Malaria is an infectious disease caused by protozoan parasites of the Plasmodium genus through the bite of female Anopheles mosquitoes, affecting 228 million people and causing 415 thousand deaths in 2018. Artemisinin-based combination therapies (ACTs) are the most recommended treatment for malaria; however, the emergence of multidrug resistance has unfortunately limited their effects and challenged the field.
- Plasmodium
- malaria
- sponge
- resistance
- antimalarial
1. Introduction
Human malaria is an infectious disease caused by single-celled protozoan parasites of the Plasmodium genus (P. falciparum, P. vivax, P. ovale, P. malariae, and P. knowlesi) through the bite of female Anopheles mosquitoes [1]. It affected 228 million people in 2018, and nearly half of the world’s population is still at risk for this disease [2]. Symptoms can range from being mild to very severe, causing chronic illness, physical disability, death and a huge health burden, especially to the most vulnerable populations.
Antimalarials based in quinolines scaffolds (i.e., chloroquine, mefloquine, amodiaquine, and piperaquine) possess a complex mechanism of action. One well-studied mechanism involves compromising the detoxification of hemoglobin degradation with heme polymerization for hemozoin crystal formation in digestive vacuole by protonated forms of quinolones [3]. It was noted that some strains of P. falciparum triggered resistance to protonated drugs due to a genetic mutation in the transporter (PfCRT) and could lead to antimalarial drug extrusion from the organelle [3].
Artemisinin-based combination therapies (ACTs) are the most recommended treatment for uncomplicated P. falciparum malaria, while artesunate is considered the most effective antimalarial drug for severe cases [4], with several biochemical processes reported as targets in parasite cells [3,5]. Despite the safety and efficiency that have been proven for the use of these drugs, the emergence of multidrug resistance has unfortunately limited their effects and challenged the field [6]. The resistance to ACTs is already spreading from Southeast Asia, as reported in 2008 [7], giving rise to a danger alert to other high-poverty regions in the world, and the identified resistance phenotype is associated with mutation of kelch domain protein gene (k13), which is postulated to be involved in protein trafficking organelles in the parasite during intraerythrocytic cycle [8], [3].
In this context, the ocean, with its rich biodiversity, has been emerging as a very promising resource of bioactive compounds and secondary metabolites from different marine organisms (bacteria, fungi, micro-algae, mollusks and other invertebrates) with multiple pharmacological properties [9,10,11]. Among them, the phylum Porifera (sponges) is the most promising for providing raw material for the development of biotechnological products for multiple human health problems [12,13,14]. Marine sponges are very primitive sessile animals with origins dated at least from the late Proterozoic over 580 million years ago [15]. Being considered representatives of the first multicellular animals, these filter-feeding organisms evolutionarily developed morphological and chemical defense mechanisms constituted mainly by secondary metabolites, compounds with a wide range of effects such as antitumor, antiviral, anti-inflammatory and antibiotic effects, which have been investigated for the treatment of human health problems [15,16]. Additionally, some authors have demonstrated the antimalarial effects of the secondary metabolites of marine sponges and have shown that these components present inhibitory activity against the malaria parasite Plasmodium falciparum [6,17].
2. Results and Discussion
2.1. Study Selection and Analysis
The flow diagram (Figure 1) demonstrated the search strategy (identification, inclusion and exclusion) used in the present study. A total of 77 articles were retrieved from the databases (PubMed, Web of Science and Scopus). Then, the duplicated records were excluded (n = 14). Thus, 66 full-text articles were assessed for eligibility, and 30 studies were excluded for different reasons, such as the following: some studies reported only the extraction of compounds and did not report the antiplasmodial activity; others described only the mechanism of the compounds; some studies were only computational. Finally, 36 studies were included and analyzed in this systematic review (Figure 1).
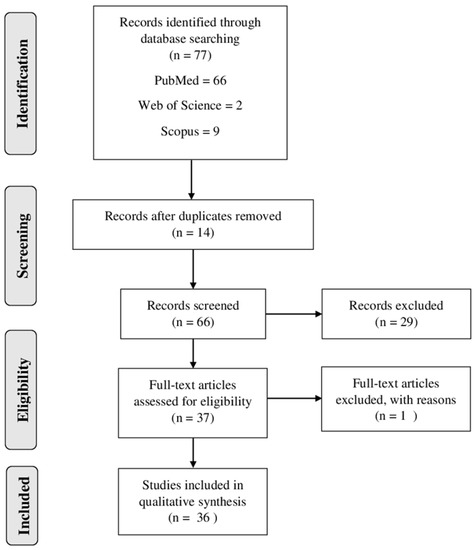
Figure 1. Flow diagram of literature search and selection criteria used in the present review adapted from PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis).
A summary of the studies is presented in Table 1. The articles analyzed were published from 1992 to 2019 in different countries. The antimalarial activity was assessed in vitro using Plasmodium falciparum culture [20] for quantification of cell viability over 24-96 h. For the in vitro assays, different lab strains were used (such as 3D7, W2, DD2, NF54), and a wide variety of methods were used for assessing P. falciparum viability ([3H] hypoxanthine, LDH, Microscopy, SYBR Green) presenting as IC50 values instead of the option of XC50. The Demospongiae sponge class was the most explored, where 30 studies evaluated their antiplasmodial activity. Among the genera in Table 1, most belong to the Demospongiae class except for Plakortis (Plakortis simplexs, Plakortis lita, Plakortis halichondrioides), which is from the Homoscleromorpha class. In addition, a great geographical variety was observed, which shows that sponges from different regions of the globe have this potential antiplasmodial activity. The inhibitory concentration for 50% of the parasites (IC50) varied from low micromolar to low nanomolar range, and the species Xestospongia sp showed the best bioactive potential, from which the compound Saringosterol was extracted, which had an IC50 of 0.25 nM. The individual IC50 for each extracted compound is reported in Table 1. The IC50 value units in µg/mL and ng/mL were converted to µM and nM for data comparison, and then some of compounds in Table 1, which IC50 was below 10 µg/mL became higher than 10 µM (see Section 3.2.2, exclusion criteria), as were compounds 10 and 11 [21], 52 [22], 2 and 3 [23], 99 [24].
Table 1. Summary of descriptions of characteristics of included articles.
| Author | Sponge Genus | Material Collection Location | Extracted Material (P. falciparum Strain and IC50 Value) |
|---|---|---|---|
| Campos et al., (2019) [21] | Fascaplysinopsis reticulata | Mayotte (Indian Ocean) | 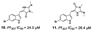 |
| Jeong., et al. (2019) [25] | Coscinoderma sp. | Chuuk Island, Federated States of Micronesia | 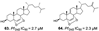 |
| Ju et al., (2019) [25] | Hyrtios erectus | Chuuk Island, Federated States of Micronesia |  |
| Murtihapsari. et al., (2019) [26] | Xestospongia sp | Kaimana, West Papua, Indonesia | 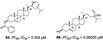 |
| Parra et al., (2018) [27] | Tedania Brasiliensis | Brazil |  |
| Campos et al., (2017) [28] | Monanchora unguiculata | Mitsio islands, Madagascar |  |
| Yang et al., (2016) [29] |
Diacarnus megaspinorhabdosa | SouthChina Sea Sponge |  |
| Gros et al., (2015) [30] |
Biemna laboutei | Madagascar | 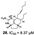 |
| Chianese et al., (2014) [31] | Plakortis simplexs | South China Sea | 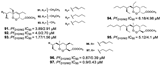 |
| Gros et al., (2014) [32] | Biemna laboutei | Madagascar at Salary Ba |  |
| Yang et al., (2014) [17] | Diacarnus megaspinorhabdosa | South China Sea |  |
| Alvarado et al., (2013) [33] |
Spongosorites sp | Not reported | 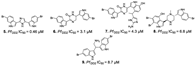 |
| Davis et al., (2013) [34] |
Plakortis lita | Not reported | 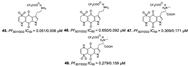 |
| Farokhi et al., (2013) [35] |
Axinyssa djiferi | Senegalese coasts |  |
| Sirirak et al., (2013) [36] |
Pachastrissa nuxs | Thailand | 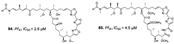 |
| Chanthathamrongsiri et al., (2012) [37] |
Stylissacf. massa | Not reported | 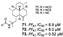 |
| Davis et al., (2012) [38] |
Zyzzya sp | Not reported |  |
| Ilias et al., (2012) [39] |
Petrosia | Eastern Fields north of Australia | 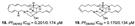 |
| Mudianta et al., (2012) [40] |
Aplysinella strongylata | Tulamben, Bali, Indonesia |  |
| El Sayed et al., (2011) [22] | Diacarnus erythraeanus | Red Sea |  |
| Galeano et al., (2011) [41] |
Verongula rigida | Urabá Gulf is located in the Southwestern Caribbean |  |
| Sirirak et al., (2011) [42] |
Pachastrissa nux | Koh-Tao, Surat-Thani ProvinceChumphon IslandsNational Park, Chumphon Province, |  |
| Xu et al., (2011) [43] |
Pseudoceratina sp | Australian biota |  |
| Jiménez-Romero et al., (2010) [44] |
Plakortis halichondrioides | Puerto Rico | 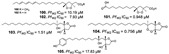 |
| Samoylenko et al., (2009) [45] |
Acanthostrongylophora ingens | Pacific |  |
| Ueoka et al., (2009) [46] |
Agelas gracilis | southern Japan |  |
| Wright et al., (2009) [47] |
Cymbastela hooperi | Not reported |  |
| Appenzeller et al., (2008) [48] |
Agelas cf. mauritiana | Solomon Islands |  |
| Desoubzdanne et al., (2008) [49] |
New Caledonian | Norfolk Rise (New Caledonia) |  |
| Tasdemir et al., (2007) [23] | Agelas oroides | Northern Aegean Sea, Turkey | - fractions: fatty acid mixtures FAME (3.4 μg/mL) and FAMF (8.7 μg/mL)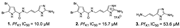 |
| Laurent et al., (2006) [50] | Xestospongia | Vanuatu |  |
| Mancini et al., (2004) [51] | Oceanapia fistulosa | New Caledonia Main Island | -crude mixture (0.98 μM) -N-methyl derivatives from the crude mixture (8 μM) |
| Fattorusso et al., (2002) [52] | Plakortis simplex | Berry Island (Bahamas) |  |
| Gochfeld et al., (2001) [24] | Plakortis sp. | Jamaica |  |
| Kirsch et al., (2000) [53] | Hyrtios cf. erecta | Fiji |  |
| Angerhofer et al., (1992) [54] | Acanthella klethra | Australia |  |
To assess the study quality, we used the GRADE method [55]. The 36 studies analyzed were categorized as moderate quality (17) because (i) there were no controls in the experiments; (ii) the toxicity of the compounds was not assessed in parallel, which made it impossible to determine the selectivity of compounds; (iii) all compounds analyzed presented a high cytotoxicity, which demonstrates the unspecified use against P. falciparum; (iv) the methods used to measure the antiplasmodial activity were not described. A total of 19 studies were classified as being of high quality (Table S1).
After this detailed review of the articles reporting the activity of compounds from marine sponges, we made a brief survey of data in the literature to compare the number of articles published reporting the activity of marine organisms with the number of articles published reporting the activity of extracts from plants. To do so, the following combinations of keywords were used: "new antimalarials and plants" or "new antimalarials and marine" and selected the works published in the last 10 years. Figure 2 represents the number of studies reporting antiplasmodial activity of new compounds found. The search for new compounds from marine sources is still uncommon compared to the search for natural products from plants. Other recent reviews have also reported this comparison, which reinforces the importance of seeking new products from marine sources, especially considering that the diverse nature of metabolites produced by these alternative sources presents a compelling case for intensive exploration [56].
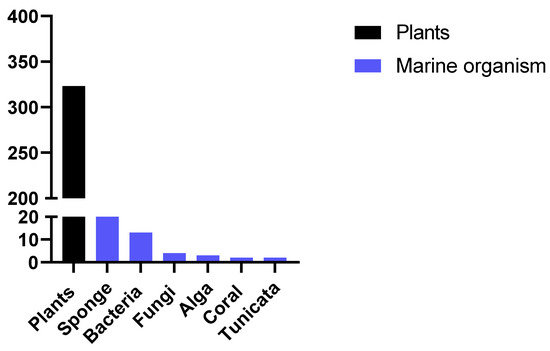
Figure 2. Number of published papers reporting the antimalarial activity of new compounds from marine sources or plants in the past 10 years.
This entry is adapted from the peer-reviewed paper 10.3390/md19030134
