The CD137 receptor is expressed by activated antigen-specific T-cells. CD137+ T-cells were identified inside TILs and PBMCs of different tumor types and have proven to be the naturally occurring antitumor effector cells, capable of expressing a wide variability in terms of TCR specificity against both shared and neoantigenic tumor-derived peptides. The aim of this review is thus summarizing and highlighting their role as drivers of patients’ immune responses in anticancer therapies as well as their potential role in future and current strategies of immunotherapy.
- CD137+ T-Cells
1. Introduction
Immunotherapy aims to re-educate the patient’s immune system to recognize and fight cancer cells. The existence of T-cells with a potential antitumor effect has laid the foundation for most of the current approaches of immunotherapy. In fact, the use of therapies such as immune checkpoint inhibitors (ICIs), DC vaccines, and adoptive T-cell transfer (ACT) finally relies on the presence of a population of effector T-cells that is capable of killing tumor cells. These immune-based drugs thus aim to unleash this population from different regulatory constraints such as T-cell exhaustion or the impossibility of reaching cancer cells, to subsequently limit tumor growth and progression. As a confirmation, the accumulation of tumor-infiltrating lymphocytes (TILs) correlates with a better clinical outcome and an improved survival in most tumor models [1][2][3][4][5][6][7][8][9][10][11], indicating their importance in predicting patients response to anticancer therapies. Nevertheless, the composition of TILs is heterogeneous [12] and it still remains challenging to identify the real population of naturally occurring antitumor T-cells [13]. Therefore, this review will discuss the emerging role of the CD137+ T-cells population as the main effector population activated against cancer cells with all the possible implications for the future of immunotherapy.
2. CD137: The Receptor
3. CD137+ T-Cells: The Natural Tumor-Specific Population
The discovery that CD137 is expressed by most of activated and antigen-specific (both against viral and tumor antigens) CD8+ T-cells, allowed the isolation of tumor-specific effector T-cells from blood, without knowing the immunogenic epitopes or the MHC-restriction complex. These cells, even if present at low frequencies, were able to kill antigen-expressing cancer cells upon expansion, although this required an ex vivo restimulation with the defined tumor antigen [60][61].
This evidence raised a strong interest in investigating this cell repertoire also inside the tumor. In fact, the tumor microenvironment (TME) is enriched for T-cells specific for defined antigens with cytolytic ability against cancer cells [62]. In addition, even if defined antigens are known for different tumor models, exomic sequencing data in different solid tumors proved that cancer cells express a various and heterogeneous set of mutated neo-antigens that are characteristic for every single patient and thus can be recognized by TILs that are able to exert an antitumor response [63]. As confirmation, T-cell receptors (TCRs) isolated from CD137+ TILs, showed a reactivity against various mutations of tumor-derived antigens [64]. Given this evidence, the possibility of identifying a tumor-specific T effector population inside the TME without the knowledge of the antigen epitopes seemed very promising.
Initial evidence proved that CD137 is strongly expressed by TILs if compared to spleen- or lymph nodes-derived T-cells and its expression is induced by hypoxia through hypoxia-inducible factor 1α [65].
Recently, Ye et al. decided to investigate the CD137+ T-cells population in ovarian cancer patients, comparing three different locations in which this subset of cells could be found: TME, ascites, and peripheral blood [66]. They demonstrated that CD137+ T-cells are present in small percentages in the peripheral blood and, to a larger extent, in ascites and even more inside the tumor, showing a progressive hierarchy with the T-cells in a closer proximity to cancer cells expressing the higher percentages of CD137 and then decreasing gradually toward the periphery. Overnight incubation with autologous cancer cells largely increased the percentage of CD137+ T-cells and their ability of producing a consistent amount of IFN-γ. Additionally, CD137 expression was further increased when T-cell lines with a known antigen specificity were used. Most importantly, when human TILs and tumor cells where transferred into immunodeficient mice, only CD137+ T-cells (but not CD137− T-cells) were able to inhibit tumor growth [66]. Thus, they demonstrated that CD137+ T-cells are those cells that naturally show the real antitumor reactivity, confirming also that they represent a subset of newly recruited antitumor T-effector cells, being CD137 expression a rapid and transient event upon specific activation. Overall, this study proposed a novel method to isolate and expand tumor reactive TILs that can be used for adoptive T-cell transfer approaches; the vast heterogeneity of TCRs is indeed conserved with this strategy thus helping to prevent the escape of those tumor cells that do not express a determined antigen or those that express mutated antigens.
These findings suggested the potential role of CD137+ T-cells as key contributors of the antitumor immune responses and thus as potential determiners of the success of immunotherapies as well as novel protagonists of immune-based approaches (Figure 1).
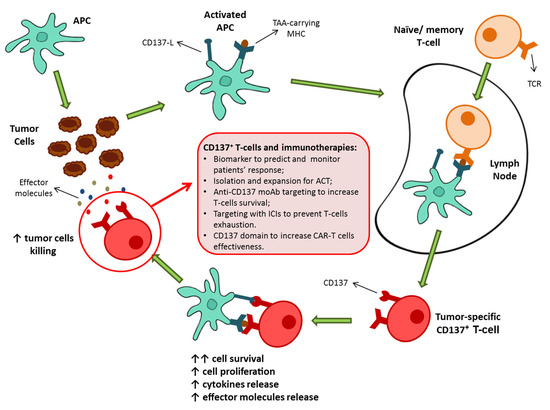
Despite these clear results showing the importance of the CD137+ T-cell population in eliciting an antitumor response, evidence about the role of these T-cells in oncologic patients have only recently emerged (Table 1).
| Cancer Type | Treatment | Results | References |
|---|---|---|---|
| Metastatic NSCLC | Anti-PD-1 ICIs | Higher percentages of CD137+ T-cells in PBMC predicted a prolonged patients’ OS and PFS. | [67,68] Ugolini et al., 2020 |
| Metastatic RCCC | Anti-VEGF-R TKIs and anti-PD-1 ICIS | Percentage of CD137+ T-cells in PBMC decreased during patients’ progression. | [69] Zizzari et al., 2018 |
| Metastatic RCCC | TKIs | Higher percentages of CD137+ T-cells in PBMC were associated with responder patients. | [70] Zizzari et al., 2020 |
| Metastatic Melanoma | Anti-PD-1 ICIS | CD137 mRNA levels at the tumor site were positively associated with a prolonged OS, PFS, and a better response to the therapy. | [71] Fröhlich et al., 2020 |
In 2020, for the first time we provided evidence about the importance of CD137+ T-cells in determining the outcome of metastatic non-small cells lung cancer (NSCLC) patients undergoing immunotherapies [67][68]. Patients that were positive for the autoantibody IgM-Rheumatoid Factor (IgM-RF) showed indeed a reduced frequency of CD137+ T-cells in peripheral blood and an increased tendency to develop an early progression, in addition to a markedly reduced progression-free survival (PFS) and overall survival (OS) after the anti-PD-1 treatment [68]. In addition, to confirm the importance of this population as an independent prognostic factor, it was reported how a higher percentage of CD137+ T-cells in peripheral blood mononuclear cells (PBMC) at baseline, was alone associated with a prolonged OS as well as PFS of patients in treatment with an anti-PD-1 ICI [68].
In addition, in 2018 it was proven that, in metastatic renal clear cell carcinoma (mRCCC) patients undergoing the anti-PD-1 treatment, the percentage of CD137+ T-cells decreased during tumor progression [69]. Moreover, patients pretreated with Tyrosin-kinase inhibitor Pazopanib, showed a robust increase in DC activation profile and a subsequent increase of the frequency of CD137+ T-cells when compared to Sunitinib [69]. Still in mRCCC, Zizzari et al. demonstrated that CD137+ T-cells were positively associated with patients response to TKI [70]. In fact, responder patients showed a markedly higher percentage of this T-cell subset when compared to non-responders. These results highlight the importance of this T-cell subset in oncologic patients response to therapies that require, even if in an indirect way, the immune system’s ability of killing tumor cells. In this scenario, the percentage of this population in peripheral blood (and most likely also in other districts as draining lymph nodes and TME) could serve as a possible biomarker able to identify those patients that would benefit the most from a determinate treatment that relies on T-cells as final effectors.
Finally, in 2020, indirect evidence of the CD137+ T-cells power in determining a prolonged survival for cancer patients came from a study on melanoma patients where it was shown that TNFRSF9 low methylation levels and the subsequent increased mRNA expression at the tumor site, that was prevalently identified inside T-cells, correlated with a better OS of patients as well as a better PFS and response to the anti-PD-1 treatment [71]. TNFRSF9 mRNA expression positively correlated also with the frequency of effector and memory tumor infiltrating lymphocytes, while it was inversely correlated with the frequency of naïve tumor infiltrating lymphocytes [71]. As a confirmation of its power as biomarker for the identification of activated effector T-cells, TNFRSF9 mRNA expression levels positively correlated with an increased IFN-γ signature [71].
These results indicate the potential role of this population as the driver of a successful immunotherapy, thus suggesting the possibility of investigating its presence in patients before undergoing immune-based treatments. In fact, a reduction in its frequency could account for the impossibility of getting a complete or even partial response at least in part of the oncologic patients. In this scenario, strategies aimed at increasing their numbers could be considered at an initial stage, in order to make the patient more prone to efficiently receive an immunotherapeutic treatment.
This entry is adapted from the peer-reviewed paper 10.3390/cancers13030456
References
- Schalper, K.A.; Brown, J.; Carvajal-Hausdorf, D.; McLaughlin, J.; Velcheti, V.; Syrigos, K.N.; Herbst, R.S.; Rimm, D.L. Objective Measurement and Clinical Significance of TILs in Non–Small Cell Lung Cancer. J. Natl. Cancer Inst. 2015, 107.
- Boxberg, M.; Leising, L.; Steiger, K.; Jesinghaus, M.; Alkhamas, A.; Mielke, M.; Pfarr, N.; Götz, C.; Wolff, K.D.; Weichert, W.; et al. Composition and Clinical Impact of the Immunologic Tumor Microenvironment in Oral Squamous Cell Carcinoma. J. Immunol. 2019, 202, 278–291.
- Zhang, L.; Conejo-Garcia, J.R.; Katsaros, D.; Gimotty, P.A.; Massobrio, M.; Regnani, G.; Makrigiannakis, A.; Gray, H.; Schlienger, K.; Liebman, M.N.; et al. Intratumoral T Cells, Recurrence, and Survival in Epithelial Ovarian Cancer. N. Engl. J. Med. 2003, 348, 203–213.
- Galon, J.; Costes, A.; Sanchez-Cabo, F.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Pagès, C.; Tosolini, M.; Camus, M.; Berger, A.; Wind, P.; et al. Type, Density, and Location of Immune Cells Within Human Colorectal Tumors Predict Clinical Outcome. Science 2006, 313, 1960–1964.
- Pagès, F.; Galon, J.; Dieu-Nosjean, M.-C.; Tartour, E.; Sautès-Fridman, C.; Fridman, W.-H. Immune infiltration in human tumors: A prognostic factor that should not be ignored. Oncogene 2009, 29, 1093–1102.
- Erdag, G.; Schaefer, J.T.; Smolkin, M.E.; Deacon, D.H.; Shea, S.M.; Dengel, L.T.; Patterson, J.W.; Slingluff, C.L. Immunotype and Immunohistologic Characteristics of Tumor-Infiltrating Immune Cells Are Associated with Clinical Outcome in Metastatic Melanoma. Cancer Res. 2012, 72, 1070–1080.
- Savas, P.P.; Salgado, R.; Denkert, C.; Sotiriou, C.; Darcy, P.K.P.; Smyth, M.J.M.; Loi, S. Clinical relevance of host immunity in breast cancer: From TILs to the clinic. Nat. Rev. Clin. Oncol. 2016, 13, 228–241.
- Santoiemma, P.P.; Powell, D.J. Tumor infiltrating lymphocytes in ovarian cancer. Cancer Biol. Ther. 2015, 16, 807–820.
- Zheng, C.; Zheng, L.; Yoo, J.-K.; Guo, H.; Zhang, Y.; Guo, X.; Kang, B.; Hu, R.; Huang, J.Y.; Zhang, Q.; et al. Landscape of Infiltrating T Cells in Liver Cancer Revealed by Single-Cell Sequencing. Cell 2017, 169, 1342–1356.e16.
- Loi, S.M.; Sirtaine, N.; Piette, F.; Salgado, R.; Viale, G.; Van Eenoo, F.; Rouas, G.; Francis, P.; Crown, J.P.; Hitre, E.; et al. Prognostic and Predictive Value of Tumor-Infiltrating Lymphocytes in a Phase III Randomized Adjuvant Breast Cancer Trial in Node-Positive Breast Cancer Comparing the Addition of Docetaxel to Doxorubicin with Doxorubicin-Based Chemotherapy: BIG 02-98. J. Clin. Oncol. 2013, 31, 860–867.
- Clemente, C.G.; Mihm, M.C.; Bufalino, R.; Zurrida, S.; Collini, P.; Cascinelli, N. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer 1996, 77, 1303–1310.
- Yu, P.; Fu, Y.-X. Tumor-infiltrating T lymphocytes: Friends or foes? Lab. Investig. 2006, 86, 231–245.
- Linette, G.P.; Carreno, B.M. Tumor-Infiltrating Lymphocytes in the Checkpoint Inhibitor Era. Curr. Hematol. Malign. Rep. 2019, 14, 286–291.
- Vinay, D.S.; Kwon, B.S. Role of 4-1BB in immune responses. Semin. Immunol. 1998, 10, 481–489.
- Cannons, J.L.; Lau, P.; Ghumman, B.; Debenedette, M.A.; Yagita, H.; Okumura, K.; Watts, T.H. 4-1BB Ligand Induces Cell Division, Sustains Survival, and Enhances Effector Function of CD4 and CD8 T Cells with Similar Efficacy. J. Immunol. 2001, 167, 1313–1324.
- Kwon, B.S.; Weissman, S.M. cDNA sequences of two inducible T-cell genes. Proc. Natl. Acad. Sci. USA 1989, 86, 1963–1967.
- Gramaglia, I.; Cooper, D.; Miner, K.T.; Kwon, B.S.; Croft, M. Co-stimulation of antigen-specific CD4 T cells by 4-1BB ligand. Eur. J. Immunol. 2000, 30, 392–402.
- Takahashi, C.; Mittler, R.S.; Vella, A.T. Cutting edge: 4-1BB is a bona fide CD8 T cell survival signal. J. Immunol. 1999, 162, 5037–5040.
- Dawicki, W.; Watts, T.H. Expression and function of 4-1BB during CD4 versus CD8 T cell responses In Vivo. Eur. J. Immunol. 2004, 34, 743–751.
- Futagawa, T.; Akiba, H.; Kodama, T.; Takeda, K.; Hosoda, Y.; Yagita, H.; Okumura, K. Expression and function of 4-1BB and 4-1BB ligand on murine dendritic cells. Int. Immunol. 2002, 14, 275–286.
- Taraban, V.Y.; Rowley, T.F.; O’Brien, L.; Chan, H.T.C.; Haswell, L.E.; Green, M.H.A.; Tutt, A.L.; Glennie, M.J.; Al-Shamkhani, A. Expression and costimulatory effects of the TNF receptor superfamily members CD134 (OX40) and CD137 (4-1BB), and their role in the generation of anti-tumor immune responses. Eur. J. Immunol. 2002, 32, 3617–3627.
- Wen, T.; Bukczynski, J.; Watts, T.H. 4-1BB Ligand-Mediated Costimulation of Human T Cells Induces CD4 and CD8 T Cell Expansion, Cytokine Production, and the Development of Cytolytic Effector Function. J. Immunol. 2002, 168, 4897–4906.
- Watts, T.H. TNF/TNFR family members in costimulation of t cell responses. Annu. Rev. Immunol. 2005, 23, 23–68.
- Diehl, L.; Van Mierlo, G.J.D.; Boer, A.T.D.; Van Der Voort, E.; Fransen, M.; Van Bostelen, L.; Krimpenfort, P.; Melief, C.J.M.; Mittler, R.; Toes, R.E.M.; et al. In Vivo Triggering Through 4-1BB Enables Th-Independent Priming of CTL in the Presence of an Intact CD28 Costimulatory Pathway. J. Immunol. 2002, 168, 3755–3762.
- Goodwin, R.G.; Din, W.S.; Davis-Smith, T.; Anderson, D.M.; Gimpel, S.D.; Sato, T.A.; Maliszewski, C.R.; Brannan, C.I.; Copeland, N.G.; Jenkins, N.A.; et al. Molecular cloning of a ligand for the inducible T cell gene 4-1BB: A member of an emerging family of cytokines with homology to tumor necrosis factor. Eur. J. Immunol. 1993, 23, 2631–2641.
- Summers, K.L.; Hock, B.D.; McKenzie, J.L.; Hart, D.N.J. Phenotypic Characterization of Five Dendritic Cell Subsets in Human Tonsils. Am. J. Pathol. 2001, 159, 285–295.
- Zapata, J.M.; Perez-Chacon, G.; Carr-Baena, P.; Martinez-Forero, I.; Azpilikueta, A.; Otano, I.; Melero, I. CD137 (4-1BB) Signalosome: Complexity Is a Matter of TRAFs. Front. Immunol. 2018, 9, 2618.
- Arch, R.H.; Thompson, C.B. 4-1BB and Ox40 Are Members of a Tumor Necrosis Factor (TNF)-Nerve Growth Factor Receptor Subfamily That Bind TNF Receptor-Associated Factors and Activate Nuclear Factor κB. Mol. Cell. Biol. 1998, 18, 558–565.
- Nam, K.-O.; Kang, H.; Shin, S.-M.; Cho, K.-H.; Kwon, B.; Kwon, B.S.; Kim, S.-J.; Lee, H.-W. Cross-Linking of 4-1BB Activates TCR-Signaling Pathways in CD8+ T Lymphocytes. J. Immunol. 2005, 174, 1898–1905.
- Cannons, J.L.; Hoeflich, K.P.; Woodgett, J.R.; Watts, T.H. Role of the stress kinase pathway in signaling via the T cell costimulatory receptor 4-1BB. J. Immunol. 1999, 163, 2990–2998.
- Saoulli, K.; Lee, S.Y.; Cannons, J.L.; Yeh, W.C.; Santana, A.; Goldstein, M.D.; Bangia, N.; Debenedette, M.A.; Mak, T.W.; Choi, Y.; et al. CD28-independent, TRAF2-dependent Costimulation of Resting T Cells by 4-1BB Ligand. J. Exp. Med. 1998, 187, 1849–1862.
- Halstead, E.S.; Mueller, Y.; Altman, J.D.; Katsikis, P.D. In Vivo stimulation of CD137 broadens primary antiviral CD8+ T cell responses. Nat. Immunol. 2002, 3, 536–541.
- Bertram, E.M.; Lau, P.; Watts, T.H. Temporal Segregation of 4-1BB Versus CD28-Mediated Costimulation: 4-1BB Ligand Influences T Cell Numbers Late in the Primary Response and Regulates the Size of the T Cell Memory Response Following Influenza Infection. J. Immunol. 2002, 168, 3777–3785.
- Maus, M.V.; Thomas, A.K.; Leonard, D.G.; Allman, D.; Addya, K.; Schlienger, K.; Riley, J.L.; June, C.H. Ex Vivo expansion of polyclonal and antigen-specific cytotoxic T lymphocytes by artificial APCs expressing ligands for the T-cell receptor, CD28 and 4-1BB. Nat. Biotechnol. 2002, 20, 143–148.
- Bukczynski, J.; Wen, T.; Watts, T.H. Costimulation of human CD28 T cells by 4-1BB ligand. Eur. J. Immunol. 2003, 33, 446–454.
- Bukczynski, J.; Wen, T.; Ellefsen, K.; Gauldie, J.; Watts, T.H. Costimulatory ligand 4-1BBL (CD137L) as an efficient adjuvant for human antiviral cytotoxic T cell responses. Proc. Natl. Acad. Sci. USA 2004, 101, 1291–1296.
- Hurtado, J.C.; Kim, Y.J.; Kwon, B.S. Signals through 4-1BB are costimulatory to previously activated splenic T cells and inhibit activation-induced cell death. J. Immunol. 1997, 158, 2600–2609.
- May, K.F.; Chen, L.; Zheng, P.; Liu, Y. Anti-4-1BB monoclonal antibody enhances rejection of large tumor burden by promoting survival but not clonal expansion of tumor-specific CD8+ T cells. Cancer Res. 2002, 62, 3459–3465.
- Lee, H.-W.; Park, S.-J.; Choi, B.K.; Kim, H.H.; Nam, K.-O.; Kwon, B.S. 4-1BB Promotes the Survival of CD8+T Lymphocytes by Increasing Expression of Bcl-xLand Bfl-1. J. Immunol. 2002, 169, 4882–4888.
- Menk, A.V.; Scharping, N.E.; Rivadeneira, D.B.; Calderon, M.J.; Watson, M.J.; Dunstane, D.; Watkins, S.C.; Delgoffe, G.M. 4-1BB costimulation induces T cell mitochondrial function and biogenesis enabling cancer immunotherapeutic responses. J. Exp. Med. 2018, 215, 1091–1100.
- Teijeira, A.; Labiano, S.; Garasa, S.; Etxeberria, I.; Santamaría, E.; Rouzaut, A.; Enamorado, M.; Azpilikueta, A.; Inogés, S.; Bolaños, E.; et al. Mitochondrial Morphological and Functional Reprogramming Following CD137 (4-1BB) Costimulation. Cancer Immunol. Res. 2018, 6, 798–811.
- Aznar, M.A.; Labiano, S.; Diaz-Lagares, A.; Molina, C.; Garasa, S.; Azpilikueta, A.; Etxeberria, I.; Sánchez-Paulete, A.R.; Korman, A.J.; Esteller, M.; et al. CD137 (4-1BB) Costimulation Modifies DNA Methylation in CD8+ T Cell–Relevant Genes. Cancer Immunol. Res. 2018, 6, 69–78.
- Freeman, Z.T.; Nirschl, T.R.; Hovelson, D.H.; Johnston, R.J.; Engelhardt, J.J.; Selby, M.J.; Kochel, C.M.; Lan, R.Y.; Zhai, J.; Ghasemzadeh, A.; et al. A conserved intratumoral regulatory T cell signature identifies 4-1BB as a pan-cancer target. J. Clin. Investig. 2020, 130, 1405–1416.
- Etxeberria, I.; Glez-Vaz, J.; Teijeira, Á.; Melero, I. New emerging targets in cancer immunotherapy: CD137/4-1BB costimulatory axis. ESMO Open 2019, 4 (Suppl. 3), e000733.
- Debenedette, M.A.; Wen, T.; Bachmann, M.F.; Ohashi, P.S.; Barber, B.H.; Stocking, K.L.; Peschon, J.J.; Watts, T.H. Cell Response to Influenza Virus. J. Immunol. 1999, 163, 4833–4841.
- Tan, J.T.; Whitmire, J.K.; Ahmed, R.; Pearson, T.C.; Larsen, C.P. 4-1BB ligand, a member of the TNF family, is important for the generation of antiviral CD8 T cell responses. J. Immunol. 1999, 163, 4859–4868.
- Kwon, B.S.; Hurtado, J.C.; Lee, Z.H.; Kwack, K.B.; Seo, S.K.; Choi, B.K.; Koller, B.H.; Wolisi, G.; Broxmeyer, H.E.; Vinay, D.S. Immune Responses in 4-1BB (CD137)-Deficient Mice. J. Immunol. 2002, 168, 5483–5490.
- Melero, I.; Bach, N.; Hellström, K.E.; Aruffo, A.; Mittler, R.S.; Chen, L. Amplification of tumor immunity by gene transfer of the co-stimulatory 4-1BB ligand: Synergy with the CD28 co-stimulatory pathway. Eur. J. Immunol. 1998, 28, 1116–1121.
- Wiethe, C.; Dittmar, K.; Doan, T.; Lindenmaier, W.; Tindle, R. Enhanced Effector and Memory CTL Responses Generated by Incorporation of Receptor Activator of NF-κB (RANK)/RANK Ligand Costimulatory Molecules into Dendritic Cell Immunogens Expressing a Human Tumor-Specific Antigen. J. Immunol. 2003, 171, 4121–4130.
- Melero, I.; Shuford, W.W.; Newby, S.A.; Aruffo, A.; Ledbetter, J.A.; Hellström, K.E.; Mittler, R.S.; Chen, L. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat. Med. 1997, 3, 682–685.
- Yonezawa, A.; Dutt, S.; Chester, C.; Kim, J.; Kohrt, H.E. Boosting Cancer Immunotherapy with Anti-CD137 Antibody Therapy. Clin. Cancer Res. 2015, 21, 3113–3120.
- Sanchez-Paulete, A.R.; Labiano, S.; Rodriguez-Ruiz, M.E.; Azpilikueta, A.; Etxeberria, I.; Bolaños, E.; Lang, V.; Rodriguez, M.; Aznar, M.A.; Jure-Kunkel, M.; et al. Deciphering CD137 (4-1BB) signaling in T-cell costimulation for translation into successful cancer immunotherapy. Eur. J. Immunol. 2016, 46, 513–522.
- Li, S.-Y.; Liu, Y. Immunotherapy of melanoma with the immune costimulatory monoclonal antibodies targeting CD137. Clin. Pharmacol. Adv. Appl. 2013, 5 (Suppl. 1), 47–53.
- Narazaki, H.; Zhu, Y.; Luo, L.; Zhu, G.; Chen, L. CD137 agonist antibody prevents cancer recurrence: Contribution of CD137 on both hematopoietic and nonhematopoietic cells. Blood 2010, 115, 1941–1948.
- Gauttier, V.; Judor, J.-P.; Le Guen, V.; Cany, J.; Ferry, N.; Conchon, S. Agonistic anti-CD137 antibody treatment leads to antitumor response in mice with liver cancer. Int. J. Cancer 2014, 135, 2857–2867.
- Sznol, M.; Hodi, F.S.; Margolin, K.; McDermott, D.F.; Ernstoff, M.S.; Kirkwood, J.M.; Wojtaszek, C.; Feltquate, D.; Logan, T. Phase I study of BMS-663513, a fully human anti-CD137 agonist monoclonal antibody, in patients (pts) with advanced cancer (CA). J. Clin. Oncol. 2008, 26, 3007.
- Massarelli, E. Clinical safety and efficacy assessment of the CD137 agonist urelumab alone and in combination with nivolumab in patients with hematologic and solid tumor malignancies. In Proceedings of the 31st Annual Meeting and Associated Programs of the Society for Immunotherapy of Cancer, National Harbor, MD, USA, 9–13 November 2016.
- Shuford, W.W.; Klussman, K.; Tritchler, D.D.; Loo, D.T.; Chalupny, J.; Siadak, A.W.; Brown, T.J.; Emswiler, J.; Raecho, H.; Larsen, C.P.; et al. 4-1BB Costimulatory Signals Preferentially Induce CD8+ T Cell Proliferation and Lead to the Amplification In Vivo of Cytotoxic T Cell Responses. J. Exp. Med. 1997, 186, 47–55.
- Wehler, T.C.; Nonn, M.; Brandt, B.; Britten, C.M.; Gröne, M.; Todorova, M.; Link, I.; Khan, S.A.; Meyer, R.G.; Huber, C.; et al. Targeting the activation-induced antigen CD137 can selectively deplete alloreactive T cells from antileukemic and antitumor donor T-cell lines. Blood 2007, 109, 365–373.
- Wolfl, M.; Kuball, J.; Ho, W.Y.; Nguyen, H.; Manley, T.J.; Bleakley, M.; Greenberg, P.D. Activation-induced expression of CD137 permits detection, isolation, and expansion of the full repertoire of CD8+ T cells responding to antigen without requiring knowledge of epitope specificities. Blood 2007, 110, 201–210.
- Watanabe, K.; Suzuki, S.; Kamei, M.; Toji, S.; Kawase, T.; Takahashi, T.; Kuzushima, K.; Akatsuka, Y. CD137-guided isolation and expansion of antigen-specific CD8 cells for potential use in adoptive immunotherapy. Int. J. Hematol. 2008, 88, 311–320.
- Romero, P.; Dunbar, P.R.; Valmori, D.; Pittet, M.; Ogg, G.S.; Rimoldi, D.; Chen, J.-L.; Liénard, D.; Cerottini, J.-C.; Cerundolo, V. Ex Vivo Staining of Metastatic Lymph Nodes by Class I Major Histocompatibility Complex Tetramers Reveals High Numbers of Antigen-experienced Tumor-specific Cytolytic T Lymphocytes. J. Exp. Med. 1998, 188, 1641–1650.
- Robbins, P.F.; Lu, Y.C.; El-Gamil, M.; Li, Y.F.; Gross, C.; Gartner, J.; Lin, J.C.; Teer, J.K.; Cliften, P.; Tycksen, E.; et al. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat. Med. 2013, 19, 747–752.
- Parkhurst, M.R.; Gros, A.; Pasetto, A.; Prickett, T.; Crystal, J.S.; Robbins, P.; Rosenberg, S.A. Isolation of T-Cell Receptors Specifically Reactive with Mutated Tumor-Associated Antigens from Tumor-Infiltrating Lymphocytes Based on CD137 Expression. Clin. Cancer Res. 2017, 23, 2491–2505.
- Palazón, A.; Martínez-Forero, I.; Teijeira, A.; Morales-Kastresana, A.; Alfaro, C.; Sanmamed, M.F.; Perez-Gracia, J.L.; Peñuelas, I.; Hervás-Stubbs, S.; Rouzaut, A.; et al. The HIF-1α Hypoxia Response in Tumor-Infiltrating T Lymphocytes Induces Functional CD137 (4-1BB) for Immunotherapy. Cancer Discov. 2012, 2, 608–623.
- Ye, Q.; Song, D.-G.; Poussin, M.; Yamamoto, T.; Best, A.; Li, C.; Coukos, G.; Powell, D.J. CD137 Accurately Identifies and Enriches for Naturally Occurring Tumor-Reactive T Cells in Tumor. Clin. Cancer Res. 2014, 20, 44–55.
- Ugolini, A.; Zizzari, I.; Ceccarelli, F.; Botticelli, A.; Colasanti, T.; Strigari, L.; Rughetti, A.; Rahimi, H.; Conti, F.; Valesini, G.; et al. 4P IgM-rheumatoid factor as a novel biomarker for a reduced survival in anti-PD-1 treated NSCLC patients through the decrease of CD137+ T-cells. Ann. Oncol. 2020, 31, S1418.
- Ugolini, A.; Zizzari, I.G.; Ceccarelli, F.; Botticelli, A.; Colasanti, T.; Strigari, L.; Rughetti, A.; Rahimi, H.; Conti, F.; Valesini, G.; et al. IgM-Rheumatoid factor confers primary resistance to anti-PD-1 immunotherapies in NSCLC patients by reducing CD137+ T-cells. EBioMedicine 2020, 62, 103098.
- Zizzari, I.G.; Napoletano, C.; Botticelli, A.; Caponnetto, S.; Calabrò, F.; Gelibter, A.; Rughetti, A.; Ruscito, I.; Rahimi, H.; Rossi, E.; et al. TK Inhibitor Pazopanib Primes DCs by Downregulation of the β-Catenin Pathway. Cancer Immunol. Res. 2018, 6, 711–722.
- Zizzari, I.G.; Napoletano, C.; Di Filippo, A.; Botticelli, A.; Gelibter, A.; Calabrò, F.; Rossi, E.; Schinzari, G.; Urbano, F.; Pomati, G.; et al. Exploratory Pilot Study of Circulating Biomarkers in Metastatic Renal Cell Carcinoma. Cancers 2020, 12, 2620.
- Fröhlich, A.; Loick, S.; Bawden, E.G.; Fietz, S.; Dietrich, J.; Diekmann, E.; Saavedra, G.; Fröhlich, H.; Niebel, D.; Sirokay, J.; et al. Comprehensive analysis of tumor necrosis factor receptor TNFRSF9 (4-1BB) DNA methylation with regard to molecular and clinicopathological features, immune infiltrates, and response prediction to immunotherapy in melanoma. EBioMedicine 2020, 52, 102647.
