
Collagen is the most studied natural polymer for bone tissue engineering scaffolds, as this biopolymer integrates about 90 wt.% of natural bone ECM proteins [73]. Collagen can actively facilitate the osteogenic process of bone progenitor cells through a series of alpha–beta integrin receptor interactions and, as a result, can promote bone mineralization and cell growth [50]. The inter- and intra-chain crosslinks of collagen are key to its mechanical properties which maintain the polypeptide chains in a tightly organized fibril structure. Although collagen has a direct impact on bone strength, this biopolymer has mechanical properties that are insufficient for creating a load-bearing scaffold. Furthermore, the mechanical and degradation properties of collagen can be customized through the process of crosslinking [74] by forming composites [75], as shown in Figure 4. It is, therefore, often combined with more robust materials to create composite scaffolds. As the major inorganic component of bone, HAp has frequently been combined with collagen in composite scaffolds. The mechanism of reaction involved in collagen surface modification and BMP-2 functionalization of 3D hydroxyapatite [76] scaffolds is displayed in Figure 4.

Figure 4. (A) Natural crosslinking of collagen (head-to-tail); (B) the intermolecular crosslink of collagen allowing for the protection of collagen from enzymatic degradation; (C) live/dead cell viability assay of PDLSCs (periodontal ligament stem cells) performed in collagen powder before implantation and 24 h after incubation showing that cells in green are alive; (D) mechanism of reaction to modify a collagen scaffold functionalized with hydroxyapatite and BMP-2, and modified scaffolds; (E) hydroxyapatite scaffold (a) micro-CT pore structure (b), surface morphology (SEM) (c), cross-sectional morphology (SEM) (d), and hydroxyapatite and collagen scaffold (SEM) (e,f); and (F) fluorescent-stained images of a collagen-hydroxyapatite-modified scaffold detecting BMP-2 after 1, 5, and 21 days [75][77][78].
Linh et al. [79] conjugated collagen and BMP-2 to the surface of a porous HAp scaffold. The composite scaffold showed higher compressive strength (50.7 MPa) compared to the HAp scaffold (45.8 MPa). Moreover, the delivery system in this composite scaffold structure more efficiently induced adipose-derived stem cell osteogenic differentiation than in HAp or HAp-collagen (without BMP incorporation) structures. HAp-collagen has been shown to be very effective in healing critical-sized bone damage in a rodent model after HAp shows high affinity to the GFs (BMP-2 and VEGF) used in combination to regenerate vascularized bone tissue [80]. This affinity allows for localized delivery of GFs at the targeted defect site.
In addition to collagen, other natural biopolymers such as silk fibroin can also be effective in bone tissue engineering applications [81][77]. Silk fibroin is a fibrous protein produced by silkworms and spiders with outstanding mechanical characteristics, high biological compatibility, and an adjustable degradation rate that can support cell differentiation [78][82] and, thus, versatility in processing. Composite silk fibroin (Antheraea mylitta) scaffolds were reinforced with functionalized carbon nanofiber to deliver BMP-2 and TGF-β1 [83]. Loaded scaffolds presented a sustained GF release profile; strong adhesion; and the development, propagation, and differentiation of MSCs into osteoblasts. Moreover, composite structures exhibited high compatibility with a targeted immune system, as evidenced by minimal pro-inflammatory cytokines release, both in vitro and in vivo. By depositing HAp on the silk fibroin nanofibrous matrices, enhanced mechanical resistance and a resourceful BMP-2 and TGF-β1 delivery system were observed [84] that induced propagation and differentiation of osteoblasts at the early stages of healing [82].
Sodium alginate is a linear anionic binary polysaccharide that consists of α-L-guluronic acid (G units) and (1-4)-linked β-D-mannuronic acid (M units) segments. This biopolymer is mostly obtained from widely available seaweeds, which makes it a great candidate for a diverse range of tissue engineering applications (Figure 5). Consecutive G (GGGGGG), M residues (MMMMMM), and alternating M and G residues (GMGMGM) compound the blocks [85]. The composition ratio of these monomers (M/G ratio) and the sequence of monomers in the polymeric backbone determine the final properties of alginate [86]. Alginate is capable of forming stabilized scaffolds through divalent cations crosslinking (i.e., Ca2+) due to the anionic nature that allows alginate complexation to these cations [87]. This modification opens avenues for a multitude of medical applications as it overcomes the hurdles faced by using native alginate, such as degradation rate and stability under aqueous conditions [88]. A partially cross-linked TEMPO-oxidized cellulose nanofibril/alginate hydrogel was used to fabricate 3D-printed scaffolds using Ca2+ crosslinking [89]. Alginate matrices were conjugated to calcium phosphate scaffolds to achieve a programmed GF delivery [90]. PDGF and BMP-2 were released sequentially with a 3-day PDGF to BMP-2 delivery overlap. It has been suggested that the sequential programming of PDGF to BMP-2 delivery promoted the differentiation of MSCs into osteoblast phenotypes and increased cellular infiltration.
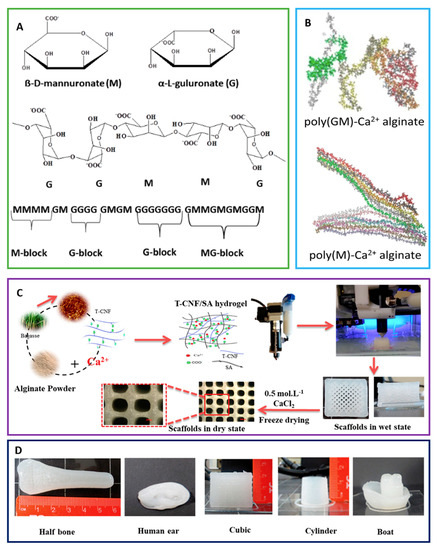
Figure 5. (A) Schematic representation of alginate showing the structure of mannuronate (M) and guluronate (G), and the chair conformation and the sequence of M block and G block arrangement in alginate are shown. (B) Poly (GM)-Ca2+ alginate and poly(M)-Ca2+ alginate are displayed. (C) The fabrication process for 3D-printed scaffolds from TEMPO-oxidized cellulose nanofibril/sodium alginate hydrogels is shown. (D) Scaffolds printed in different forms and designs from optimal TEMPO-oxidized cellulose nanofibril/sodium alginate hydrogel formulation are shown [91][92][93].
An alternative to overcoming the challenges faced by composite biomaterials is the use of cellulose and other nature-derived polymers once vast manufacturing approaches and sources are available [94]. Cellulose occurs naturally and is an accessible polymer after it is refined from lignocellulose or synthesized from bacteria [91]. Hydrogels with specific structures and diverse functionalities that have biomedical applications can be prepared by manipulating the functional groups in the structure of cellulose and its derivatives (methylcellulose, carboxymethylcellulose, and hydroxypropylmethylcellulose) [92]. Nonetheless, cellulose hydrogels show restricted mechanical attributes that hold back their utilization in hard tissue applications. To surpass this limitation of cellulose-based scaffolds and to build on the functional properties for hard tissue application, mineralization of cellulose hydrogels with HAp and other materials has been actively investigated in recent years [90][94][91][92][95][93][96][97][98][99][100]. Bacterial cellulose was successfully combined with HAp to deliver BMP-2 [95]. The system kinetics was studied in vitro and showed a gradual release of BMP-2 and mineralization spots. Also, BMP-2-loaded aligned electrospun cellulose nanocomposite nanofibers were studied for in vivo bone regeneration in a rabbit model [93]. The results suggest a slight difference between the GF release of aligned and random scaffolds. The aligned scaffold delivered the GFs (0.74 μg/mm2) slightly slower than the random scaffold (0.76 μg/mm2) after seven days.
Chitin–chitosan is a nitrogen-containing polysaccharide-based biopolymer group derived from diverse natural raw materials such as fungi, crustaceans, and insects [96][97]. Chitin and chitosan are structurally similar to glycosaminoglycans (GAGs, the major component of the bone ECM), which make them suitable biopolymers for tissue engineering scaffolds [96][97][98]. Chitin used in combination with chitosan/poly(vinyl alcohol) to fabricate nanofibers showed enhanced mechanical properties and offered osteoblast cell growth with HAp biomineralization [99]. Chitosan nanoparticles loaded with BMP-2 were dispersed into collagen hydrogel and added to the scaffolds. The system showed active osteoinduction through the controlled delivery of GFs [99]. Drug delivery systems using β-tricalcium-phosphate/gelatin containing chitosan-based nanoparticles [100] and dextran sulfate-chitosan microspheres [101][102] were designed to promote the sustained delivery of BMP-2 for bone tissue regeneration. Both systems showed that alginate composite scaffolds were able to attain the controlled release profile of GFs and to act as a mechanically and biologically compatible framework with prominent osteoinductive activity.
Recent studies have suggested GAGs as potential biomaterials for tissue engineering application, as this biopolymer predominantly exists in the ECM, has low immunogenicity, and can perform strong interactions with GFs [103]. The structural composition (degree of sulfation and polymer length) of GAGs are varied and determine the precise performance of GAGs. Cell-binding motifs, native-like mechanical properties, bone mineralization-specific sites, and robust GF binding and signaling capacity are among the GAG properties [104][105]. Notwithstanding, investigations on GAGs as molecules for engineering tissue scaffolds have been conducted as of late. GAGs isolated from mammalian sources such as heparin [47][106], heparan sulfate [76][107], chondroitin sulfate [108][109], keratan sulfate [110], and hyaluronic acid [111][112] (non-sulfated) are the most widely explored in regeneration medicine. Strong ionic interactions are expected between GAGs and proteins. Among the GAGs, hyaluronic acid is the predominant GAG in the skin whereas chondroitin sulfate is the major GAG found in bone. GAGs interact with residues that are prominently exposed on the surface of proteins. Clusters of positively charged basic amino acids on proteins form ion pairs with spatially defined negatively charged sulphate or carboxylate groups on GAG chains. The main contribution to binding affinity comes from ionic interactions between the highly acidic sulphate groups and the basic side chains of the protein. Despite incomplete understanding of the interactions between cells and ECM, namely, at the molecular level, it is known that GAGs modulate the adhesion of progenitor cells and their subsequent differentiation and gene expression. These regulatory roles are related to the GAG ability to interact with GFs and to protect GFs from proteolytic degradation, increasing the half-life of GFs. For instance, during osteogenesis, heparan sulfate provides matrix-bound or cell surface-bound reservoirs for specific binding proteins, including GFs such as BMPs [47]. In vivo BMP-2 retention can be improved via heparin microparticles (HMPs). HMPs can improve the safety profile of scaffold-based BMP-2 delivery systems and, consequently, can reduce the heterotopic ossification. Moreover, these microparticles can improve the spatial localization of bone formation in large bone defects. Overall, GAGs play an important regulatory role in the development and regeneration of skin and bone tissue by performing complex effects on skin and bone cells at all stages of their differentiation, including the attraction and adhesion of precursor cells, their subsequent differentiation, their activity and immune responses, and their interactions with other proteins. Thus, GAGs are part of a new genesis of biomimetic biomaterials.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22020903
References
- Neves, M.I.; Araújo, M.; Moroni, L.; Da Silva, R.M.P.; Barrias, C.C. Glycosaminoglycan-Inspired Biomaterials for the Development of Bioactive Hydrogel Networks. Molecules 2020, 25, 978.
- Wang, L.; Fang, M.; Xia, Y.; Hou, J.; Nan, X.; Zhao, B.; Wang, X. Preparation and biological properties of silk fibroin/nano-hydroxyapatite/graphene oxide scaffolds with an oriented channel-like structure. RSC Adv. 2020, 10, 10118–10128.
- Nie, L.; Deng, Y.; Li, P.; Hou, R.; Shavandi, A.; Yang, S. Hydroxyethyl Chitosan-Reinforced Polyvinyl Alcohol/Biphasic Calcium Phosphate Hydrogels for Bone Regeneration. ACS Omega 2020, 5, 10948–10957.
- Ratnayake, J.T.; Ross, E.D.; Dias, G.J.; Shanafelt, K.M.; Taylor, S.S.; Gould, M.L.; Guan, G.; Cathro, P.R. Preparation, characterisation and in-vitro biocompatibility study of a bone graft developed from waste bovine teeth for bone regeneration. Mater. Today Commun. 2020, 22, 100732.
- Shavandi, A.; Bekhit, A.E.-D.A.; Ali, M.A.; Sun, Z. Bio-mimetic composite scaffold from mussel shells, squid pen and crab chitosan for bone tissue engineering. Int. J. Biol. Macromol. 2015, 80, 445–454.
- Shavandi, A.; Bekhit, A.E.-D.A.; Sun, Z.; Ali, M.A. Injectable gel from squid pen chitosan for bone tissue engineering applications. J. Sol-Gel Sci. Technol. 2016, 77, 675–687.
- Bessa, P.C.; Casal, M.; Reis, R.L. Bone morphogenetic proteins in tissue engineering: The road from laboratory to clinic, part II (BMP delivery). J. Tissue Eng. Regen. Med. 2008, 2, 81–96.
- Khojasteh, A.; Behnia, H.; Naghdi, N.; Esmaeelinejad, M.; Alikhassy, Z.; Stevens, M. Effects of different growth factors and carriers on bone regeneration: A systematic review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, e405–e423.
- Termaat, M.F.; Boer, D.; Bakker, F.C.; Patka, P.; Haarman, H.J. Bone morphogenetic proteins: Development and clinical efficacy in the treatment of fractures and bone defects. J. Bone Jt. Surg. Am. 2005, 87, 1367–1378.
- Cochran, D.L.; Jones, A.A.; Lilly, L.C.; Fiorellini, J.P.; Howell, H. Evaluation of Recombinant Human Bone Morphogenetic Protein-2 in Oral Applications Including the Use of Endosseous Implants: 3-Year Results of a Pilot Study in Humans. J. Periodontol. 2000, 71, 1241–1257.
- Krishnan, L.; Priddy, L.B.; Esancy, C.; Klosterhoff, B.S.; Stevens, H.Y.; Tran, L.; Guldberg, R.E. Delivery vehicle effects on bone regeneration and heterotopic ossification induced by high dose BMP-2. Acta Biomater. 2017, 49, 101–112.
- Yamamoto, M.; Takahashi, Y.; Tabata, Y. Enhanced bone regeneration at a segmental bone defect by controlled release of bone morphogenetic protein-2 from a biodegradable hydrogel. Tissue Eng. 2006, 12, 1305–1311.
- Yuan, Q.; Kubo, T.; Doi, K.; Morita, K.; Takeshita, R.; Katoh, S.; Shiba, T.; Gong, P.; Akagawa, Y. Effect of combined application of bFGF and inorganic polyphosphate on bioactivities of osteoblasts and initial bone regeneration. Acta Biomater. 2009, 5, 1716–1724.
- Fujioka-Kobayashi, M.; Schaller, B.; Saulacic, N.; Pippenger, B.E.; Zhang, Y.; Miron, R.J. Absorbable collagen sponges loaded with recombinant bone morphogenetic protein 9 induces greater osteoblast differentiation when compared to bone morphogenetic protein 2. Clin. Exp. Dent. Res. 2017, 3, 32–40.
- Hertweck, J.; Ritz, U.; Götz, H.; Schottel, P.C.; Rommens, P.M.; Hofmann, A. CD34+ cells seeded in collagen scaffolds promote bone formation in a mouse calvarial defect model. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1505–1516.
- Kowalczewski, C.J.; Saul, J.M. Biomaterials for the Delivery of Growth Factors and Other Therapeutic Agents in Tissue Engineering Approaches to Bone Regeneration. Front. Pharmacol. 2018, 9, 513.
- Dao, D.T.; Vuong, J.T.; Anez-Bustillos, L.; Pan, A.; Mitchell, P.D.; Fell, G.L.; Baker, M.A.; Bielenberg, D.R.; Puder, M. Intranasal delivery of VEGF enhances compensatory lung growth in mice. PLoS ONE 2018, 13, e0198700.
- Martino, M.M.; Briquez, P.S.; Maruyama, K.; Hubbell, J.A. Extracellular matrix-inspired growth factor delivery systems for bone regeneration. Adv. Drug Deliv. Rev. 2015, 94, 41–52.
- Hu, K.; Olsen, B.R. Vascular Endothelial Growth Factor Control Mechanisms in Skeletal Growth and Repair. Dev. Dyn. 2017, 246, 227–234.
- Onishi, T.; Shimizu, T.; Akahane, M.; Omokawa, S.; Okuda, A.; Kira, T.; Inagaki, Y.; Tanaka, Y. Osteogenic extracellular matrix sheet for bone tissue regeneration. Eur. Cells Mater. 2018, 36, 69–80.
- Yan, H.J.; Casalini, T.; Hulsart-Billström, G.; Wang, S.; Oommen, O.P.; Salvalaglio, M.; Larsson, S.; Hilborn, J.; Varghese, O.P. Synthetic design of growth factor sequestering extracellular matrix mimetic hydrogel for promoting in vivo bone formation. Biomaterials 2018, 161, 190–202.
- Spiller, K.L.; Vunjak-Novakovic, G. Clinical translation of controlled protein delivery systems for tissue engineering. Drug Deliv. Transl. Res. 2015, 5, 101–115.
- Dang, M.; Saunders, L.; Niu, X.; Fan, Y.; Ma, P.X. Biomimetic delivery of signals for bone tissue engineering. Bone Res. 2018, 6, 25.
- Timin, A.S.; Muslimov, A.R.; Zyuzin, M.V.; Peltek, O.O.; Karpov, T.E.; Sergeev, I.S.; Dotsenko, A.I.; Goncharenko, A.A.; Yolshin, N.D.; Sinelnik, A.; et al. Multifunctional scaffolds with improved antimicrobial properties and osteogenicity based on piezoelectric electrospun fibers decorated with bioactive composite microcapsules. ACS Appl. Mater. Interfaces 2018, 10, 34849–34868.
- Briquez, P.S.; Hubbell, J.A.; Martino, M.M. Extracellular matrix-inspired growth factor delivery systems for skin wound healing. Adv. Wound Care 2015, 4, 479–489.
- Sluzalska, K.D.; Slawski, J.; Sochacka, M.; Lampart, A.; Otlewski, J.; Zakrzewska, M. Intracellular Partners of Fibroblast Growth Factors 1 and 2—Implications for Functions. Cytokin Growth Factor Rev. 2020.
- Tian, H.; Zhao, J.; Brochmann, E.J.; Wang, J.C.; Murray, S.S. Bone Morphogenetic Protein-2 and Tumor Growth: Diverse Effects and Possibilities for Therapy. Cytokin Growth Factor Rev. 2017, 34, 73–91.
- Azevedo, H.S.; Pashkuleva, I. Biomimetic Supramolecular Designs for the Controlled Release of Growth Factors in Bone Regeneration. Adv. Drug Deliv. Rev. 2015, 94, 63–76.
- Katagiri, T.; Watabe, T. Bone morphogenetic proteins. Cold Spring Harb. Perspect. Biol. 2016, 8.
- Tong, Z.; Guo, J.; Glen, R.C.; Morrell, N.W.; Li, W. A bone morphogenetic protein (BMP)-derived peptide based on the type I receptor-binding site modifies cell-type dependent BMP signalling. Sci. Rep. 2019, 9, 13446.
- Schmidt-Bleek, K.; Willie, B.M.; Schwabe, P.; Seemann, P.; Duda, G.N. BMPs in Bone Regeneration: Less is More Effective, a Paradigm-Shift. Cytokin Growth Factor Rev. 2016, 27, 141–148.
- Botega, I.I.; Zamarioli, A.; Guedes, P.M.S.G.; da Silva, R.A.B.; Issa, J.P.M.; Butezloff, M.M.; Sousa, Y.T.C.S.; Ximenez, J.P.B.; Volpon, J.B. Bone callus formation is highly disrupted by dietary restriction in growing rats sustaining a femoral fracture. Acta Cir. Bras. 2019, 34.
- Shah, N.J.; Hyder, M.N.; Quadir, M.A.; Courchesne, N.M.D.; Seeherman, H.J.; Nevins, M.; Spector, M.; Hammond, P.T. Adaptive growth factor delivery from a polyelectrolyte coating promotes synergistic bone tissue repair and reconstruction. Proc. Natl. Acad. Sci. USA 2014, 111, 12847–12852.
- Udomluck, N.; Lee, H.; Hong, S.; Lee, S.-H.; Park, H. Surface functionalization of dual growth factor on hydroxyapatite-coated nanofibers for bone tissue engineering. Appl. Surf. Sci. 2020, 520, 146311.
- Scarfì, S. Use of Bone Morphogenetic Proteins in Mesenchymal Stem Cell Stimulation of Cartilage and Bone Repair. World J. Stem Cells 2016, 8, 1–12.
- Sun, N.; Chen, Y.; Yu, F.; Zhixin, F.; Lin, J.; Sun, B.; Yu, B.; Cheng, X.; Zheng, X.; Wu, B. Monocrotaline pyrrole enhanced bone morphogenetic protein 7 signaling transduced by alternative activin A receptor type 2A in pulmonary arterial smooth muscle cells. Eur. J. Pharmacol. 2019, 863.
- Huang, B.; Yuan, Y.; Liu, C. Biomaterial-guided immobilization and osteoactivity of bone morphogenetic protein-2. Appl. Mater. Today 2020, 19, 1–22.
- Cui, Y.; Xu, B.; Yin, Y.; Chen, B.; Zhao, Y.; Xiao, Z.; Yang, B.; Shi, Y.; Fang, Y.; Ma, X.; et al. Collagen particles with collagen-binding bone morphogenetic protein-2 promote vertebral laminar regeneration in infant rabbits. Biomed. Mater. 2020, 15, 055008.
- Subbiah, R.; Guldberg, R.E. Materials science and design principles of growth factor delivery systems in tissue engineering and regenerative medicine. Adv. Healthc. Mater. 2019, 8, 1801000.
- El Bialy, I.; Jiskoot, W.; Reza Nejadnik, M. Formulation, Delivery and Stability of Bone Morphogenetic Proteins for Effective Bone Regeneration; Springer: New York, NY, USA, 2017; Volume 34, pp. 1152–1170.
- Chen, D.; Zhang, C.; Huo, H.; Ji, C.; Sun, M.; Nie, L. Injectable temperature-sensitive hydrogel with VEGF loaded microspheres for vascularization and bone regeneration of femoral head necrosis. Mater. Lett. 2018, 229, 138–141.
- Nie, L.; Chen, D.; Zhong, S.; Shi, Q.; Sun, Y.; Politis, C.; Shavandi, A. Injectable cell-laden poly(N-isopropylacrylamide)/chitosan hydrogel reinforced via graphene oxide and incorporated with dual-growth factors. Mater. Lett. 2020, 280, 128572.
- Farokhi, M.; Mottaghitalab, F.; Shokrgozar, M.A.; Ou, K.L.; Mao, C.; Hosseinkhani, H. Importance of dual delivery systems for bone tissue engineering. J. Control. Release 2016, 225, 152–169.
- Kitasato, S.; Tanaka, T.; Chazono, M.; Komaki, H.; Kakuta, A.; Inagaki, N.; Akiyama, S.; Marumo, K. Local application of alendronate controls bone formation and β-tricalcium phosphate resorption induced by recombinant human bone morphogenetic protein-2. J. Biomed. Mater. Res. Part A 2020, 108, 528–536.
- Visser, R.; Rico-Llanos, G.A.; Pulkkinen, H.; Becerra, J. Peptides for Bone Tissue Engineering. J. Control. Release 2016, 244, 122–135.
- Bien, N.D.; Miura, K.-I.; Sumita, Y.; Nakatani, Y.; Shido, R.; Kajii, F.; Kamakura, S.; Asahina, I. Bone regeneration by low-dose recombinant human bone morphogenetic protein-2 carried on octacalcium phosphate collagen composite. J. Hard Tissue Biol. 2020, 29, 123–130.
- Hettiaratchi, M.H.; Chou, C.; Servies, N.; Smeekens, J.M.; Cheng, A.; Esancy, C.; Wu, R.; McDevitt, T.C.; Guldberg, R.E.; Krishnan, L. Competitive protein binding influences heparin-based modulation of spatial growth factor delivery for bone regeneration. Tissue Eng. Part A 2017, 23, 683–695.
- Newman, M.R.; Benoit, D.S.W. Local and Targeted Drug Delivery for Bone Regeneration. Curr. Opin. Biotechnol. 2016, 40, 125–132.
- De Witte, T.-M.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Regen. Biomater. 2018, 5, 197–211.
- Fernandez-Yague, M.A.; Abbah, S.A.; McNamara, L.; Zeugolis, D.I.; Pandit, A.; Biggs, M.J. Biomimetic approaches in bone tissue engineering: Integrating biological and physicomechanical strategies. Adv. Drug Deliv. Rev. 2015, 84, 1–29.
- Gan, Q.; Zhu, J.; Yuan, Y.; Liu, H.; Qian, J.; Li, Y.; Liu, C. A dual-delivery system of pH-responsive chitosan-functionalized mesoporous silica nanoparticles bearing BMP-2 and dexamethasone for enhanced bone regeneration. J. Mater. Chem. B 2015, 3, 2056–2066.
- Hussein, K.H.; Park, K.M.; Kang, K.S.; Woo, H.M. Biocompatibility Evaluation of Tissue-Engineered Decellularized Scaffolds for Biomedical Application. Mater. Sci. Eng. C 2016, 67, 766–778.
- Mishra, R.; Sefcik, R.S.; Bishop, T.J.; Montelone, S.M.; Crouser, N.; Welter, J.F.; Caplan, A.I.; Dean, D. Growth factor dose tuning for bone progenitor cell proliferation and differentiation on Resorbable Poly(propylene fumarate) Scaffolds. Tissue Eng. Part C Methods 2016, 22, 904–913.
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D Bioactive Composite Scaffolds for Bone Tissue Engineering. Bioprinting 2018, 3, 278–314.
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current Development of Biodegradable Polymeric Materials for Biomedical Applications. Drug Des. Dev. Ther. 2018, 12, 3117–3145.
- Boskey, A.L. Bone composition: Relationship to bone fragility and antiosteoporotic drug effects. BoneKEy Rep. 2013, 2.
- Shavandi, A.; Bekhit, A.E.-D.A.; Sun, Z.F.; Ali, A. A Review of Synthesis Methods, Properties and Use of Hydroxyapatite as a Substitute of Bone. J. Biomim. Biomater. Biomed. Eng. 2015, 25, 98–117.
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding Strategies for Tissue Engineering and Regenerative Medicine Applications. Materials 2019, 12, 1824.
- Venkataraman, N.; Bansal, S.; Bansal, P.; Narayan, S. Dynamics of bone graft healing around implants. J. Int. Clin. Dent. Res. Organ. 2015, 7, 40.
- Chocholata, P.; Kulda, V.; Babuska, V. Fabrication of Scaffolds for Bone-Tissue Regeneration. Materials 2019, 12, 568.
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for bone tissue engineering: State of the art and new perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262.
- Nikolova, M.P.; Chavali, M.S. Recent Adv. biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292.
- Agrawal, S.; Srivastava, R. Osteoinductive and Osteoconductive Biomaterials; Springer: Berlin/Heidelberg, Germany, 2020; pp. 355–395.
- Gao, C.; Peng, S.; Feng, P.; Shuai, C. Bone Biomaterials and Interactions with Stem Cells. Bone Res. 2017, 5, 1–33.
- Shuai, C.; Yang, W.; He, C.; Peng, S.; Gao, C.; Yang, Y.; Qi, F.; Feng, P. A magnetic micro-environment in scaffolds for stimulating bone regeneration. Mater. Des. 2020, 185, 108275.
- Eivazzadeh-Keihan, R.; Bahojb Noruzi, E.; Khanmohammadi Chenab, K.; Jafari, A.; Radinekiyan, F.; Hashemi, S.M.; Ahmadpour, F.; Behboudi, A.; Mosafer, J.; Mokhtarzadeh, A.; et al. Metal-based nanoparticles for bone tissue engineering. J. Tissue Eng. Regen. Med. 2020, 14, 1687–1714.
- Fan, B.; Guo, Z.; Li, X.; Li, S.; Gao, P.; Xiao, X.; Wu, J.; Shen, C.; Jiao, Y.; Hou, W. Electroactive barium titanate coated titanium scaffold improves osteogenesis and osseointegration with low-intensity pulsed ultrasound for large segmental bone defects. Bioact. Mater. 2020, 5, 1087–1101.
- Lv, J.; Xiu, P.; Tan, J.; Jia, Z.; Cai, H.; Liu, Z. Enhanced angiogenesis and osteogenesis in critical bone defects by the controlled release of BMP-2 and VEGF: Implantation of electron beam melting-fabricated porous Ti 6 Al 4 V scaffolds incorporating growth factor-doped fibrin glue. Biomed. Mate. 2015, 10, 035013.
- Vehof, J.W.; Haus, M.T.; de Ruijter, A.E.; Spauwen, P.H.; Jansen, J.A. Bone formation in transforming growth factor beta-I-loaded titanium fiber mesh implants. Clin. Oral Implants Res. 2002, 13, 94–102.
- Comesaña, R.; Lusquiños, F.; Del Val, J.; Quintero, F.; Riveiro, A.; Boutinguiza, M.; Jones, J.R.; Hill, R.G.; Pou, J. Toward smart implant synthesis: Bonding bioceramics of different resorbability to match bone growth rates. Sci. Rep. 2015, 5, 10677.
- Blackwood, K.A.; Bock, N.; Dargaville, T.R.; Ann Woodruff, M. Scaffolds for growth factor delivery as applied to bone tissue engineering. Int. J. Polym. Sci. 2012, 2012, 174942.
- Geiger, M.; Li, R.H.; Friess, W. Collagen sponges for bone regeneration with rhBMP-2. Adv. Drug Deliv. Rev. 2003, 55, 1613–1629.
- Lin, X.; Patil, S.; Gao, Y.G.; Qian, A. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front. Pharmacol. 2020, 11, 757.
- Gu, L.; Shan, T.; Ma, Y.x.; Tay, F.R.; Niu, L. Novel biomedical applications of crosslinked collagen. Trends Biotechnol. 2019, 37, 464–491.
- Wang, Q.; Zhang, Y.; Li, B.; Chen, L. Controlled dual delivery of low doses of BMP-2 and VEGF in a silk fibroin–nanohydroxyapatite scaffold for vascularized bone regeneration. J. Mater. Chem. B 2017, 5, 6963–6972.
- Lee, J.H.; Luo, X.; Ren, X.; Tan, T.C.; Smith, R.A.A.; Swaminathan, K.; Sekar, S.; Bhakoo, K.; Nurcombe, V.; Hui, J.H.; et al. A Heparan Sulfate Device for the Regeneration of Osteochondral Defects. Tissue Eng. Part A 2019, 25, 352–363.
- Choi, J.H.; Kim, D.K.; Song, J.E.; Oliveira, J.M.; Reis, R.L.; Khang, G. Silk Fibroin-Based Scaffold for Bone Tissue Engineering; Springer: New York, NY, USA, 2018; Volume 1077, pp. 371–387.
- Walsh, D.P.; Raftery, R.M.; Chen, G.; Heise, A.; O’Brien, F.J.; Cryan, S.A. Rapid healing of a critical-sized bone defect using a collagen-hydroxyapatite scaffold to facilitate low dose, combinatorial growth factor delivery. J. Tissue Eng. Regen. Med. 2019, 13, 1843–1853.
- Shen, X.; Zhang, Y.; Gu, Y.; Xu, Y.; Liu, Y.; Li, B.; Chen, L. Sequential and sustained release of SDF-1 and BMP-2 from silk fibroin-nanohydroxyapatite scaffold for the enhancement of bone regeneration. Biomaterials 2016, 106, 205–216.
- Naskar, D.; Ghosh, A.K.; Mandal, M.; Das, P.; Nandi, S.K.; Kundu, S.C. Dual growth factor loaded nonmulberry silk fibroin/carbon nanofiber composite 3D scaffolds for in vitro and in vivo bone regeneration. Biomaterials 2017, 136, 67–85.
- Bhattacharjee, P.; Naskar, D.; Maiti, T.K.; Bhattacharya, D.; Kundu, S.C. Investigating the potential of combined growth factors delivery, from non-mulberry silk fibroin grafted poly(ε-caprolactone)/hydroxyapatite nanofibrous scaffold, in bone tissue engineering. Appl. Mater. Today 2016, 5, 52–67.
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Progr. Polym. Sci. 2012, 37, 106–126.
- Tohamy, K.M.; Mabrouk, M.; Soliman, I.E.; Beherei, H.H.; Aboelnasr, M.A. Novel alginate/hydroxyethyl cellulose/hydroxyapatite composite scaffold for bone regeneration: In vitro cell viability and proliferation of human mesenchymal stem cells. Int. J. Biol. Macromol. 2018, 112, 448–460.
- Hecht, H.; Srebnik, S. Structural characterization of sodium alginate and calcium alginate. Biomacromolecules 2016, 17, 2160–2167.
- Nataraj, D.; Narendra, R. Chemical modifications of alginate and its derivatives. Int. J. Chem. Res. 2019, 1–17.
- Abouzeid, R.E.; Khiari, R.; Beneventi, D.; Dufresne, A. Biomimetic mineralization of three-dimensional printed alginate/TEMPO-oxidized cellulose nanofibril scaffolds for bone tissue engineering. Biomacromolecules 2018, 19, 4442–4452.
- Bayer, E.A.; Jordan, J.; Roy, A.; Gottardi, R.; Fedorchak, M.V.; Kumta, P.N.; Little, S.R. Programmed platelet-derived growth factor-BB and bone morphogenetic protein-2 delivery from a hybrid calcium phosphate/alginate scaffold. Tissue Eng. Part A 2017, 23, 1382–1393.
- Coelho, F.; Cavicchioli, M.; Specian, S.S.; Scarel-Caminaga, R.M.; Penteado, L.D.A.; Medeiros, A.I.D.; Ribeiro, S.J.D.L.; Capote, T.S.D.O. Bacterial cellulose membrane functionalized with hydroxiapatite and anti-bone morphogenetic protein 2: A promising material for bone regeneration. PLoS ONE 2019, 14, e0221286.
- Dutta, S.D.; Patel, D.K.; Lim, K.T. Functional Cellulose-Based Hydrogels as Extracellular Matrices for Tissue Engineering. J. Biol. Eng. 2019, 13, 1–19.
- Zhang, X.; Wang, C.; Liao, M.; Dai, L.; Tang, Y.; Zhang, H.; Coates, P.; Sefat, F.; Zheng, L.; Song, J.; et al. Aligned electrospun cellulose scaffolds coated with rhBMP-2 for both in vitro and in vivo bone tissue engineering. Carbohydr. Polym. 2019, 213.
- Deepthi, S.; Venkatesan, J.; Kim, S.K.; Bumgardner, J.D.; Jayakumar, R. An overview of chitin or chitosan/nano ceramic composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1338–1353.
- Tao, J.; Zhang, Y.; Shen, A.; Yang, Y.; Diao, L.; Wang, L.; Cai, D.; Hu, Y. Injectable chitosan-based thermosensitive hydrogel/nanoparticle-loaded system for local delivery of vancomycin in the treatment of osteomyelitis. Int. J. Nanomed. 2020, 15, 5855–5871.
- Gohil, S.V.; Padmanabhan, A.; Deschamps, J.; Nair, L.S. Chitosan-Based Scaffolds for Growth Factor Delivery. Tissue Eng. Ther. 2017, 2, 175–207.
- Bastami, F.; Paknejad, Z.; Jafari, M.; Salehi, M.; Rezai Rad, M.; Khojasteh, A. Fabrication of a three-dimensional β-tricalcium-phosphate/gelatin containing chitosan-based nanoparticles for sustained release of bone morphogenetic protein-2: Implication for bone tissue engineering. Mater. Sci. Eng. C 2017, 72, 481–491.
- Xia, Y.J.; Xia, H.; Chen, L.; Ying, Q.S.; Yu, X.; Li, L.H.; Wang, J.H.; Zhang, Y. Efficient delivery of recombinant human bone morphogenetic protein (rhBMP-2) with dextran sulfate-chitosan microspheres. Exp. Ther. Med. 2018, 15, 3265–3272.
- Celikkin, N.; Rinoldi, C.; Costantini, M.; Trombetta, M.; Rainer, A.; Święszkowski, W. Naturally Derived Proteins and Glycosaminoglycan Scaffolds for Tissue Engineering Applications. Mater. Sci. Eng. C 2017, 78, 1277–1299.
- Hachim, D.; Whittaker, T.E.; Kim, H.; Stevens, M.M. Glycosaminoglycan-based biomaterials for growth factor and cytokine delivery: Making the right choices. J. Control. Release 2019, 313, 131–147.
- Dinoro, J.; Maher, M.; Talebian, S.; Jafarkhani, M.; Mehrali, M.; Orive, G.; Foroughi, J.; Lord, M.S.; Dolatshahi-Pirouz, A. Sulfated Polysaccharide-Based Scaffolds for Orthopaedic Tissue Engineering. Biomaterials 2019, 214, 119214.
- Thanyaphoo, S.; Kaewsrichan, J. A new biocompatible delivery scaffold containing heparin and bone morphogenetic protein 2. Acta Pharm. 2016, 66, 373–385.
- Hettiaratchi, M.H.; Krishnan, L.; Rouse, T.; Chou, C.; McDevitt, T.C.; Guldberg, R.E. Heparin-mediated delivery of bone morphogenetic protein-2 improves spatial localization of bone regeneration. Sci. Adv. 2020, 6, eaay1240.
- Ma, C.; Jing, Y.; Sun, H.; Liu, X. Hierarchical nanofibrous microspheres with controlled growth factor delivery for bone regeneration. Adv. Healthc. Mater. 2015, 4, 2699–2708.
- Liu, Y.; Gu, J.; Fan, D. Fabrication of high-strength and porous hybrid scaffolds based on nano-hydroxyapatite and human-like collagen for bone tissue regeneration. Polymers 2020, 12, 61.
- Andrews, S.; Cheng, A.; Stevens, H.; Logun, M.T.; Webb, R.; Jordan, E.; Xia, B.; Karumbaiah, L.; Guldberg, R.E.; Stice, S. Chondroitin sulfate glycosaminoglycan scaffolds for cell and recombinant protein-based bone regeneration. Stem Cells Transl. Med. 2019, 8, 575–585.
- Fenbo, M.; Sijing, L.; Ruiz-Ortega, L.I.; Yuanjun, Z.; Lei, X.; Kui, W.; Lijun, L.; Bin, T. Effects of alginate/chondroitin sulfate-based hydrogels on bone defects healing. Mater. Sci. Eng. C 2020, 116, 111217.
- Caterson, B.; Melrose, J. Keratan Sulfate, a Complex Glycosaminoglycan with Unique Functional Capability. Glycobiology 2018, 28, 182–206.
- Li, M.; Zhang, X.; Jia, W.; Wang, Q.; Liu, Y.; Wang, X.; Wang, C.; Jiang, J.; Gu, G.; Guo, Z.; et al. Improving in vitro biocompatibility on biomimetic mineralized collagen bone materials modified with hyaluronic acid oligosaccharide. Mater. Sci. Eng. C 2019, 104, 110008.
- Makvandi, P.; Ali, G.W.; Della Sala, F.; Abdel-Fattah, W.I.; Borzacchiello, A. Hyaluronic acid/corn silk extract based injectable nanocomposite: A biomimetic antibacterial scaffold for bone tissue regeneration. Mater. Sci. Eng. C 2020, 107, 110195.
